Cognitive and Learning Outcomes in Late Preterm Infants at School Age: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Source and Search Strategy
2.2. Selection of Eligible Studies: Inclusion and Exclusion Criteria
2.3. Data Extraction
2.4. Quality Assessment
3. Results
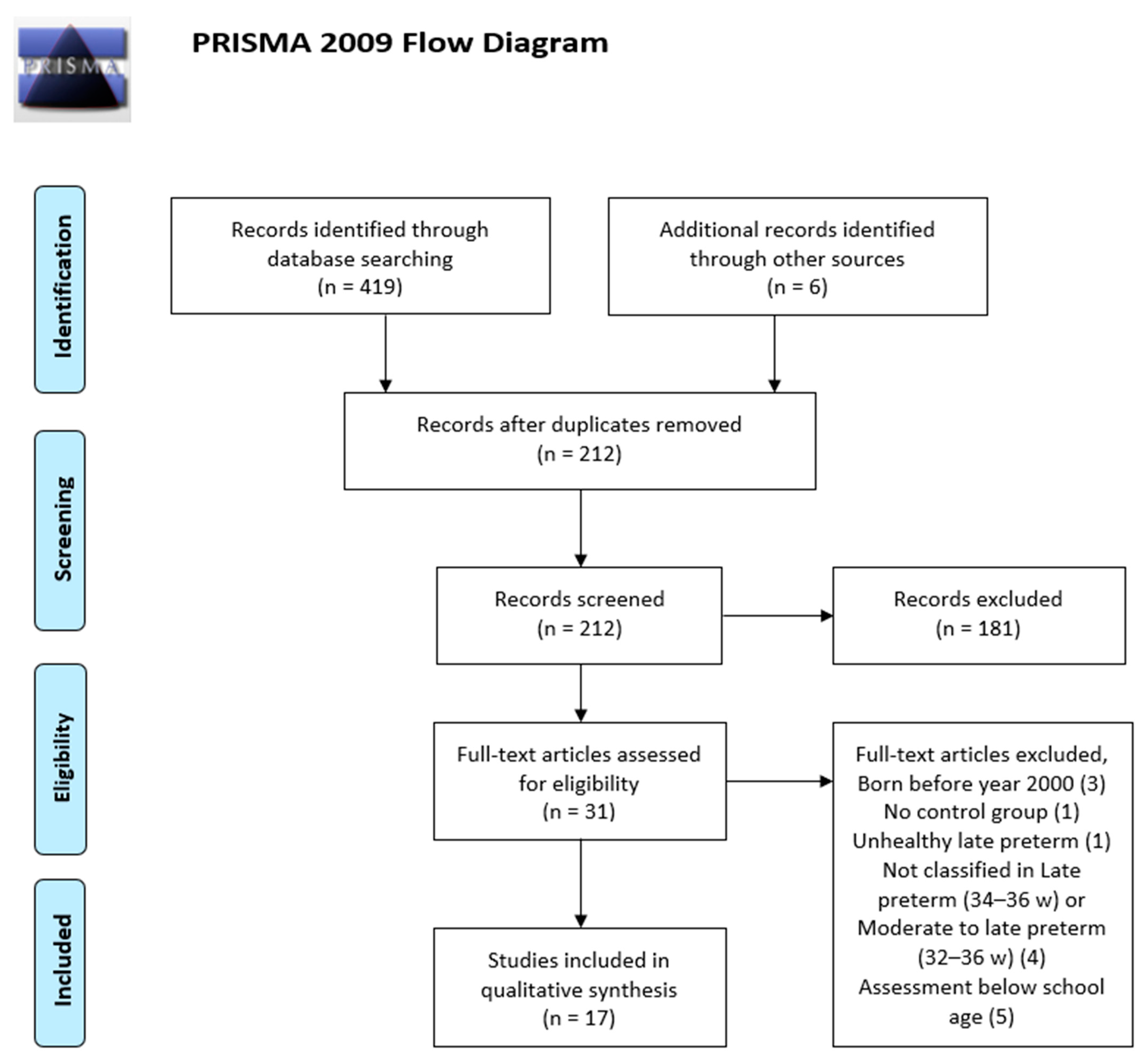
3.1. Included Studies
3.2. Description of Included Studies
3.2.1. Neuropsychological Outcomes: Cognitive Functioning and Language
3.2.2. Academic Outcomes
3.3. Quality of Papers
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Raju, T.N.; Higgins, R.D.; Stark, A.R.; Leveno, K.J. Optimizing care and outcome for late-preterm (near-term) infants: A summary of workshop sponsored by National Institute of Child Health and Human Development. Pediatrics 2006, 118, 1207–1214. [Google Scholar] [CrossRef]
- Wang, M.L.; Dorer, D.J.; Fleming, M.P.; Catlin, E.A. Clinical outcomes of near-term infants. Pediatrics 2004, 114, 372–376. [Google Scholar] [CrossRef]
- Engle, W.A.; Tomashek, K.M.; Wallman, C. Comittee on Fetus and Newborn, American Academy of Pediatrics. “Late-preterm” infants: A population at risk. Pediatrics 2007, 120, 1390–1401. [Google Scholar] [CrossRef] [Green Version]
- Shapiro-Mendoza, C.K.; Tomashek, K.M.; Kotelchuk, M.; Barfield, W.; Weiss, J.; Evans, S. Risk factors for neonatal morbidity and mortality among ‘healthy’ late preterm newborns. Semin. Perinatol. 2006, 30, 54–60. [Google Scholar]
- Vohr, B. Long-term outcomes of moderately preterm, late preterm, and early term infants. Clin. Perinatol. 2013, 40, 739–751. [Google Scholar] [CrossRef]
- Peacock, P.J.; Henderson, J.; Odd, D.; Emond, A. Early school attainment in late-preterm infants. Arch. Dis. Child. 2012, 97, 118–120. [Google Scholar] [CrossRef]
- Huddy, C.L.J.; Johnson, A.; Hope, P.L. Educational and behavioural problems in babies of 32–35 weeks gestation. Arch. Dis. Child. Fetal Neonatal Ed. 2001, 85, F23–F28. [Google Scholar] [CrossRef] [Green Version]
- Lipkind, H.S.; Slopen, M.E.; Pfeiffer, M.R.; McVeigh, K.H. School-age outcomes of late preterm infants in New York City. Am. J. Obstet. Gynecol. 2012, 206, 222.e1–222.e6. [Google Scholar] [CrossRef]
- Chyi, L.; Lee, H.C.; Hintz, S.R.; Gould, J.B.; Sutcliffe, T.L. School outcomes of late preterm infants: Special needs and challenges for infants born at 32 to 36 weeks of gestation. J. Pediatr. 2008, 153, 25–31. [Google Scholar] [CrossRef]
- van Baar, A.L.; Vermaas, J.; Knots, E.; de Kleine, M.J.; Soons, P. Functioning at school age of moderately preterm children born at 32 to 36 weeks’ gestational age. Pediatrics 2009, 124, 251–257. [Google Scholar] [CrossRef]
- Demestre, X.; Schonhaut, L.; Morillas, J.; Martínez-Nadal, S.; Vila, C.; Raspall, F.; Sala, P. Riesgo de déficit del desarrollo en prematuros tardíos. Evaluación a los 48 meses mediante el Ages and Stages Questionnaires. An. Pediatr. 2016, 84, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Nadal, S.; Demestre, X.; Schonhaut, L.; Muñoz, S.R.; Sala, P. Impact of neonatal morbidity on the risk of development delay in late preterm infants. Early Hum. Dev. 2018, 116, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Heinonen, K.; Eriksson, J.G.; Kajantie, E.; Pesonen, A.V.; Barker, D.J.; Osmond, C.; Raikkonen, K. Late-preterm birth and lifetime socioeconomic attainments: The Helsinki birth cohort study. Pediatrics 2013, 132, 647–655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heinonen, K.; Eriksson, J.G.; Lahti, J.; Kajantie, E.; Pesonen, A.K.; Tuovinen, S.; Osmond, C.; Raikkonen, K. Late preterm birth and neurocognitive performance in late adulthood: A birth cohort study. Pediatrics 2015, 135, e818–e825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Jong, M.; Verhoeven, M.; van Baar, A.L. School outcome, cognitive functioning, and behaviour problems in moderate and late preterm children and adults: A review. Semin. Fetal Neonatal Med. 2012, 17, 163–169. [Google Scholar] [CrossRef]
- Moster, D.; Lie, R.T.; Markestad, T. Long-term medical and social consequences of preterm birth. N. Engl. J. Med. 2008, 359, 262–273. [Google Scholar] [CrossRef] [Green Version]
- Lindström, K.; Winbladh, B.; Haglund, B.; Hjern, A. Preterm infants as young adults: A Swedish national cohort study. Pediatrics 2007, 120, 70–77. [Google Scholar] [CrossRef]
- Martin, J.A.; Osterman, M.J.K. Describing the increase in preterm births in the United States, 2014–2016. NCHS Data Brief 2018, 312, 1–8. [Google Scholar]
- García-Reymundo, M.; Demestre, X.; Calvo, M.J.; Ginovart, G.; Jiménez, A.; Hurtado, J.A. Prematuro tardío en España: Experiencia del Grupo SEN34-36. An. Pediatr. 2018, 88, 246–252. [Google Scholar] [CrossRef]
- Matthews, T.J.; MacDorman, M.F.; Thoma, M.E. Infant mortality statistics from the 2013 period linked birth/infant death data set. Natl. Vital Stat. Rep. 2015, 64, 1–30. [Google Scholar]
- Cheong, J.L.Y.; Thompson, D.K.; Olsen, J.E.; Spittle, A.J. Late preterm births: New insights from neonatal neuroimaging and neurobehaviour. Semin. Fetal Neonatal Med. 2019, 24, 60–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kinney, H.C. The near-term (late preterm) human brain and risk for periventricular leukomalacia: A review. Semin. Perinatol. 2006, 30, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Kapellou, O.; Counsell, S.J.; Kennea, N.; Dyet, L.; Saeed, N.; Stark, J.; Maalouf, E.; Duggan, P.; Ajayi-Obe, M.; Hajnal, J.; et al. Abnormal cortical development after premature birth shown by altered allometric scaling of brain growth. PLoS Med. 2006, 3, e265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walsh, J.M.; Doyle, L.W.; Anderson, P.J.; Lee, K.J.; Cheong, J.L. Moderate and late preterm birth: Effect on brain size and maturation at term-equivalent age. Radiology 2014, 273, 232–240. [Google Scholar] [CrossRef]
- Kelly, C.E.; Cheong, J.L.; Gabra Fam, L.; Leemans, A.; Seal, M.L.; Doyle, L.W.; Anderson, P.J.; Spittle, A.J.; Thompson, D.K. Moderate and late preterm infants exhibit widespread brain white matter microestructure alterations at term-equivalent age relative to term-born controls. Brain Imaging Behav. 2016, 10, 41–49. [Google Scholar] [CrossRef]
- Batalle, D.; Hughes, E.J.; Zhang, H.; Tournier, J.D.; Tusor, N.; Aljabar, P.; Wali, L.; Alexander, D.C.; Hajnal, J.V.; Nosarti, C.; et al. Early development of structural networks and the impact of prematurity on brain connectivity. Neuroimage 2017, 149, 379–392. [Google Scholar] [CrossRef] [Green Version]
- Hurtado, J.A.; García-Reymundo, M.; Calvo, M.J.; Ginovart, G.; Jimenez, A.; Tirado, M.; Demestre, X. Recomendaciones para el manejo perinatal y seguimiento del recién nacido prematuro tardío. An. Pediatr. 2014, 81, 327.e1–327.e7. [Google Scholar] [CrossRef]
- Martín, Y.; García-Reymundo, M.; Hurtado, J.A.; Calvo, M.J.; Soriano, F.J.; Ginnovart, G.; Moya, A.J.; Guasch, X.D. Recomendaciones de seguimiento del prematuro tardío. Rev. Pediatr. Aten Primaria 2018, 20, 195–200. [Google Scholar]
- Lipkin, P.H.; Macias, M.M.; AAP Council on children with disabilities, section on developmental and behavioral pediatrics. Promoting optimal development: Identifying infants and young children with developmental disorders through developmental surveillance and screening. Pediatrics 2020, 145, e20193449. [Google Scholar] [CrossRef] [Green Version]
- NICE Guideline. Developmental Follow-Up of Children and Young People Born Preterms. 2017. Available online: www.nice.org.uk/guidance/ng72 (accessed on 30 May 2020).
- McGowan, J.E.; Alderdice, F.A.; Holmes, V.A.; Johnston, L. Early childhood development of late-preterm infants: A systematic review. Pediatrics 2011, 127, 1111–1124. [Google Scholar] [CrossRef] [Green Version]
- Tripathi, T.; Dusing, S. Long-term neurodevelopmental outcomes of infants born late preterm: A systematic review. Res. Rep. Neonatol. 2015, 5, 91–111. [Google Scholar]
- Chan, E.; Leong, P.; Malouf, R.; Quigley, M.A. Long-term cognitive and school outcomes of late-preterm and early-term births: Systematic review. Child. Care Health Dev. 2016, 42, 297–312. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tezlaff, J.; Altman, D.G.; The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [Green Version]
- Critical Appraisal Skills Programme (2018). CASP Cohort Study Checklist. Available online: https://casp-uk.net/wp-content/uploads/2018/03/CASP-Cohort-Study-Checklist-2018_fillable_form.pdf (accessed on 30 May 2020).
- Baron, I.S.; Kerns, K.A.; Müller, U.; Ahronovich, M.D.; Litman, F.R. Executive functions in extremely low birth weight and late-preterm preschoolers: Effects on working memory and response inhibition. Child. Neuropsychol. 2012, 18, 586–599. [Google Scholar] [CrossRef]
- Stene-Larsen, K.; Brandlistuen, R.E.; Lang, A.M.; Landolt, M.A.; Latal, B.; Vollrath, M.E. Communication impairments in early term and late preterm children: A prospective cohort study following children to age 36 months. J. Pediatr. 2014, 165, 1123–1128. [Google Scholar] [CrossRef] [Green Version]
- Brown, H.K.; Speechley, K.N.; Macnab, J.; Natale, R.; Campbell, M.K. Mild prematurity, proximal social processes, and development. Pediatrics 2014, 134, e814–e824. [Google Scholar] [CrossRef] [Green Version]
- Brumbaugh, J.E.; Hodel, A.S.; Thomas, K.M. The impact of late preterm birth on executive function at preschool age. Am.J. Perinatol. 2014, 31, 305–314. [Google Scholar] [PubMed]
- Shah, P.; Kaciroti, N.; Richards, B.; Oh, W.; Lumeng, J.C. Developmental Outcomes of Late Preterm Infants from Infancy to Kindergarten. Pediatrics 2016, 138, e20153496. [Google Scholar] [CrossRef] [Green Version]
- Sejer, E.P.F.; Bruun, F.J.; Slavensky, J.A.; Mortensen, E.L.; Schiøler Kesmodel, U. Impact of gestational age on child intelligence, attention and executive function at age 5: A cohort study. BMJ Open 2019, 9, e028982. [Google Scholar] [CrossRef]
- Hodel, A.S.; Brumbaugh, J.E.; Morris, A.R.; Thomas, K.M. Hot executive function following moderate-to-late preterm birth: Altered delay discounting at 4 years of age. Dev. Sci. 2016, 19, 221–234. [Google Scholar] [CrossRef]
- Hornman, J.; de Winter, A.F.; Kerstjens, J.M.; Bos, A.F.; Reijneveld, S.A. Stability of Developmental Problems after School Entry of Moderately-Late Preterm and Early Preterm-Born Children. J. Pediatr. 2017, 187, 73–79. [Google Scholar] [CrossRef]
- Quigley, M.A.; Poulsen, G.; Boyle, E.; Wolke, D.; Field, D.; Alfirevic, Z.; Kurinczuk, J.J. Early term and late preterm birth are associated with poorer school performance at age 5 years: A cohort study. Arch. Dis. Child. Fetal Neonatal Ed. 2012, 97, F167–F173. [Google Scholar] [CrossRef]
- Baron, I.S.; Weiss, B.A.; Litman, F.R.; Ahronovich, M.D.; Baker, R. Latent mean differences in executive function in at-risk preterm children: The delay-deficit dilemma. Neuropsychology 2014, 28, 541–551. [Google Scholar] [CrossRef]
- Rider, G.N.; Weiss, B.A.; McDermott, A.T.; Hopp, C.A.; Baron, I.S. Test of visuospatial construction: Validity evidence in extremely low birth weight and late preterm children at early school age. Child. Neuropsychol. 2016, 22, 587–599. [Google Scholar] [CrossRef]
- Bogičević, L.; Verhoeven, M.; van Baar, A.L. Toddler skills predict moderate-to-late preterm born children’s cognition and behaviour at 6 years of age. PLoS ONE 2019, 14, e0223690. [Google Scholar] [CrossRef]
- Woythaler, M.; McCormick, M.C.; Mao, W.Y.; Smith, V.C. Late Preterm Infants and Neurodevelopmental Outcomes at Kindergarten. Pediatrics 2015, 136, 424–431. [Google Scholar] [CrossRef] [Green Version]
- Chan, E.; Quigley, M.A. School performance at age 7 years in late preterm and early term birth: A cohort study. Arch. Dis. Child. Fetal Neonatal Ed. 2014, 99, F451–F457. [Google Scholar] [CrossRef] [Green Version]
- Sheehan, J.C.; Kerns, K.A.; Müller, U. The effect of task complexity on planning in preterm-born children. Clin. Neuropsychol. 2017, 31, 438–458. [Google Scholar] [CrossRef]
- Brumbaugh, J.E.; Conrad, A.L.; Lee, J.K.; DeVolder, I.J.; Zimmerman, M.B.; Magnotta, V.A.; Axelson, E.D.; Nopoulos, P.C. Altered brain function, structure, and developmental trajectory in children born late preterm. Pediatr. Res. 2016, 80, 197–203. [Google Scholar] [CrossRef]
- Wachtel, E.V.; Zaccario, M.; Mally, P. Impact of Respiratory Morbidities on Neurodevelopmental Outcome of Late Preterm Infants. Am. J. Perinatol. 2015, 32, 1164–1168. [Google Scholar] [CrossRef]
- Kerstjens, J.M.; Bocca-Tjeertes, I.F.; de Winter, A.F.; Reijneveld, S.A.; Bos, A.F. Neonatal morbidities and developmental delay in moderately preterm-born children. Pediatrics 2012, 130, e265–e272. [Google Scholar] [CrossRef] [Green Version]
- Baron, I.S.; Erickson, K.; Ahronovich, M.D.; Baker, R.; Litman, F.R. Cognitive deficit in preschoolers born late-preterm. Early Hum. Dev. 2011, 87, 115–119. [Google Scholar] [CrossRef]
| Authors, Year and Location | Study Design/ Year of Birth | Study Population | Primary Objective | Assessment Ages/Tests or Tasks/ Confounders Adjusted for | Results/ Short Synopsis |
|---|---|---|---|---|---|
| Baron et al., 2012.US [36] | Longitudinal cohort study from PETIT (Prematurity’s Effects on Toddlers, Infants and Teens) Born 2004–2006 | ELBW n = 52 LPT n = 196 Control group FT n = 121 | To compare ELBW, LPT and FT children with a FT control group on a novel battery of experimental computerized EF tasks. | 3 years Selected EF tasks: -P-CPT -Boy-Girl Stroop -Go/No-Go -Jack’s Boxes -DAS Confounders: GA. Maternal educational level | LPT < FT on complex working memory. LPT = FT on response inhibition measures. LPT < FT on General conceptual ability (GCA) LPT > FT on omission errors in the P-CPT task Selective EF tasks can distinguish between preterm groups of different GA and FT children in preschool years. LPT performed worse than FT on complex working memory. |
| Stene-Larsen et al., 2014. Norway [37] | Cohort study from Norwegian Mother and Child Cohort Study Born 1999–2008 | LPT n = 1673 ET n = 7109 Control group FTn = 30,641 | To investigate the risk of communication impairments at age 18 and 36 months in children born ET and LPT. | 18 months and 3 years -ASQ (Questionnaire) Confounders: Child gender, maternal age, maternal level of education, maternal gestational diabetes, preeclampsia/HELLP syndrome, multiple gestation, SGA. | LPT (and ET): increased risk of communication impairments at both ages. -Communication impairment (18 months): ET aRR 1.27 (95%CI 1.12–1.44), LPT aRR 1.74 (95%CI 1.41–2.14) -Expressive language impairment (36 months): ET aRR 1.22 (95%CI 1.07–1.39), LPT aRR 1.37 (95%CI 1.09–1.73) LPT are at increased risk for communication impairments. Given the large number of children potentially affected, this may result in significant health care costs. |
| Brown et al., 2014. Canada [38] | Cohorts study Secondary analysis of National Longitudinal Survey of Children and Youth (NLSCY) Born 1994–2009 | 2 to 3 years LPT n = 1102 ET n = 4333 4 to 5 years LPT n = 866 ET n = 3478 Control group FT 2 to 3 years N = 9664 4 to 5 years N = 7859 | To elucidate the role that GA plays in determining risks for poor developmental outcomes in LPT and ET in the context of proximal social processes | 2–3 years and 4–5 years - 2–3 years: MSD - 4–5 years: PPVT-R - Parenting Scale Confounders: Smoking, alcohol use during pregnancy, placental ischemia and other hypoxia, maternal diabetes or other medical condition during pregnancy. Social context as described in terms of family structure. Family resources and family functioning. Child gender. | 2–3 years: LPT > FT rate of developmental delay (16.7% LPT, 13.9% FT). LPT: aRR 1.13 (95%CI 0.90-1.42) 4–5 years: LPT > FT rate of receptive vocabulary delay (13.1% LPT, 12.7% FT) LPT: aRR 1.06 (95%CI 0.70–1.43). LPT closer to FT. Social factors (not GA) maybe the most important factor influencing developmental outcomes |
| Brumbaugh, et al., 2014.US [39] | Prospective cohort study Born 2005–2006 | LPT n = 39 Control group FT n = 44 | To assess whether LPT children demonstrate impaired EF compared with full term children | 4 years -Battery of EF tasks -PPVT -BRIEF-P Confounders: Verbal IQ, GA | LPT < FT on verbal inhibitory control and short term verbal memory tasks. LPT = FT on nonverbal inhibitory control or spatial memory. Parents of LPT and FT rated children’s behaviour similarly. GA as a predictor of the task performance. LPT demonstrated compromised verbal inhibitory control and short-term verbal memory compared with full-term peers. LPT may not be spared from altered brain development. |
| Shah et al., 2016. US [40] | Cohort study Early Childhood Longitudinal Study, Birth Cohort (ECLS-B) Born 2001 | LPT n = 1000 Control group ET n = 1800 FTn = 3200 | To compare developmental outcomes of LPT with ET and FT from infancy to kindergarten | 9 and 24 months, 3 years, 4–6 years -9 and 24 months: BSF-R -Preschool-Kindergarten: -PPVT -Pre-CTOPP -Preschool pre-reading assessment and mathematics Confounders: -Maternal age, maternal race or ethnicity, socioeconomic status at 9 months, parenting, infant gender, birth weight, early intervention services -At preschool and Kindergarten: age at assessment, month of school | -9 months: LPT < FT (and ET) in developmental outcomes (T = 47.31) vs. ET (T = 49.12) and FT (T = 50.09). -24 months: LPT = FT in developmental outcomes -Preschool age (3 years): LPT < FT in pre-reading skills and mathematics. -Kindergarten (4–6 years): LPT < FT in reading. Although LPT seem to catch up and demonstrate comparable developmental outcomes to FT at 24 months, later on they demonstrate less optimal pre-reading and reading skills and maths at preschool and kindergarten time points. |
| Sejer et al., 2019. Denmark [41] | Cohort study Lifestyle During Pregnancy Study (LDPS) Born 2003–2008 | VPT to MPT n = 8 LPT n = 40 Control group FT n = 1728 | To assess the impact of GA on intelligence, attention and executive function at 5 years | 5 years -WPPSI-R -TEACh -BRIEF Confounders: Maternal age at birth, maternal IQ, average alcohol consumption in pregnancy, smoking in pregnancy, parity, maternal marital status, parental educational level, child gender. | Very to moderate preterm obtain -10.6 IQ vs. full term and -5.3 in teacher-assessed Global Executive Composite, adjusted results. No association with poor cognition were shown in LPT. No associations between LPT and poor cognitive outcomes were shown at age 5. GA may play an important role in determining cognitive abilities independent of maternal intelligence and parental education. |
| Hodel et al., 2016. US [42] | Observational cohort study Born: NA | MLPT n = 45 Control group FT n = 46 | To determine whether low-risk, healthy children born MLPT also exhibit impairments in the development of prefrontal-dependent hot EF skills in comparison to term children at preschool age. | 4.5–5 years -Hot EF tasks -Cool EF tasks -WPPSI-III -BRIEF-P Confounders: Intelligence, processing speed | M-LPT at age 4.5 years< FT less likely to choose larger, delayed rewards across all levels of reward magnitude on a delay discontinuing task using tangible rewards. MLPT = FT on a delay aversion task involving abstract rewards and on measures of cool EFs. Evidence of disrupted hot EFs in children born MLPT at preschool age as measured on a delay discontinuing tasks. |
| Hornman et al., 2017. Netherlands [43] | Cohort study Longitudinal Preterm Outcome Project (LOLLIPOP) Born 2002–2003 | EPT n = 376 MLPT n = 688 Control group FT n = 403 | To assess the stability of developmental problems before school entry at 4 years and one year after school entry at 5 years | 4 and 5 years -ASQ Confounders: Sex, SGA, multiple birth, low education level of the parents, non-Dutch birth country of parent or children, single parent family | 4 years: MLPT < FT 7.9% (p = 0.016); EPT 13% (p < 0.001), FT 4.1% 5 years: MLPT = FT On underlying domains, MLPT and EPT had mainly emerging motor problems and resolving communication problems, but the changing rates of MLPT were lower. After school entry, the overall development of MLPT shows stability patterns comparable with FT. On the underlying domains, MLPT had patterns comparable with EPT but lower rates. |
| Quigley et al., 2012. UK [44] | Cohort study Millennium Cohort Study (MCS) Born 2000–2002 | VPT n = 84 MPT n = 92 LPT n = 471 ET n = 1596 Control group FT n = 5407 | To compare school performance at age 5 years in four groups of preterm children differing in GA (ET, LPT, MPT and VPT) and a FT control group. | 5 years -FSP Confounders: Child gender, ethnicity, whether firstborn, breastfeeding duration, month of birth, mother’s age at, delivery, marital status, education, social class, languages spoken in the child’s home | % not reaching a good level of overall achievement: -FT: 51% -ET: 55% aRR 1.05 (95%CI 1–1.11) -LPT: 59% aRR 1.12 (95%CI 1.04–1.22) -MPT: 63% aRR 1.19 (95%CI 0.98–1.45) -VPT: 66% aRR 1,19 (95%CI 1–1.42) LPT birth is associated with an increased risk of poorer educational achievement at age 5 years. |
| Baron et al., 2014. US [45] | Retrospective observational cross-sectional cohort study from PETIT (Prematurity’s Effects on Toddlers, Infants and Teens) Born 2000–2009 3 years assessment: Born 1998–2006 6 years assessment: | 3 years -ELBW n = 93 -LPT n = 398 Control group FT n = 177 6 years -ELBW n = 126 -LPT n = 102 Control group FT n = 183 | To use latent means analysis in structural equation modeling (SEM) to make between-group comparisons (ELBW, LPT and FT) in EF at two time points: preschool (3 years) and early school age (6–7 years) | 3 and 6 years - Baron-Hopkins Board Test -DAS EF indicators: 1.Noun fluency 2.Action-verb fluency 3.Similarities reasoning 4.Matrices reasoning 5.Working memory Confounders: No | 3 years: LPT < FT (0.61 SD). 6 years: LPT = FT (0.10 SD) Statistically significant between-group differences at age 3, but no longer present at age 6. LPT showed higher risk for EFsdeficits than FT at an early age. Deficitscould represent a transient developmental delay likely to resolve at an older age, or a more subtle adverse effect likely to persist over the life span. |
| Rider et al., 2016. US [46] | Retrospective cohort study from PETIT (Prematurity’s Effects on Toddlers, Infants and Teens) Born 3 years assessment: 2006–2010 6 years assessment: 2004–2007 | 3 years -ELBW n = 53 -LPT n = 228 Control group FT n = 74 6 years -ELBW n = 42 -LPT n = 141 Control group FT n=82 | To provide convergent validity evidence for TVSC To examine performance differences between participants born ELBW, LPT or at FT at preschool (3 years) and early school age (6 years) | 3 and 6 years -TVSC -Developmental Test of Visual-Motor Integration (VMI, 5th ed.) -DAS-II -Baron-Hopkins Board Test -Purdue Pegboard Test of Manual Dexterity Confounders: No | 3 years: LPT < FT 6 years: LPT = FT TVSC practical differences between LPT and FT were small at age 3 and trivial at age 6 years. Although LPT at 6 years performed comparably to FT on the TVSC, LPT should not be considered absent of risk. |
| Bogicevic et al., 2019. Netherlands [47] | Prospective cohort study, Study in Attention of Preterm children (STAP Project) Born 2010–2011 | MLPT n = 88 Control group FT n = 83 | To compare cognitive and behavioural functioning at 6 years. To assess which toddler skills predict later cognitive and behavioural functioning | 18–24 months and 6 years 18 months: UTATE. 24 months: Bayley-III-NL 6 years:WPPSI-III-NL; CBCL/6-18 Confounders: Maternal education | - MLPT < FT on processing speed - MLPT > FT on behavioural problems. -At 6 years: Attention problems were predicted by poorer orienting of attention skills at 18 months and lower performance IQ was predicted by lower alerting of attention at 18 months. Full scale and verbal IQ were predicted by language skills at 24 months. Poorer functioning in MLPT at primary school-age reveals vulnerabilities specifically in processing speed and attention problems, which suggests the need for specific assessment of these skills. Poorer orienting of attention skills at toddler age as early predictors of later attention problems. |
| Woythaler et al., 2015. US [48] | Cohort study Early Childhood Longitudinal Study, Birth Cohort (ECLS-B) Born 2001 | LPT n = 950 Control group Term n = 4900 | To assess neurodevelopmental outcomes from infancy to school age and determine predictive values of earlier developmental testing compared with school-age testing | 24 months, 4–6 years (kindergarten) 24 months: MDI of BSF-R 4–6 years: TSRS Confounders: Maternal race, education, marital status, prenatal care, primary language, impoverished household, gender, fetal growth, plurality, delivery type, gestational age, and breastfeeding | - LPT >FT in aOR of worse TSRSs (aOR 1.52 (95%CI 1.06–2.18) -Positive predictive value of MDI <70 at 24 months and a TSRS <5% at 4–6 years was 10.4% LPT continue to be delayed at kindergarten compared with FT. The predictive validity of having a TSRS in the bottom 5% given a MDI<70 at 24 months was poor. A child who tested within the normal range (>85) at 24 months had an excellent chance of testing in the normal range at kindergarten. |
| Chan & Quigley, 2014. UK [49] | Cohort study Millenium Cohort Study (MCS) Born 2000–2001 | VPT n = 69 MPT n = 67 LPT n = 360 ET n = 1258 Control group Term n = 4277 | To investigate the effect of GA particularly in LPT and ET on school performance at 7 years | 7 years -KS1 Confounders: Maternal age at delivery, maternal education, maternal socioeconomic status, marital status, multiple births, whether firstborn, smoking during pregnancy. Gender, age within school year. | Increased risk of poor performance: -VPT: aRR 1.78 (95%CI 1.24–2.54) -MPT: aRR 1.71 (95%CI 1.15–2.54) -LPT: aRR 1.36 (95%CI 1.09–1.68) -ET: aRR 1.07 (95%CI 0.94–1.23) LPT birth negatively impacts academic outcomes at age 7 years as measured by KS1 school assessment. |
| Sheehan et al., 2017. Canada [50] | Cohort study Born 2000–2011 | ELBW n = 105 LPT n = 248 Control group FT n = 132 | To examine the effect of preterm birth on planning skills in early and middle childhood using problem solving tasks with different cognitive workload demands | 3, 6 and 9 years -Monkey Tree task (MTT) -DAS/DAS-II -Beery-Buktenica Developmental Test -B-HB -BRIEF-P Confounders: Gender, maternal education | - 3 years: LPT < FT in problem solving (MTT). - 6 to 9 years: LPT = FT in problem solving efficiency. Significant correlations between MTT measures and performance on other EF tasks. MTT captured significant performance differences in planning skills between LPT and FT. Cognitive workload, as a function of problem complexity, affects planning skills in young LPT who show a subtle, but distinguishable, adverse neuropsychological outcome. |
| Brumbaugh, et al., 2016. US [51] | Observational cohort study Born 2000–2006 | LPT n = 52 Control group FT n = 74 | To analyze the potential occurrence of altered brain development in LPT | 6–13 years -PBS-30 -WISC-IV -WRAT -GPT-PANESS Confounders: No | -LPT < FT in processing speed, visuo-spatial perception and memory. -LPT > FT on behavioural difficulties from parental reports -LPT = FT in cognitive ability/academic achievement. -LPT = FT on intracranial volumes, but less total tissue and more cerebrospinal fluid in LPT. Tissue differences in the cerebrum are distributed across cortical and subcortical tissue. LPT had a relatively smaller thalamus than FT. Only FT demonstrated significant decreases in cortical tissue volume and thickness with age. LPT demonstrated more difficulty in processing speed, visual-spatial perception, and memory. Together the behavioural, cognitive and brain structural findings suggest the potential insult of LPT birth on the developing brain given the differences persist at school age. |
| Reference | Did the Study Address a Clearly Focused Issue? | Was the Cohort Recruited in An Acceptable Way? | Was the Exposure Accurately Measured to Minimize Bias? | Was the Outcome Accurately Measured to Minimize Bias? | Have the Authors Identified All Important Confounding Factors? | Have They Taken Account of the Confounding Factors in the Design and/or Analysis? | Was the Follow Up of Subjects Complete Enough? | Was the Follow Up of Subjects Long Enough? |
|---|---|---|---|---|---|---|---|---|
| Baron et al. [36] | Yes | Yes | Yes | Yes | No | Yes | No | No |
| Stene-Larsen et al. [37] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No |
| Brown et al. [38] | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes |
| Brumbaugh et al. [39] | Yes | Yes | Yes | Yes | No | No | Yes | Yes |
| Shah et al. [40] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Sejer et al. [41] | Yes | Yes | Yes | Yes | Yes | Yes | Can’t tell | Yes |
| Hodel et al. [42] | Yes | Yes | Yes | Yes | No | No | Yes | Yes |
| Hornman et al. [43] | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes |
| Quigley et al. [44] | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes |
| Baron et al. [45] | Yes | Yes | Yes | Yes | No | No | Yes | Yes |
| Rider et al. [46] | Yes | Yes | Yes | Yes | No | No | Yes | Yes |
| Bogicevic et al. [47] | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes |
| Woythaler et al. [48] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Chan &Quigley [49] | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes |
| Sheehan et al. [50] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Brumbaugh et al. [51] | Yes | Yes | Yes | Yes | No | No | No | Yes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Nadal, S.; Bosch, L. Cognitive and Learning Outcomes in Late Preterm Infants at School Age: A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 74. https://doi.org/10.3390/ijerph18010074
Martínez-Nadal S, Bosch L. Cognitive and Learning Outcomes in Late Preterm Infants at School Age: A Systematic Review. International Journal of Environmental Research and Public Health. 2021; 18(1):74. https://doi.org/10.3390/ijerph18010074
Chicago/Turabian StyleMartínez-Nadal, Sílvia, and Laura Bosch. 2021. "Cognitive and Learning Outcomes in Late Preterm Infants at School Age: A Systematic Review" International Journal of Environmental Research and Public Health 18, no. 1: 74. https://doi.org/10.3390/ijerph18010074
APA StyleMartínez-Nadal, S., & Bosch, L. (2021). Cognitive and Learning Outcomes in Late Preterm Infants at School Age: A Systematic Review. International Journal of Environmental Research and Public Health, 18(1), 74. https://doi.org/10.3390/ijerph18010074






