Effects of Soy Isoflavones on Biochemical Markers of Bone Metabolism in Postmenopausal Women: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy and Study Selection
2.2. Data Extraction
2.3. Quality Assessment and Bias Risk of the Trials
2.4. Statistical Analysis and Meta-Analysis
2.5. Subgroup Analysis
3. Results
3.1. Characteristics of Included Trials
3.2. Assessment of Study Quality
3.3. Association between Soy Isoflavones Supplementation and Bone Turnover Markers
4. Discussion
4.1. Limitations of this Study
4.2. Mechanisms of Action of Soy Isoflavones
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clarke, B. Normal bone anatomy and physiology. Clin. J. Am. Soc. Nephrol. 2008, 3, 131–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kenkre, J.S.; Bassett, J. The bone remodelling cycle. Ann. Clin. Biochem. 2018, 55, 308–327. [Google Scholar] [CrossRef] [PubMed]
- Smit, T.H.; Burger, E.H.; Huyghe, J.M. Is BMU-coupling a strain-regulated phenomenon? A finite element analysis. J. Bone Miner. Res. 2002, 15, 301–307. [Google Scholar] [CrossRef]
- Jilka, R.L. Biology of the basic multicellular unit and the pathophysiology of osteoporosis. Med. Pediatr. Oncol. 2003, 41, 182–185. [Google Scholar] [CrossRef]
- Seeman, E.; Delmas, P.D. Bone quality—The material and structural basis of bone strength and fragility. N. Engl. J. Med. 2006, 354, 2250–2261. [Google Scholar] [CrossRef] [Green Version]
- Pérez-López, F.R.; Chedraui, P.; Cuadros-López, J.L. Bone mass gain during puberty and adolescence: Deconstructing gender characteristics. Curr. Med. Chem. 2010, 17, 453–466. [Google Scholar] [CrossRef]
- Zemel, B. Bone mineral accretion and its relationship to growth, sexual maturation and body composition during childhood and adolescence. World Rev. Nutr. Diet. 2013, 106, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Mora, S.; Gilsanz, V. Establishment of peak bone mass. Endocrinol. Metab. Clin. N. Am. 2003, 32, 39–63. [Google Scholar] [CrossRef]
- Krall, E.A.; Dawson-Hughes, B. Heritable and life-style determinants of bone mineral density. J. Bone Miner. Res. 1993, 8, 1–9. [Google Scholar] [CrossRef]
- Heaney, R.P.; Abrams, S.; Dawson-Hughes, B.; Looker, A.; Marcus, R.; Matkovic, V.; Weaver, C. Peak bone mass. Osteoporos. Int. 2000, 11, 985–1009. [Google Scholar] [CrossRef]
- Cooper, C.; Eastell, R. Bone gain and loss in premenopausal women. BMJ 1993, 306, 1357–1358. [Google Scholar] [CrossRef] [Green Version]
- Randolph, J.F., Jr.; Sowers, M.; Bondarenko, I.V.; Harlow, S.D.; Luborsky, J.L.; Little, R.J. Change in estradiol and follicle-stimulating hormone across/the early menopausal transition: Effects of ethnicity and age. J. Clin. Endocrinol. Metab. 2004, 89, 1555–1561. [Google Scholar] [CrossRef] [Green Version]
- Yoldemir, T.; Erenus, M.; Durmusoglu, F. The impact of serum FSH and estradiol on postmenopausal osteoporosis related to time since menopause. Gynecol. Endocrinol. 2012, 28, 884–888. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, W.; Yu, C.; Zhang, X.; Zhang, H.; Guan, Q.; Zhao, J.; Xu, J. Follicle-stimulating hormone increases the risk of postmenopausal osteoporosis by stimulating osteoclast differentiation. PLoS ONE 2015, 10, e0134986. [Google Scholar] [CrossRef] [Green Version]
- Eghbali-Fatourechi, G.; Khosla, S.; Sanyal, A.; Boyle, W.J.; Lacey, D.L.; Riggs, B.L. Role of RANK ligand in mediating increased bone resorption in early postmenopausal women. J. Clin. Investig. 2003, 111, 1221–1230. [Google Scholar] [CrossRef]
- Seifert-Klauss, V.; Fillenberg, S.; Schneider, H.; Luppa, P.; Mueller, D.; Kiechle, M. Bone loss in premenopausal, perimenopausal and postmenopausal women: Results of a prospective observational study over 9 years. Climacteric 2012, 15, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Riis, B.J.; Hansen, M.A.; Jensen, A.M.; Overgaard, K.; Christiansen, C. Low bone mass and fast rate of bone loss at menopause: Equal risk factors for future fracture: A 15-year follow-up study. Bone 1996, 19, 9–12. [Google Scholar] [CrossRef]
- Seibel, M.J. Biochemical markers of bone turnover: Part I: Biochemistry and variability. Clin. Biochem. Rev. 2005, 26, 97–122. [Google Scholar]
- Greenblatt, M.B.; Tsai, J.N.; Wein, M.C. Bone turnover markers in the diagnosis and monitoring of metabolic bone disease. Clin. Chem. 2017, 63, 464–474. [Google Scholar] [CrossRef] [Green Version]
- Garnero, P.; Sornay-Rendu, E.; Claustrat, B.; Delmas, P.J. Biochemical markers of bone turnover, endogenous hormones and the risk of fractures in postmenopausal women: The OFELY study. J. Bone Miner. Res. 2000, 15, 1526–1536. [Google Scholar] [CrossRef] [PubMed]
- Chaki, O.; Yoshikata, I.; Kikuchi, R.; Nakayama, M.; Uchiyama, Y.; Hirahara, F.; Gorai M.D., I. The predictive value of biochemical markers of bone turnover for bone mineral density in postmenopausal Japanese women. J. Bone Miner Res. 2000, 15, 1537–1544. [Google Scholar] [CrossRef] [PubMed]
- Karlamangla, A.S.; Burnett-Bowie, S.M.; Crandall, C.J. Bone health during the menopause transition and beyond. Obstet. Gynecol. Clin. N. Am. 2018, 45, 695–708. [Google Scholar] [CrossRef]
- De Franciscis, P.; Colacurci, N.; Riemma, G.; Conte, A.; Pittana, E.; Guida, M.; Schiattarella, A. A nutraceutical approach to menopausal complaints. Med. Kaunas 2019, 55, 544. [Google Scholar] [CrossRef] [Green Version]
- De Franciscis, P.; Conte, A.; Schiattarella, A.; Riemma, G.; Cobellis, L.; Colacurci, N. Non-hormonal treatments for menopausal symptoms and sleep disturbances: A comparison between purified pollen extracts and soy isoflavones. Curr. Pharm. Des. 2020, 26, 4509–4514. [Google Scholar] [CrossRef] [PubMed]
- Pabich, M.; Materska, M. Biological effect of soy isoflavones in the prevention of civilization diseases. Nutrients 2019, 11, 1660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions Version 6.0. Available online: https://training.cochrane.org/handbook/current (accessed on 30 June 2020).
- Begg, C.B.; Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [Green Version]
- Cumpston, M.; Li, T.; Page, M.J.; Chandler, J.; Welch, V.A.; Higgins, J.P.T.; Thomas, J. Updated guidance for trusted systematic reviews: A new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev. 2019, 10, ED000142. [Google Scholar] [CrossRef] [Green Version]
- Follmann, D.; Elliott, P.; Suh, I.; Cutler, J. Variance imputation for overviews of clinical trials with continuous response. J. Clin. Epidemiol. 1999, 45, 769–773. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials revisited. Contemp. Clin. Trials 2015, 45, 139–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Hernández, A.V.; Steyerberg, E.W.; Habbema, J.D. Covariate adjustment in randomized controlled trials with dichotomous outcomes increases statistical power and reduces sample size requirements. J. Clin. Epidemiol. 2004, 57, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Baker, W.J.; White, C.M.; Cappelleri, J.C.; Kluger, J.; Coleman, C.I. Health Outcomes, Policy, and Economics (HOPE) Collaborative Group. Understanding heterogeneity in meta-analysis: The role of meta-regression. Int. J. Clin. Pract. 2009, 63, 1426–1434. [Google Scholar] [CrossRef] [PubMed]
- Knight, D.C.; Howes, J.B.; Eden, J.A.; Howes, L.G. Effects on menopausal symptoms and acceptability of isoflavone-containing soy powder dietary supplementation. Climacteric 2001, 4, 13–18. [Google Scholar] [CrossRef]
- Morabito, N.; Crisafulli, A.; Vergara, C.; Gaudio, A.; Lasco, A.; Frisina, N.; D’Anna, R.; Corrado, F.; Pizzoleo, M.A.; Cincotta, M.; et al. Effects of genistein and hormone-replacement therapy on bone loss in early postmenopausal women: A randomized double-blind placebo-controlled study. J. Bone Miner. Res. 2002, 17, 1904–1912. [Google Scholar] [CrossRef] [PubMed]
- Arjmandi, B.H.; Khalil, D.A.; Smith, B.J.; Lucas, E.A.; Juma, S.; Payton, M.E.; Wild, R.A. Soy protein has a greater effect on bone in postmenopausal women not on hormone replacement therapy, as evidenced by reducing bone resorption and urinary calcium excretion. J. Clin. Endocrinol. Metab. 2003, 88, 1048–1054. [Google Scholar] [CrossRef] [Green Version]
- Dalais, F.S.; Ebeling, P.R.; Kotsopoulos, D.; McGrath, B.P.; Teede, H.J. The effects of soy protein containing isoflavones on lipids and indices of bone resorption in postmenopausal women. Clin. Endocrinol. Oxf. 2003, 58, 704–709. [Google Scholar] [CrossRef]
- Uesugi, T.; Toda, T.; Okuhira, T.; Chen, J.T. Evidence of estrogenic effect by the three-month-intervention of isoflavone on vaginal maturation and bone metabolism in early postmenopausal women. Endocr. J. 2003, 50, 613–619. [Google Scholar] [CrossRef] [Green Version]
- Brooks, J.D.; Ward, W.E.; Lewis, J.E.; Hilditch, J.; Nickell, L.; Wong, E.; Thompson, L.U. Supplementation with flaxseed alters estrogen metabolism in postmenopausal women to a greater extent than does supplementation with an equal amount of soy. Am. J. Clin. Nutr. 2004, 79, 318–325. [Google Scholar] [CrossRef]
- Nikander, E.; Metsä-Heikkilä, M.; Ylikorkala, O.; Tiitinen, A. Effects of phytoestrogens on bone turnover in postmenopausal women with a history of breast cancer. J. Clin. Endocrinol. Metab. 2004, 89, 1207–1212. [Google Scholar] [CrossRef] [Green Version]
- Kreijkamp-Kaspers, S.; Kok, L.; Grobbee, D.E.; de Haan, E.H.; Aleman, A.; Lampe, J.W.; Schouw, Y.T. Effect of soy protein containing isoflavones on cognitive function, bone mineral density, and plasma lipids in postmenopausal women: A randomized controlled trial. JAMA 2004, 292, 65–74. [Google Scholar] [CrossRef] [Green Version]
- Harkness, L.S.; Fiedler, K.; Sehgal, A.R.; Oravec, D.; Lerner, E. Decreased bone resorption with soy isoflavone supplementation in postmenopausal women. J. Womens Health Larchmt 2004, 13, 1000–10007. [Google Scholar] [CrossRef]
- Arjmandi, B.H.; Lucas, E.A.; Khalil, D.A.; Devareddy, L.; Smith, B.J.; McDonald, J.; Arquitt, A.B.; Payton, M.E.; Mason, C. One year soy protein supplementation has positive effects on bone formation markers but not bone density in postmenopausal women. Nutr. J. 2005, 4, 8. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Oka, J.; Higuchi, M.; Tabata, I.; Toda, T.; Fujioka, M.; Fuku, N.; Teramoto, T.; Okuhira, T.; Ueno, T.; et al. Cooperative effects of isoflavones and exercise on bone and lipid metabolism in postmenopausal Japanese women: A randomized placebo-controlled trial. Metabolism 2006, 55, 423–433. [Google Scholar] [CrossRef]
- Ye, Y.B.; Tang, X.Y.; Verbruggen, M.A.; Su, Y.X. Soy isoflavones attenuate bone loss in early postmenopausal Chinese women: A single-blind randomized, placebo-controlled trial. Eur. J. Nutr. 2006, 45, 327–334. [Google Scholar] [CrossRef]
- Evans, E.M.; Racette, S.B.; Van Pelt, R.E.; Peterson, L.R.; Villareal, D.T. Effects of soy protein isolate and moderate exercise on bone turnover and bone mineral density in postmenopausal women. Menopause 2007, 14, 481–488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brink, E.; Coxam, V.; Robins, S.; Wahala, K.; Cassidy, A.; Branca, F. PHYTOS Investigators. Long-term consumption of isoflavone-enriched foods does not affect bone mineral density, bone metabolism, or hormonal status in early postmenopausal women: A randomized, double-blind, placebo controlled study. Am. J. Clin. Nutr. 2008, 87, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Marini, H.; Bitto, A.; Altavilla, D.; Burnett, B.P.; Polito, F.; Di Stefano, V.; Minutoli, L.; Atteritano, M.; Levy, R.M.; D’Anna, R.; et al. Breast safety and efficacy of genistein aglycone for postmenopausal bone loss: A follow-up study. J. Clin. Endocrinol. Metab. 2008, 93, 4787–4796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turhan, N.O.; Bolkan, F.; Duvan, C.I.; Ardicoglu, Y. The effect of isoflavones on bone mass remodelling markers in postmenopausal women. Turk. J. Med. Sci. 2008, 38, 145–152. [Google Scholar]
- Kenny, A.M.; Mangano, K.M.; Abourizk, R.H.; Bruno, R.S.; Anamani, D.E.; Kleppinger, A.; Walsh, S.J.; Prestwood, K.M.; Kerstetter, J.E. Soy proteins and isoflavones affect bone mineral density in older women: A randomized controlled trial. Am. J. Clin. Nutr. 2009, 90, 234–242. [Google Scholar] [CrossRef]
- Vupadhyayula, P.M.; Gallagher, J.C.; Templin, T.; Logsdon, S.M.; Smith, L.M. Effects of soy protein isolate on bone mineral density and physical performance indices in postmenopausal women--a 2-year randomized, double-blind, placebo-controlled trial. Menopause 2009, 16, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Levis, S.; Strickman-Stein, N.; Ganjei-Azar, P.; Xu, P.; Doerge, D.R.; Krischer, J. Soy isoflavones in the prevention of menopausal bone loss and menopausal symptoms: A randomized, double-blind trial. Arch. Intern. Med. 2011, 171, 1363–1369. [Google Scholar] [CrossRef] [Green Version]
- Tai, T.Y.; Tsai, K.S.; Tu, S.T.; Wu, J.S.; Chang, C.I.; Chen, C.L.; Shaw, N.S.; Peng, H.Y.; Wang, S.Y.; Wu, C.H. The effect of soy isoflavone on bone mineral density in postmenopausal Taiwanese women with bone loss: A 2-year randomized double-blind placebo-controlled study. Osteoporos. Int. 2012, 23, 1571–1580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.; Choue, R.; Lim, H. Effect of soy isoflavones supplement on climacteric symptoms, bone biomarkers, and quality of life in Korean postmenopausal women: A randomized clinical trial. Nutr. Res. Pract. 2017, 11, 223–231. [Google Scholar] [CrossRef] [Green Version]
- Sathyapalan, T.; Aye, M.; Rigby, A.S.; Fraser, W.D.; Thatcher, N.J.; Kilpatrick, E.S.; Atkin, S.L. Soy reduces bone turnover markers in women during early menopause: A randomized controlled trial. J. Bone Miner. Res. 2017, 32, 157–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Liu, Y.; Xu, Q.; Zhang, Y.; Liu, L.; Li, H.; Li, F.; Liu, Z.; Yang, X.; Yu, X.; et al. The effect of soy isoflavone combined with calcium on bone mineral density in perimenopausal Chinese women: A 6-month randomised double-blind placebo-controlled study. Inter. J. Food Sci. Nutr. 2020, 71, 473–481. [Google Scholar] [CrossRef]
- Ma, D.F.; Qin, L.Q.; Wang, P.Y.; Katoh, R. Soy isoflavone intake inhibits bone resorption and stimulates bone formation in menopausal women: Meta-analysis of randomized controlled trials. Eur. J. Clin. Nutr. 2008, 62, 155–161. [Google Scholar] [CrossRef] [Green Version]
- Uesugi, T.; Fukui, Y.; Yamori, Y. Beneficial effects of soybean isoflavone supplementation on bone metabolism and serum lipids in postmenopausal japanese women: A four-week study. J. Am. Coll. Nutr. 2002, 21, 97–102. [Google Scholar] [CrossRef]
- Yamori, Y.; Moriguchi, E.H.; Teramoto, T.; Miura, A.; Fukui, Y.; Honda, H.; Fukui, M.; Nara, Y.; Taira, K.; Moriguchi, Y. Isoflavones reduce postmenopausal bone resorption in female Japanese immigrants in Brazil: A ten-week study. J. Am. Coll. Nutr. 2002, 21, 560–563. [Google Scholar] [CrossRef]
- Mori, M.; Sagara, M.; Ikeda, K.; Miki, T.; Yamori, Y. Soy isoflavones improve bone metabolism in postmenopausal Japanese women. Clin. Exp. Pharmacol. Physiol. 2004, 31, 44–46. [Google Scholar] [CrossRef] [PubMed]
- Taku, K.; Melby, M.K.; Kurzer, M.S.; Mizuno, S.; Watanabe, S.; Ishimi, Y. Effects of soy isoflavone supplements on bone turnover markers in menopausal women: Systematic review and meta-analysis of randomized controlled trials. Bone 2010, 47, 413–423. [Google Scholar] [CrossRef]
- Albertazzi, P.; Steel, S.A.; Bottazzi, M. Effect of pure genistein on bone markers and hot flushes. Climacteric 2005, 8, 371–379. [Google Scholar] [CrossRef]
- Xu, F.; Jin, B.Q.; Wu, W.P.; Lv, I.; Tang, D. Effects of phtyto-estrogens on the bone mineral density and biochemical markers of bone metabolism in postmenopausal women (in Chinese). Matern. Child Health Care China 2007, 22, 1517–1519. [Google Scholar]
- Wei, P.; Liu, M.; Chn, Y.; Chen, D.C. Systematic review of soy isoflavone supplements on osteoporosis in women. Asian Pac. J. Tropl. Med. 2012, 5, 243–248. [Google Scholar] [CrossRef] [Green Version]
- Uesugi, S.; Watanabe, S.; Ishiwata, N.; Uehara, M.; Ouchi, K. Effects of isoflavone supplements on bone metabolic markers and climacteric symptoms in Japanese women. Biofactors 2004, 22, 221–228. [Google Scholar] [CrossRef]
- Shao, X.J.; Ye, Z.Q.; Luo, C.X.; Cai, D.Z. Effect of soy isoflavones on osteoporosis in postmenopausal women. Acad. J. Sun Yat-Sen Univ. 2007, 28, 241–242. [Google Scholar]
- Arcoraci, V.; Atteritano, M.; Squadrito, F.; D’Anna, R.; Marini, H.S.D.; Minutoli, L.; Messina, S.; Altavilla, D.; Bitto, A. Antiosteoporotic cctivity of genistein aglycone in postmenopausal women: Evidence from a post-hoc analysis of a multicenter randomized controlled trial. Nutrients 2017, 9, 179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuiper, G.G.; Lemmen, J.G.; Carlsson, B.; Corton, J.C.; Safe, S.H.; van der Saag, P.T.; Burg, B.; Gustafsson, J.-Å. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology 1998, 139, 4252–4263. [Google Scholar] [CrossRef]
- Mueller, S.O.; Simon, S.; Chae, K.; Metzler, M.; Korach, K.S. Phytoestrogens and their human metabolites show distinct agonistic and antagonistic properties on estrogen receptor alpha (Eralpha) and Erbeta in human cells. Toxicol. Sci. 2004, 80, 14–25. [Google Scholar] [CrossRef] [Green Version]
- Okazaki, R.; Inoue, D.; Shibata, M.; Saika, M.; Kido, S.; Ooka, H.; Tomiyama, H.; Sakamoto, Y.; Matsumoto, T. Estrogen promotes early osteoblast differentiation and inhibits adipocyte differentiation in mouse bone marrow stromal cell lines that express estrogen receptor (ER) alpha or beta. Endocrinology 2002, 143, 2349–2356. [Google Scholar] [CrossRef]
- Abdelrazek, H.M.A.; Mahmoud, M.M.A.; Tag, H.M.; Greish, S.M.; Eltamany, D.A.; Soliman, M.T.A. Soy isoflavones ameliorate metabolic and immunological alterations of ovariectomy in female wistar rats: Antioxidant and estrogen sparing potential. Oxid. Med. Cell Longev. 2019, 2019, 5713606. [Google Scholar] [CrossRef] [Green Version]
- Marini, H.; Minutoli, L.; Polito, F.; Bitto, A.; Altavilla, D.; Atteritano, M.; Gaudio, A.; Mazzaferro, S.; Frisina, A.; Frisina, N.; et al. OPG and sRANKL serum concentrations in osteopenic, postmenopausal women after 2-year genistein administration. J. Bone Miner. Res. 2008, 23, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Ju, W.C.; Yeo, J.H.; Kim, J.Y.; Seo, H.S.; Uchida, Y.; Cho, Y. Increased OPG/RANKL ratio in the conditioned medium of soybean-treated osteoblasts suppresses RANKL-induced osteoclast differentiation. Int. J. Mol. Med. 2014, 33, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Mezei, O.; Banz, W.J.; Steger, R.W.; Peluso, M.R.; Winters, T.A.; Shay, N. Soy isoflavones exert antidiabetic and hypolipidemic effects through the PPAR pathways in obese Zucker rats and murine RAW 264.7 cells. J. Nutr. 2003, 133, 1238–1243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giaginis, C.; Tsantili-Kakoulidou, A.; Theocharis, S. Peroxisome proliferator-activated receptors (PPARs) in the control of bone metabolism. Fundam. Clin. Pharmacol. 2007, 21, 231–244. [Google Scholar] [CrossRef] [PubMed]
- Suh, K.S.; Koh, G.; Park, C.Y.; Woo, J.T.; Kim, S.W.; Kim, N.P.; Park, I.K.; Kim, Y.S. Soybean isoflavones inhibit tumor necrosis factor-alpha-induced apoptosis and the production of interleukin-6 and prostaglandin E2 in osteoblastic cells. Phytochemistry 2003, 63, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.F.; Wong, M.S. Genistein modulates the effects of parathyroid hormone in human osteoblastic SaOS-2 cells. Brit. J. Nutr. 2006, 95, 1039–1047. [Google Scholar] [CrossRef] [Green Version]
- Joshua, J.; Kalyanaraman, H.; Marathe, N.; Pilz, R.B. Nitric oxide as a mediator of estrogen effects in osteocytes. Vitam. Horm. 2014, 96, 247–263. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.R.; Xiao, C.L.; He, G.W.; Yin, X.F.; Chen, N.P.; Cao, Y.; He, Q.-Y. Global phosphoproteomic effects of natural tyrosine kinase inhibitor, genistein, on signaling pathways. Proteomics 2010, 10, 976–986. [Google Scholar] [CrossRef]
- Jung, H.; Ahn, S.; Kim, B.S.; Shib, S.Y.; Lee, Y.H.; Lim, T. Isoflavones as modulators of adenosine monophosphate-activated protein kinase. Appl. Biol. Chem. 2016, 59, 217–225. [Google Scholar] [CrossRef]
- Jeyabalan, J.; Shah, M.; Viollet, B.; Chenu, C. AMP-activated protein kinase pathway and bone metabolism. J. Endocrinol. 2011, 212, 277–290. [Google Scholar] [CrossRef]
- Sugimoto, T.; Nishiyama, K.; Kuribayashi, F.; Chihara, K. Serum levels of insulin-like growth factor (IGF) I, IGF-binding protein (IGFBP)-2, and IGFBP-3 in osteoporotic patients with and without spinal fractures. J. Bone Miner. Res. 1997, 12, 1272–1279. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Feng, Y.; Feng, J.; Chen, X. The effect of soy intervention on insulin-like growth factor 1 levels: A meta-analysis of clinical trials. Phytother. Res. 2020, 34, 1570–1577. [Google Scholar] [CrossRef]
- Kim, H.; Peterson, T.G.; Bernes, S. Mechanisms of action of the soy isoflavone genistein: Emerging role for effects via transformiang growth factor beta signaling pathaway. Am. J. Clin. Nutr. 1999, 68, 1418–1425. [Google Scholar] [CrossRef] [Green Version]
- Chang, K.L.; Hu, Y.C.; Hsieh, B.S.; Cheng, H.L.; Hsu, H.W.; Huang, L.W.; Su, S.J. Combined effect of soy isoflavones and vitamin D3 on bone loss in ovariectomized rats. Nutrition 2013, 29, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Mazumder, M.A.R.; Hongsprabhas, P. Genistein as antioxidant and antibrowning agents in In Vivo and In Vitro: A review. Biomed. Pharmacother. 2016, 82, 379–392. [Google Scholar] [CrossRef]
- Unfer, V.; Casini, M.L.; Costabile, L.; Mignosa, M.; Gerli, S.; Di Renzo, G.C. Endometrial effects of long-term treatment with phytoestrogens: A randomized, double-blind, placebo-controlled study. Fertil. Steril. 2004, 82, 145–148. [Google Scholar] [CrossRef]
- Murray, M.J.; Meyer, W.R.; Lessey, B.A.; Oi, R.H.; DeWire, R.E. Soy protein isolate with isoflavones does not prevent estradiol-induced endometrial hyperplasia in postmenopausal women: A pilot trial. Menopause 2003, 10, 456–464. [Google Scholar] [CrossRef]
- Palacios, S.; Pornel, B.; Bergeron, C.; Chantre, P.; Nogales, F.; Aubert, L.; Vazquez, F.; Eden, J.; Mares, P. Endometrial safety assessment of a specific and standardized soy extract according to international guidelines. Menopause 2007, 14, 1006–1011. [Google Scholar] [CrossRef] [PubMed]
- Alekel, D.L.; Genschel, U.; Koehler, K.J.; Hofmann, H.; Van Loan, M.D.; Beer, B.S.; Hanson, L.N.; Peterson, C.T.; Kurzer, M.S. Soy Isoflavones for Reducing Bone Loss Study: Effects of a 3-year trial on hormones, adverse events, and endometrial thickness in postmenopausal women. Menopause 2015, 22, 185–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quaas, A.M.; Kono, N.; Mack, W.J.; Hodis, H.N.; Felix, J.C.; Paulson, R.J.; Shoupe, D. Effect of isoflavone soy protein supplementation on endometrial thickness, hyperplasia, and endometrial cancer risk in postmenopausal women: A randomized controlled trial. Menopause 2013, 20, 840–844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
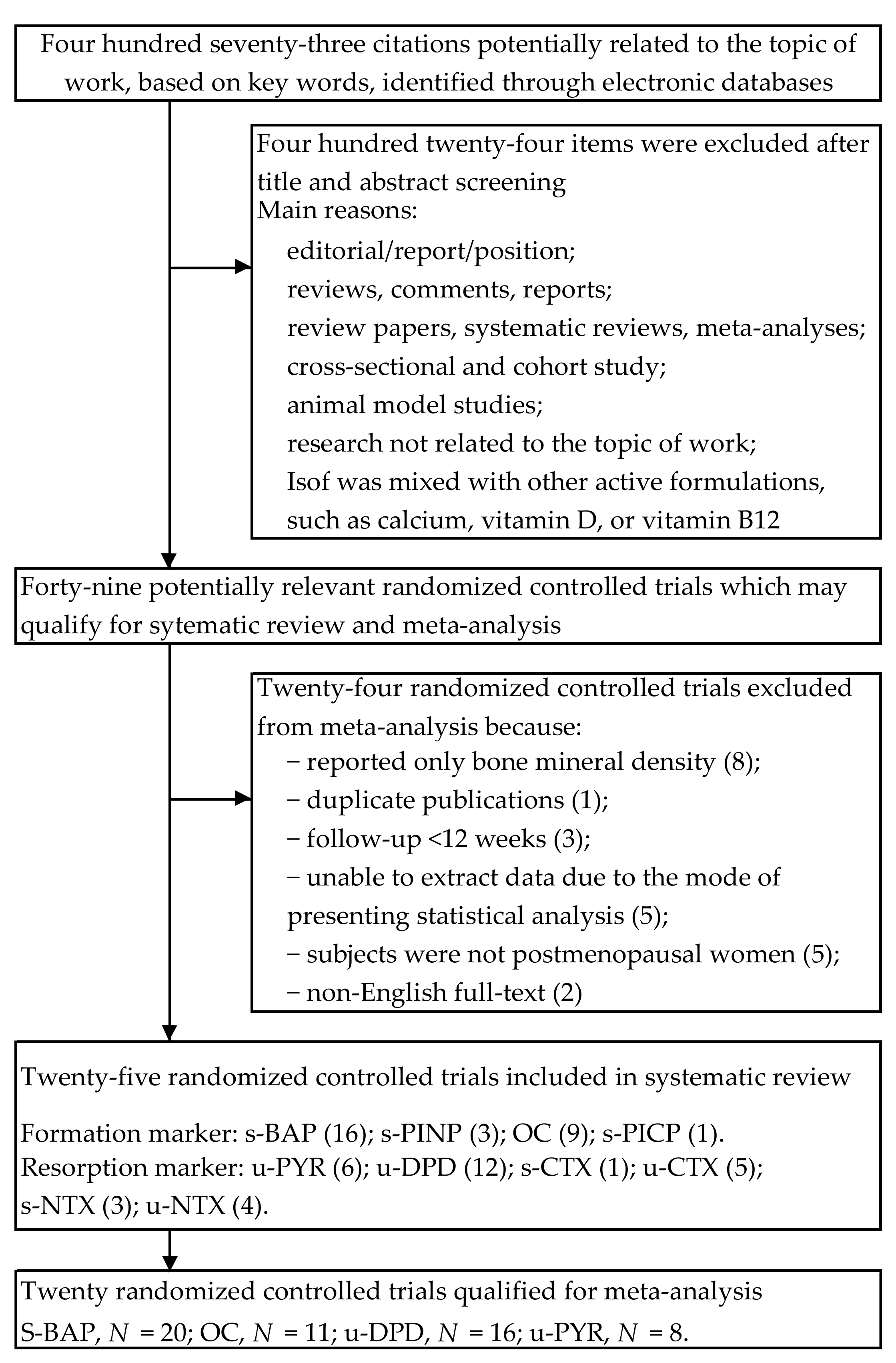







| First Author Year Country | Study Design Follow-up | Participants a Age, y (Range) Treated/Control | Intervention form Therapy and Daily Dosage b vs. Control | Indices c Formation Markers Resorption Markers |
|---|---|---|---|---|
| Knight [36] 2001 UK | Double-blind, parallel group 3-months | 53.1 ± 4.6 (40–65) 3.6 ± 4.5 ysm 9/11 | Beverage powder 60 g SP, 134.4 mg Isof (aglycones: 77.4 mg) vs. casein protein | OC |
| Morabito [37] 2002 Italy | Double-blind, parallel group 12-months | 51.5 ± 4.5 (47–57) 6.5 ± 2.5 ysm BMI 24 ± 3 T-score = 1 SD 30/30 | Tablet 54 mg Isof (Gen, purity ~98%) vs. placebo | OC, BAP YD, DPD |
| Arjmandi [38] 2003 USA | Double-blind, parallel group 3-months | 62.1 ± 11.0 BMI 32.2 ± 8.0 20/22 | Powder in drink mix 40 g SP, 88.4 mg Isof vs. 40 g milk protein | BAP DPD |
| Dalais [39] 2003 Australia | Double-blind, parallel group 3-months | 60.0 ± 6.2 (50–75) BMI 25.5 ± 4.6 38/40 | Powder mixed with drinks or food 40 g SP, 118 mg Isof (aglycones: 69 mg) vs. 40 g casein protein | PYD, DPD |
| Uesugi [40] 2003 Japan | Double-blind, parallel group 3-months | 53.7 ± 6.9 (45–65) 6.0 ± 6.0 ysm BMI 22.6 ± 2.8 11/10 | Capsule 61.8 mg Isof (glycosides: 31.2 mg Dai, 6.8 mg Gen, 21.6 Gly) vs. placebo (dextrin) | PYD |
| Brooks [41] 2004 Canada | Double-blind, parallel group 16-weeks | 53.4 ± 3.02 <5 ysm BMI 27.4 ± 5.3 13/15 | Muffins 25 g SP, 41.9 mg Isof (15.5 mg Dai, 25.7 mg Gen, 0.7 mg Gly) vs. 25 g whole-wheat flour | BAP DPD |
| Nikander [42] 2004 Finland | Double-blind, cross-over trial 3-months | 55 ± 6 (35–69) ≥ 5 ysm BMI 26.3 ± 3.3 28/28 | Tablet 114 mg Isof (aglycones: 58% Gly, 36% Dai,6% Gen) vs. placebo | BAP, PINP, PICP PYD, DPD, NTX |
| Kreijkamp-Kaspers [43] 2004 Netherlands | Double-blind, parallel group 12-months | 66.6 ± 4.8 (60–75) 18.0 ± 7.0 ysm BMI 26.2 ± 3.8 88/87 | Powder mixed with drinks or food 25.6 g SP: 99 mg Isof (aglycones: 52 mg Gen, 41 mg Dai, 6 mg Gly) vs. 25.6 g milk protein | BAP |
| Harkness [44] 2004 USA | Double-blind, cross-over trial 6-months | 70.6 ± 6.3 19.1 ± 5.5 ysm BMI 26.1 ± 4.8 T = score ≥ −2.5 10/9 | Pill 110 mg Isof (98% glycosides and 2% aglycones) vs. placebo | OC, BAP |
| Arjmandi [45] 2005 USA | Double-blind, parallel group 12-months | 54.5 ± 5.5 (<65) 5.5 ± 5.0 ysm BMI 27.9 ± 5.3 35/27 | Snack, drink mix or cereal 25 g SP, 60 mg Isof vs. 20 g protein (devoid of soy and isof) | OC, BAP DPD |
| Wu [46] 2006 Japan | Double-blind, parallel group 6-months | 54.3 ± 2.9 (45–60) 3.2 ± 1.7 ysm BMI 21.1 ± 2.4 33/33 | Capsule 75 mg Isof (47 mg aglycones) vs. placebo (dextrin) | OC, BAP DPD |
| Ye [47] 2006 China | Single-blind, parallel group, 3 arms trial 6-months | 52.3 ± 3.3 (45–60) 2.6 ± 1.5 ysm BMI 22.6 ± 2.3 25/26/27 | Capsule (a) 126 mg Isof, (b) 84 mg isoflavones (aglycones: 52% Dai, 15% Gen, 33% Gly) vs. placebo (starch) | OC, BAP DPD |
| Evans [48] 2007 USA | Double-blind, parallel group 9-months | 63.1 ± 5.1 (50–65) 8.2 ± 5.1 ysm BMI 26.8 ± 2.8 21/22 | Protein beverage products 25.6 g SP, 91.5 mg Isof (aglycones) vs. 25.6 g milk protein | BAP CTX |
| Brink [49] 2008 Netherlands, Italy, France | Double-blind, parallel group, international, multicenter trial 12-months | 53 ± 3 2.7 ± 1.3 ysm BMI 24.5 ± 2.1 118 (NL 45; I, 39; F, 34)/119 (NL, 46; I, 39; F, 34) | Biscuits or bars 110 mg Isof (aglycones: 60–75% Gen, 25–35% Dai, 1–5% Gly) vs. control | BAP, PINP PYD, DPD |
| Marini [50] 2008 Italy | Double-blind, parallel group 24-months | 53.7 ± 2.5 3.6 ± 2.6 ysm BMI 24.9 ± 3.7 71/67 | Tablet 54 mg Isof (Gen, purity ~98% vs. placebo | BAP PYD, DPD, CTX |
| Turhan [51] 2008 Turkey | Single-blind, parallel group 6-months | 51.5 ± 5.2 (42–59) 3.7 ± 1.8 ysm BMI 27.0 ± 3.1 45/45 | Tablet 80 mg isof (aglycones: 59.6 mg Gen, 15.6 mg Dai, 4.8 Gly) vs. placebo (starch) | OC CTX |
| Kenny [52] 2009 United States | Double-blind, parallel group 12-months | 72.9 ± 5.9 (60–93) 23.1 ± 9.0 ysm BMI 28.9 ± 5.9 25/22 | Dietary protein + pill 8 g SP + 105 mg Isof (aglycones) vs. 18 g mix of protein (whey, caseinate, egg white) + placebo (maltodextrin) | BAP NTX |
| Vupadhyayula [53] 2009 USA | Double-blind, parallel group 24-monts | 66.5 ± 4.5 (>55) 14.3 ± 5.4 ysm BMI 26.3 ± 3.8 T-score > −2.5 30/35 | Powder 25 g SP, 90 mg Isof vs. 25 g milk protein | NTX |
| Levis [54] 2011 USA | Double-blind, parallel group 24-months | 52.5 ± 3.3 (45–60) BMI 26.3 ± 3.3 T-score > −2.0 99/83 | Tablet 200 mg Isof (aglycones: 91 mg Gen, 103 mg Dai) vs. placebo | NTX |
| Tai [55] 2012 Taiwan | Double-blind, parallel group 24-months | 55.9 ± 3.8 (45–65) 5.1 ± 2.7 ysm BMI 22.9 ± 2.6 200/199 | Capsule 300 mg Isof (aglycones: 57.5% Gen, 42.5% Dai) vs. placebo (cellulose) | BAP NTX |
| Lee [56] 2017 Korea | Double-blind, parallel group 3-months | 53.6 ± 3.4 (45–60) 3.6 ± 2.3 ysm BMI 19.0–30.0 41/43 | Tablet 70 mg Isof (glycosides: 38 mg Gly, 20 mg Dai, 12.4 mg Gen)vs. placebo (dextrin) | OC, BAP DPD, NTX, CTX |
| Sathyapalan [57] 2017 UK | Double-blind, parallel group 6-months | 50 y≤ 2 ysm, BMI, 26.9 ± 5.8 60/60 | Snack bar 15 g SP, 66 mg Isof (90% glycosides, 10% aglycones) vs. 15 g soy protein alone | PINP CTX |
| Zhang [58] 2020 China | Double-blind, parallel group 6-months | 58 ± 3.1 (40–55) 4.0 ± 3.2 ysm BMI 23.3 ± 3.2 38/37 | Tablet 60 mg Isof vs. placebo (microcrystalline cellulose, dextrin) | OC |
| Subgroup Outcomes | Osteocalcin (OC) | Bone Alkaline Phosphatase (BAP) | Pyridinoline (PYD) | Deoxypyridinoline (DPD) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | n | WMD (95% CI) | p | I2 (%) | N | n | WMD (95% CI) | p | I2 (%) | N | n | WMD (95% CI) | p | I2 (%) | N | n | WMD (95% CI) | p | I2 (%) | |
| Overall effects | 10 | 581 | 4.16 (−7.72, 16.04) | 0.49 | 85.35 | 18 | 1560 | 5.50 (−3.81, 14.82) | 0.25 | 92.39 | 8 | 589 | −12.09 (−25.37,1.20) | 0.07 | 89.12 | 14 | 959 | −7.48 (−15.37, 0.41) | 0.06 | 66.83 |
| Follow-up period | ||||||||||||||||||||
| ≤6 months | 8 | 459 | 0.27 (−10.93, 11.47) | 0.96 | 83.68 | 8 | 400 | 2.07 (−1.85, 5.98) | 0.30 | 0.00 | 3 | 154 | −17.04 (−42.00, 8.31) | 0.08 | 77.94 | 8 | 459 | −8.56 (−19.56, 2.43) | 0.13 | 56.68 |
| >6 months | 2 | 122 | 6.47 (−81.55, 94.48) | 0.88 | 83.60 | 10 | 1160 | 8.10 (−7.16, 23.36) | 0.30 | 95.34 | 5 | 435 | −9.85 (−26.70, 7.00) | 0.25 | 92.08 | 6 | 500 | −6.48 (−18.82, 5.85) | 0.30 | 78.14 |
| Menopausal status | ||||||||||||||||||||
| <5 years | 7 | 440 | −0.96 (−12.70, 10.78) | 0.87 | 85.31 | 11 | 1112 | 6.97 (−5.83, 19.76) | 0.29 | 94.80 | 5 | 431 | −4.79 (−18.40, 8.83) | 0.49 | 86.09 | 10 | 715 | −4.28 (−13.05, 4.48) | 0.34 | 67.53 |
| ≥5 years | 3 | 141 | 14.98 (−24.91, 54.87) | 0.46 | 72.71 | 7 | 448 | 1.90 (−6.80, 10.60) | 0.67 | 58.24 | 3 | 158 | −25.87 (−52.81, 1.07) | 0.06 | 87.73 | 4 | 244 | −16.49 (−32.17, −0.81) | 0.04 | 53.73 |
| Age | ||||||||||||||||||||
| <54 years | 6 | 359 | 14.18 (−13.96, 42.33) | 0.32 | 90.13 | 9 | 652 | 10.25 (−6.58, 27.08) | 0.23 | 95.44 | 6 | 456 | −14.45 (−30.70, 1.81) | 0.08 | 91.97 | 9 | 655 | −8.65 (−20.25, 2.95) | 0.14 | 78.34 |
| ≥54 years | 4 | 222 | −3.57 (−13.30, 6.16) | 0.47 | 63.70 | 9 | 908 | 0.72 (−3.51, 4.94) | 0.74 | 19.02 | 2 | 133 | −3.50 (−16.02, 9.03) | 0.13 | 0.00 | 5 | 304 | −4.87 (−12.95, 3.20) | 0.24 | 0.00 |
| BMI § | ||||||||||||||||||||
| ≤24.9 kg/m2 | 6 | 390 | 14.17 (−1.27, 29.61) | 0.07 | 89.01 | 10 | 1088 | 10.18 (−2.88, 23.25) | 0.13 | 95.34 | 6 | 456 | −14.45 (−30.70, 1.81) | 0.08 | 91.97 | 9 | 693 | −7.18 (−17.86, 3.50) | 0.19 | 78.85 |
| >24.9 kg/m2 | 3 | 171 | −15.13 (−46.96, 16.69) | 0.35 | 70.69 | 8 | 472 | −3.09 (−8.31, 2.13) | 0.25 | 0.00 | 2 | 133 | −3.50 (−16.02, 9.03) | 0.58 | 0.00 | 5 | 266 | −9.53 (−19.73, 0.68) | 0.07 | 0.00 |
| Intervention type | ||||||||||||||||||||
| Soy protein | 2 | 82 | −16.11 (−44.40, 12.19) | 0.26 | 0.00 | 9 | 634 | −1.29 (−5.77, 3.20) | 0.57 | 0.00 | 4 | 314 | 2.79 (−2.64, 8.22) | 0.31 | 0.00 | 7 | 446 | 0.53 (−6.03, 7.10) | 0.87 | 5.10 |
| Isoflavone extract | 8 | 499 | 7,03 (−5.79, 19,84) | 0.28 | 88.93 | 9 | 926 | 10.61 (−4.01, 25.23) | 0.15 | 95.75 | 4 | 275 | −29.16 (−39.59, −18.73) | 0.00 | 50.68 | 7 | 513 | −14.63 (−27.88, −1.38) | 0.03 | 77.90 |
| Isoflavone dose | ||||||||||||||||||||
| <80 mg/day | 5 | 347 | 8.07 (−7.79, 23.92) | 0.32 | 89.88 | 6 | 438 | 14.97 (−7.65, 37.60) | 0.19 | 94.96 | 3 | 219 | −32.04 (−40.42, −23.66) | 0.00 | 24.47 | 6 | 441 | −9.02 (−22.09, 4.05) | 0.18 | 75.61 |
| ≥80 mg/day | 5 | 234 | 3.87 (−23.35, 31.09) | 0.78 | 78.27 | 12 | 1122 | 0.15 (−2.74, 3.04) | 0.92 | 0.00 | 5 | 370 | 1.91 (−3.34, 7.16) | 0.48 | 0.00 | 8 | 518 | −6.33 (−16.72, 4.06) | 0.23 | 60.38 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanadys, W.; Barańska, A.; Błaszczuk, A.; Polz-Dacewicz, M.; Drop, B.; Malm, M.; Kanecki, K. Effects of Soy Isoflavones on Biochemical Markers of Bone Metabolism in Postmenopausal Women: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Int. J. Environ. Res. Public Health 2021, 18, 5346. https://doi.org/10.3390/ijerph18105346
Kanadys W, Barańska A, Błaszczuk A, Polz-Dacewicz M, Drop B, Malm M, Kanecki K. Effects of Soy Isoflavones on Biochemical Markers of Bone Metabolism in Postmenopausal Women: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. International Journal of Environmental Research and Public Health. 2021; 18(10):5346. https://doi.org/10.3390/ijerph18105346
Chicago/Turabian StyleKanadys, Wiesław, Agnieszka Barańska, Agata Błaszczuk, Małgorzata Polz-Dacewicz, Bartłomiej Drop, Maria Malm, and Krzysztof Kanecki. 2021. "Effects of Soy Isoflavones on Biochemical Markers of Bone Metabolism in Postmenopausal Women: A Systematic Review and Meta-Analysis of Randomized Controlled Trials" International Journal of Environmental Research and Public Health 18, no. 10: 5346. https://doi.org/10.3390/ijerph18105346
APA StyleKanadys, W., Barańska, A., Błaszczuk, A., Polz-Dacewicz, M., Drop, B., Malm, M., & Kanecki, K. (2021). Effects of Soy Isoflavones on Biochemical Markers of Bone Metabolism in Postmenopausal Women: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. International Journal of Environmental Research and Public Health, 18(10), 5346. https://doi.org/10.3390/ijerph18105346







