Assessing Biocompatibility of Face Mask Materials during COVID-19 Pandemic by a Rapid Multi-Assays Strategy
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Cytotoxicity Assay
2.3. Colorimetric Griess Assay
2.4. AlphaLISA
2.5. Statistical Analysis
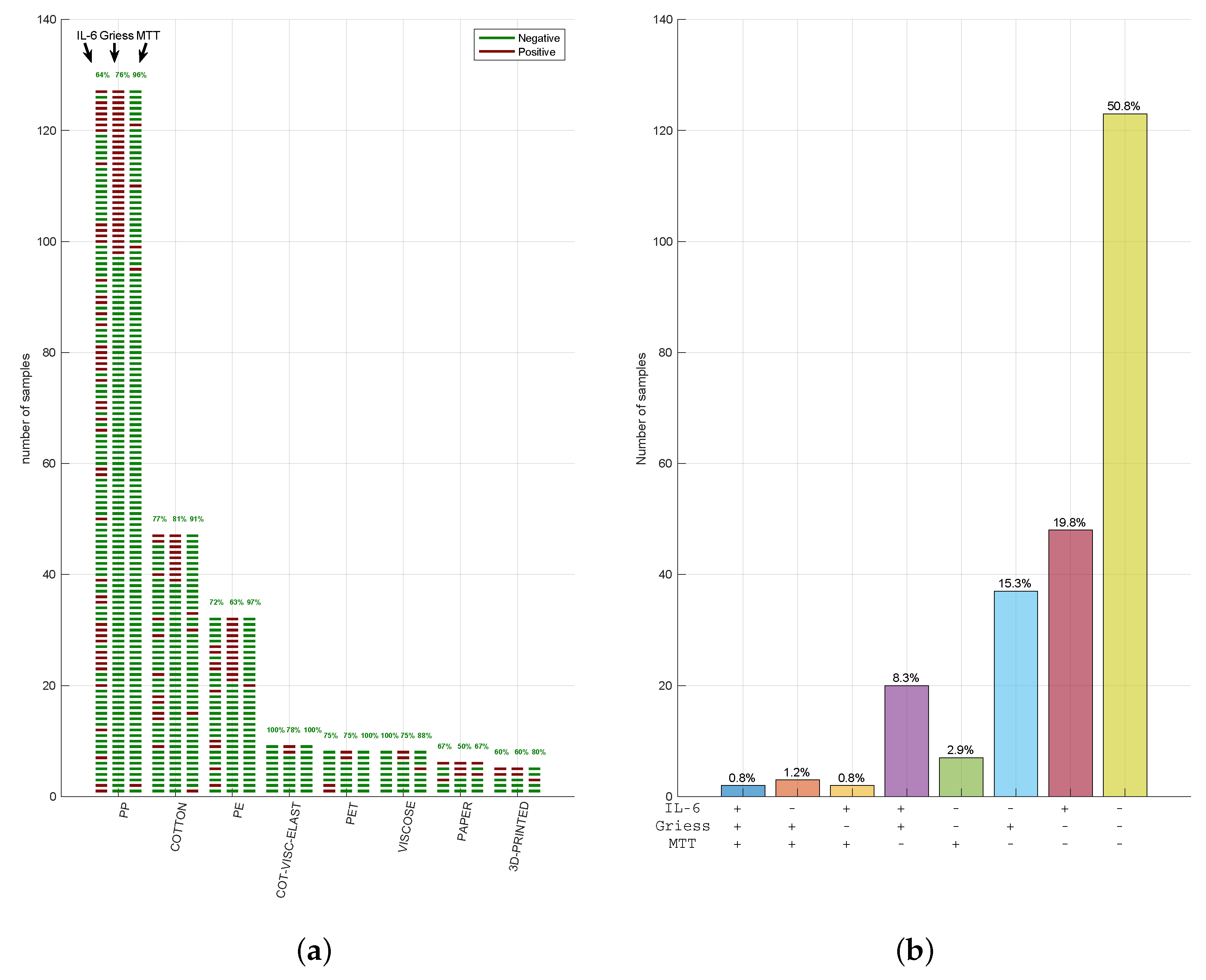
3. Results
3.1. Face Mask Material Identification and Testing
3.2. Face Mask Materials Do Not Reveal Cytotoxicity Showing a Variable Inflammatory Potential
3.3. Cytotoxicity, Nitrite, and IL-6 Assays Have Reproducible Results in Three CE-Marked Masks
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leung, N.H.L.; Chu, D.K.W.; Shiu, E.Y.C.; Chan, K.-H.; McDevitt, J.J.; Hau, B.J.P.; Yen, H.-L.; Li, Y.; Ip, D.K.M.; Peiris, J.S.M.; et al. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat. Med. 2020, 26, 676–680. [Google Scholar] [CrossRef] [PubMed]
- Tessarolo, F.; Nollo, G.; Maniglio, D.; Rigoni, M.; Benedetti, L.; Helfer, F.; Corradi, I.; Rovati, L.; Ferrari, A.; Piccini, M.; et al. Testing Surgical Face Masks in an Emergency Context: The Experience of Italian Laboratories during the COVID-19 Pandemic Crisis. Int. J. Environ. Res. Public Health 2021, 18, 1462. [Google Scholar] [CrossRef] [PubMed]
- ISO 10993-1:2018 Biological Evaluation of Medical Devices—Part 1: Evaluation and Testing within a Risk Management Process 2018. Available online: https://www.iso.org/standard/68936.html (accessed on 8 May 2021).
- ISO/TR 15499:2016. Biological Evaluation of Medical Devices—Guidance on the Conduct of Biological Evaluation within a Risk Management Process 2016. Available online: https://www.iso.org/standard/69217.html (accessed on 8 May 2021).
- UNI CEI EN ISO 14971:2020 Dispositivi Medici—Applicazione della Gestione dei Rischi ai Dispositivi Medici 2020. Available online: https://www.iso.org/standard/72704.html (accessed on 8 May 2021).
- Sipahl, H.; Bayram, F.E.O.; Palabiyik, S.S.; Bayram, D.; Aydin, A. Investigation of the Biocompatibility of Surgical Masks. Pteridines 2018, 29, 80–86. [Google Scholar] [CrossRef]
- Cals-Grierson, M.-M.; Ormerod, A.D. Nitric oxide function in the skin. Nitric Oxide Biol. Chem. 2004, 10, 179–193. [Google Scholar] [CrossRef] [PubMed]
- Shiva, S. Nitrite: A physiological store of nitric oxide and modulator of mitochondrial function. Redox Biol. 2013, 1, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Griess, P. Bemerkungen zu der Abhandlung der HH. Weselsky und Benedikt „Ueber einige Azoverbindungen”. Ber. Dtsch. Chem. Ges. 1879, 12, 426–428. [Google Scholar] [CrossRef]
- Rincon, M. Interleukin-6: From an inflammatory marker to a target for inflammatory diseases. Trends Immunol. 2012, 33, 571–577. [Google Scholar] [CrossRef] [PubMed]
- ISO 10993-10:2010 Biological Evaluation of Medical Devices—Part 10: Tests for Irritation and Skin Sensitization 2010. Available online: https://www.iso.org/standard/40884.html (accessed on 8 May 2021).
- Makene, V.W.; Pool, E.J. The assessment of inflammatory activity and toxicity of treated sewage using RAW264.7 cells. Water Environ. J. 2015, 29, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Piva, S.J.; Tatsch, E.; De Carvalho, J.A.M.; Bochi, G.V.; Kober, H.; Duarte, T.; Duarte, M.M.M.F.; da Cruz, I.B.M.; Moretto, M.B.; Moresco, R.N. Assessment of inflammatory and oxidative biomarkers in obesity and their associations with body mass index. Inflammation 2013, 36, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Avdagić, N.; Zaćiragić, A.; Babić, N.; Hukić, M.; Seremet, M.; Lepara, O.; Nakaš-Ićindić, E. Nitric oxide as a potential biomarker in inflammatory bowel disease. Bosn. J. Basic Med. Sci. 2013, 13, 5–9. [Google Scholar] [CrossRef] [PubMed]
- López-García, S.; Pecci-Lloret, M.P.; García-Bernal, D.; Guerrero-Gironés, J.; Pecci-Lloret, M.R.; Rodríguez-Lozano, F.J. Are Denture Adhesives Safe for Oral Cells? J. Prosthodont. 2021, 30, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Lozano, F.J.; Serrano-Belmonte, I.; Pérez Calvo, J.C.; Coronado-Parra, M.T.; Bernabeu-Esclapez, A.; Moraleda, J.M. Effects of two low-shrinkage composites on dental stem cells (viability, cell damaged or apoptosis and mesenchymal markers expression). J. Mater. Sci. Mater. Med. 2013, 24, 979–988. [Google Scholar] [CrossRef] [PubMed]
- ISO 10993-12:2012 Biological Evaluation of Medical Devices—Part 12: Sample Preparation and Reference Materials 2012. Available online: https://www.iso.org/standard/53468.html (accessed on 8 May 2021).
- ISO 10993-5:2009 Biological Evaluation of Medical Devices—Part 5: Tests For In Vitro Cytotoxicity 2009. Available online: https://www.iso.org/standard/36406.html (accessed on 8 May 2021).
- Juráňová, J.; Aury-Landas, J.; Boumediene, K.; Baugé, C.; Biedermann, D.; Ulrichová, J.; Franková, J. Modulation of Skin Inflammatory Response by Active Components of Silymarin. Molecules 2018, 24, 123. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in Inflammation, Immunity, and Disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration. Guidance for Industry Bioanalytical Method Validation. 2013. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/bioanalytical-method-validation-guidance-industry (accessed on 8 May 2021).
- Earl, L.K.; Dickens, A.D.; Rowson, M.J. A critical analysis of the rabbit eye irritation test variability and its impact on the validation of alternative methods. Toxicol. In Vitro 1997, 11, 295–304. [Google Scholar] [CrossRef]
- Weil, C.S.; Scala, R.A. Study of intra- and interlaboratory variability in the results of rabbit eye and skin irritation tests. Toxicol. Appl. Pharmacol. 1971, 19, 276–360. [Google Scholar] [CrossRef]
- EN 14683:2019+AC:2019: Medical Face Masks—Requirements and Test Methods 2019. Available online: http://store.uni.com/catalogo/uni-en-14683-2019-29 (accessed on 8 May 2021).
- Carlos Rubio-Romero, J.; Del Carmen Pardo-Ferreira, M.; Antonio Torrecilla García, J.; Calero-Castro, S. Disposable masks: Disinfection and sterilization for reuse, and non-certified manufacturing, in the face of shortages during the COVID-19 pandemic. Saf. Sci. 2020, 129, 104830. [Google Scholar] [CrossRef] [PubMed]
- Illeperuma, R.P.; Park, Y.J.; Kim, J.M.; Bae, J.Y.; Che, Z.M.; Son, H.K.; Han, M.R.; Kim, K.M.; Kim, J. Immortalized gingival fibroblasts as a cytotoxicity test model for dental materials. J. Mater. Sci. Mater. Med. 2012, 23, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Nowarski, R.; Jackson, R.; Flavell, R.A. The Stromal Intervention: Regulation of Immunity and Inflammation at the Epithelial-Mesenchymal Barrier. Cell 2017, 168, 362–375. [Google Scholar] [CrossRef] [PubMed]
- Quan, C.; Cho, M.K.; Shao, Y.; Mianecki, L.E.; Liao, E.; Perry, D.; Quan, T. Dermal fibroblast expression of stromal cell-derived factor-1 (SDF-1) promotes epidermal keratinocyte proliferation in normal and diseased skin. Protein Cell 2015, 6, 890–903. [Google Scholar] [CrossRef] [PubMed]


| CFM1 | |||||||
|---|---|---|---|---|---|---|---|
| Blank | HDPE | Latex | Extract100 | Extract46.41 | Extract21.54 | Extract10 | |
| PART#1 | 100 | 90.86 ± 14.04 | 6.54 ± 0.39 | 95.28 ± 14.22 | 97.05 ± 12.68 | 98.39 ± 16.92 | 98.25 ± 16.57 |
| PART#2 | 100 | 93.26 ± 11.15 | 5.66 ± 0.38 | 90.58 ± 9.93 | 93.23 ± 15.52 | 93.32 ± 9.70 | 94.59 ± 7.10 |
| PART#3 | 100 | 92.14 ± 6.58 | 8.45 ± 0.34 | 94.68 ± 10.33 | 98.08 ± 9.03 | 101.39 ±10.13 | 101.46 ± 7.87 |
| CFM2 | |||||||
| Blank | HDPE | Latex | Extract100 | Extract46.41 | Extract21.54 | Extract10 | |
| PART#1 | 100 | 90.79 ± 3.64 | 6.23 ± 0.49 | 90.68 ± 5.52 | 91.57 ± 9.37 | 92.19 ± 9.03 | 92.56 ± 6.87 |
| PART#2 | 100 | 95.44 ± 6.65 | 6.04 ± 0.43 | 97.22 ± 9.97 | 98.43 ± 11.78 | 100.59 ± 6.51 | 100.64 ± 4.24 |
| PART#3 | 100 | 87.39 ± 6.65 | 5.54 ± 0.37 | 9.87 ± 6.66 | 97.04 ± 3.76 | 97.37 ± 6.17 | 97.98 ±12.12 |
| CFM3 | |||||||
| Blank | HDPE | Latex | Extract100 | Extract46.41 | Extract21.54 | Extract10 | |
| PART#1 | 100 | 87.38 ± 5.54 | 5.53 ± 0.34 | 84.26 ± 4.93 | 84.92 ± 2.42 | 85.87 ± 4.46 | 86.22 ± 3.50 |
| PART#2 | 100 | 90.83 ± 6,12 | 6.59 ± 0.52 | 93.20 ± 4.51 | 95.99 ± 6.47 | 96.09 ± 7.62 | 96.26 ± 3.10 |
| PART#3 | 100 | 92.11 ± 14.90 | 6.82 ± 0.47 | 92.78 ± 12.43 | 102.52 ± 14.06 | 110.19 ± 6.76 | 111.49 ± 12.47 |
| AVERAGE (µg/mL) ± SD% | |||
|---|---|---|---|
| Negative Control | −0.98 ± 0 | ||
| Triton X-100 | 115.69 ± 0.09 | ||
| part#1 | part#2 | part#3 | |
| CFM1 | −0.98 ± 0 | −0.98 ± 0 | −0.86 ± 0.09 |
| CFM2 | −0.70 ± 0.09 | −0.98 ± 0 | −0.92 ± 0.09 |
| CFM3 | −0.92 ± 0.09 | −0.92 ± 0.09 | −0.89 ± 0.09 |
| AVERAGE (pg/mL) ± RSD% | |||
|---|---|---|---|
| Negative Control | 116.99 ± 10.51 | ||
| Lipopolysaccharide | 897.63 ± 0.60 | ||
| part#1 | part#2 | part#3 | |
| CFM1 | 133.06 ± 2.94 | 124.85 ± 12.58 | 104.41 ± 15.32 |
| CFM2 | 97.70 ± 3.00 | 90.79 ± 12.07 | 89.38 ± 12.99 |
| CFM3 | 123.72 ± 12.28 | 120.03 ± 2.90 | 104.19 ± 6.58 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrachi, T.; Ganzerli, F.; Cuoghi, A.; Ferrari, A.; Resca, E.; Bergamini, V.; Accorsi, L.; Burini, F.; Pasini, D.; Arnaud, G.F.; et al. Assessing Biocompatibility of Face Mask Materials during COVID-19 Pandemic by a Rapid Multi-Assays Strategy. Int. J. Environ. Res. Public Health 2021, 18, 5387. https://doi.org/10.3390/ijerph18105387
Petrachi T, Ganzerli F, Cuoghi A, Ferrari A, Resca E, Bergamini V, Accorsi L, Burini F, Pasini D, Arnaud GF, et al. Assessing Biocompatibility of Face Mask Materials during COVID-19 Pandemic by a Rapid Multi-Assays Strategy. International Journal of Environmental Research and Public Health. 2021; 18(10):5387. https://doi.org/10.3390/ijerph18105387
Chicago/Turabian StylePetrachi, Tiziana, Francesco Ganzerli, Aurora Cuoghi, Alberto Ferrari, Elisa Resca, Valentina Bergamini, Luca Accorsi, Francesco Burini, Davide Pasini, Gaelle Françoise Arnaud, and et al. 2021. "Assessing Biocompatibility of Face Mask Materials during COVID-19 Pandemic by a Rapid Multi-Assays Strategy" International Journal of Environmental Research and Public Health 18, no. 10: 5387. https://doi.org/10.3390/ijerph18105387
APA StylePetrachi, T., Ganzerli, F., Cuoghi, A., Ferrari, A., Resca, E., Bergamini, V., Accorsi, L., Burini, F., Pasini, D., Arnaud, G. F., Piccini, M., Aldrovandi, L., Mari, G., Tomasi, A., Rovati, L., Dominici, M., & Veronesi, E. (2021). Assessing Biocompatibility of Face Mask Materials during COVID-19 Pandemic by a Rapid Multi-Assays Strategy. International Journal of Environmental Research and Public Health, 18(10), 5387. https://doi.org/10.3390/ijerph18105387







