Damage to the Testicular Structure of Rats by Acute Oral Exposure of Cadmium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Design
2.3. Experimental Duration (Animal Trails)
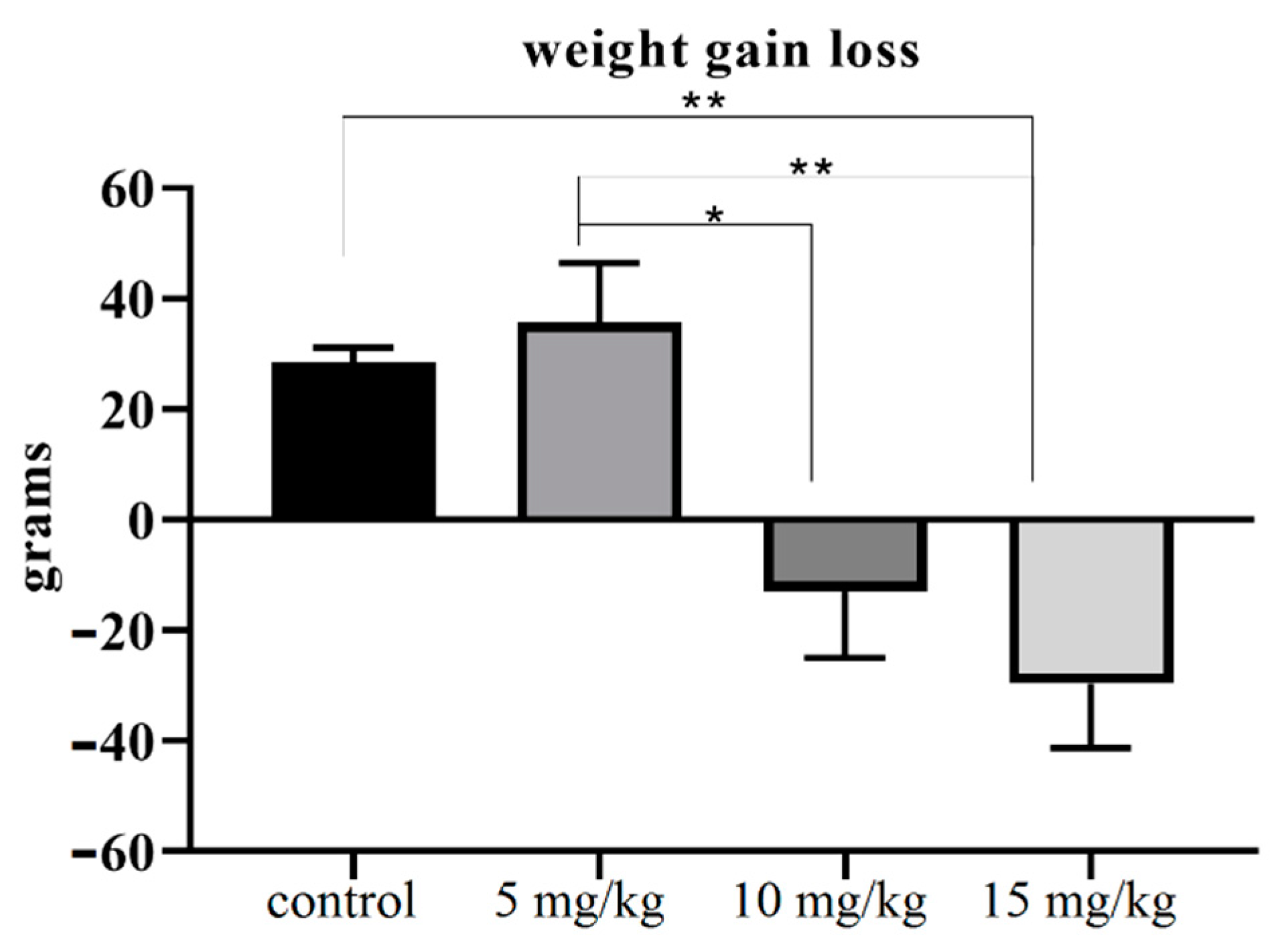
2.4. The Relative Weight of Testes
2.5. Histology
2.6. Image J Software Application
2.7. Daily Sperm Production
2.8. Comet Assay
2.9. Statistical Analysis
3. Results
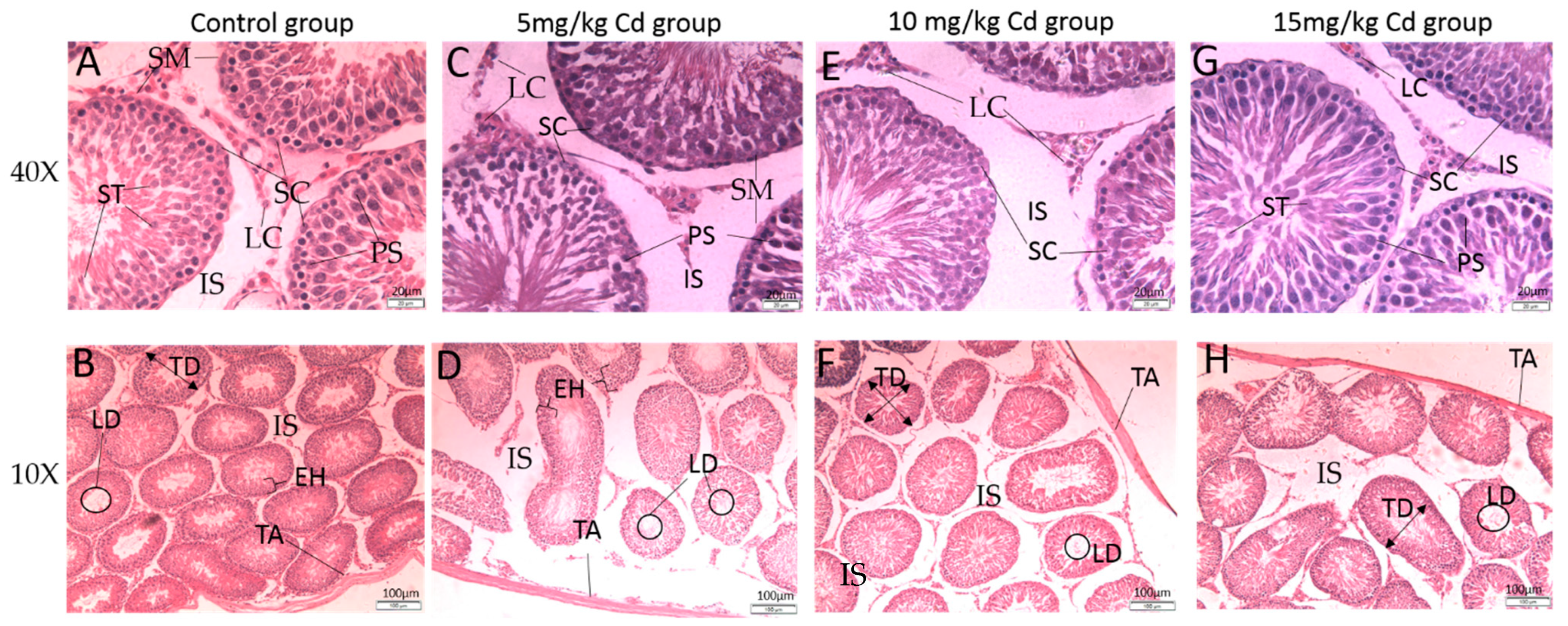
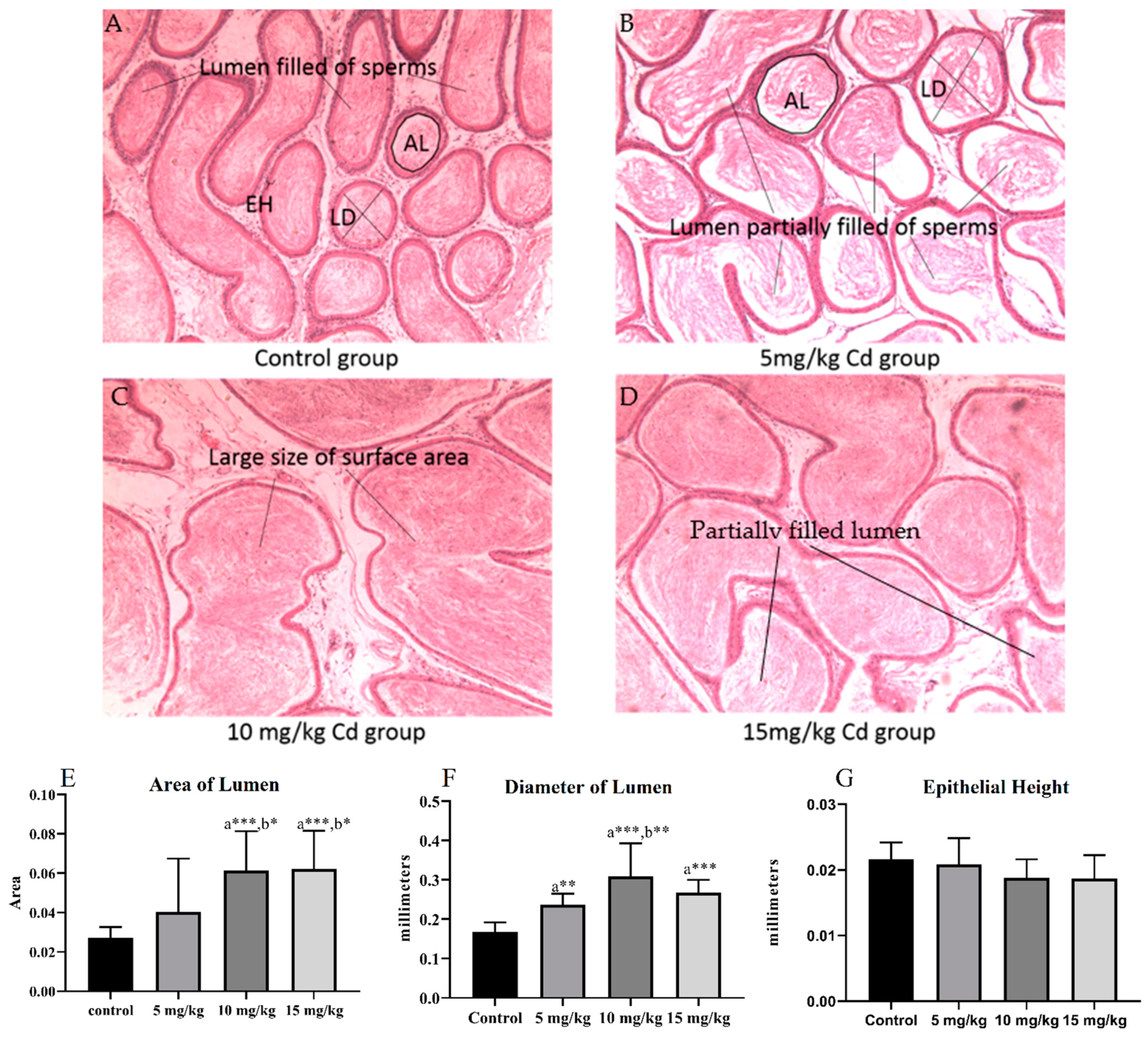
3.1. Histology
3.1.1. Testes
3.1.2. Epididymis
3.2. Daily Sperm Production
3.3. Comet Assay
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cheng, Y.; Zhang, J.; Wu, T.; Jiang, X.; Jia, H.; Qing, S.; An, Q.; Zhang, Y.; Su, J. Reproductive toxicity of acute Cd exposure in mouse: Resulting in oocyte defects and decreased female fertility. Toxicol. Appl. Pharmacol. 2019, 379, 114684. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Romić, D.; Akber, M.A.; Romić, M. Trace metals accumulation in soil irrigated with polluted water and assessment of human health risk from vegetable consumption in Bangladesh. Environ. Geochem. Health 2018, 40, 59–85. [Google Scholar] [CrossRef] [PubMed]
- Mance, G.; Mance, G. Toxicity of Metals to Freshwater Invertebrates. Pollut. Threat Heavy Met. Aquat. Environ. 1987, 127–173. [Google Scholar] [CrossRef]
- Li, P.; Lin, C.; Cheng, H.; Duan, X.; Lei, K. Contamination and health risks of soil heavy metals around a lead/zinc smelter in southwestern China. Ecotoxicol. Environ. Saf. 2015, 113, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wu, T.; Zhang, C.; Luo, L.; Xie, M.; Huang, H. Cadmium exposure in newborn rats ovary induces developmental disorders of primordial follicles and the differential expression of SCF/c-kit gene. Toxicol. Lett. 2017, 280, 20–28. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Air Quality Guidelines for Europe World Health Organization Regional Office for Europe; WHO: Geneva, Switzerland, 1987; pp. 136–139. [Google Scholar]
- World Health Organization. Exposure to Cadmium: A Major Public Health Concern; WHO: Geneva, Switzerland, 2010; pp. 3–6. [Google Scholar]
- Trejo, N.; Matus, I.; del Pozo, A.; Walter, I.; Hirzel, J. Cadmium phytoextraction capacity of white lupine (Lupinus albus L.) and narrow-leafed lupine (Lupinus angustifolius L.) in three contrasting agroclimatic conditions of Chile. Chil. J. Agric. Res. 2016, 76, 228–235. [Google Scholar] [CrossRef] [Green Version]
- Järup, L.; Åkesson, A. Current status of cadmium as an environmental health problem. Toxicol. Appl. Pharmacol. 2009, 238, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Rahimzadeh, M.R.; Rahimzadeh, M.R.; Kazemi, S.; Moghadamnia, A. Cadmium toxicity and treatment: An update. Casp. J. Intern. Med. 2017, 8, 135. [Google Scholar]
- Bai, S.; Fu, K.; Yin, H.; Cui, Y.; Yue, Q.; Li, W.W.; Cheng, L.; Tan, H.; Liu, X.; Guo, Y.; et al. 2. Wahl eines theoretischen Rahmens 1. Zielsetzung. Development 2018, 145, dev164855. [Google Scholar] [CrossRef] [Green Version]
- Vahter, M.; Åkesson, A.; Lidén, C.; Ceccatelli, S.; Berglund, M. Gender differences in the disposition and toxicity of metals. Environ. Res. 2007, 104, 85–95. [Google Scholar] [CrossRef]
- Van Maele-Fabry, G.; Lombaert, N.; Lison, D. Dietary exposure to cadmium and risk of breast cancer in postmenopausal women: A systematic review and meta-analysis. Environ. Int. 2016, 86, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Arora, M.; Weuve, J.; Schwartz, J.; Wright, R.O. Association of evironmental cadmium exposure with pediatric dental caries. Environ. Health Perspect. 2008, 116, 821–825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willers, S.; Gerhardsson, L.; Lundh, T. Environmental tobacco smoke (ETS) exposure in children with asthma—Relation between lead and cadmium, and cotinine concentrations in urine. Respir. Med. 2005, 99, 1521–1527. [Google Scholar] [CrossRef] [Green Version]
- Berglund, M.; Akesson, A.; Nermell, B.; Vahter, M. Intestinal absorption of dietary cadmium in women depends on body iron stores and fiber intake. Environ. Health Perspect. 1994, 102, 1058–1066. [Google Scholar] [CrossRef]
- Egan, S.K.; Bolger, P.M.; Carrington, C.D. Update of US FDA’s Total Diet Study food list and diets. J. Exp. Sci. Environ. Epidemiol. 2007, 17, 573–582. [Google Scholar] [CrossRef] [Green Version]
- Larsen, E.H.; Andersen, N.L.; Møller, A.; Petersen, A.; Mortensen, G.K.; Petersen, J. Monitoring the content and intake of trace elements from food in Denmark. Food Addit. Contam. 2002, 19, 33–46. [Google Scholar] [CrossRef]
- Llobet, J.M.; Falcó, G.; Casas, C.; Teixidó, A.; Domingo, J.L. Concentrations of arsenic, cadmium, mercury, and lead in common foods and estimated daily intake by children, adolescents, adults, and seniors of Catalonia, Spain. J. Agric. Food Chem. 2003, 51, 838–842. [Google Scholar] [CrossRef]
- MacIntosh, D.L.; Spengler, J.D.; Özkaynak, H.; Tsai, L.H.; Barry Ryan, P. Dietary exposures to selected metals and pesticides. Environ. Health Perspect. 1996, 104, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Olsson, I.M.; Bensryd, I.; Lundh, T.; Ottosson, H.; Skerfving, S.; Oskarsson, A. Cadmium in blood and urine—Impact of sex, age, dietary intake, iron status, and former smoking—Association of renal effects. Environ. Health Perspect. 2002, 110, 1185–1190. [Google Scholar] [CrossRef]
- Thomas, K.W.; Pellizzari, E.D.; Berry, M.R. Population-based dietary intakes and tap water concentrations for selected elements in the EPA Region V National Human Exposure Assessment Survey (NHEXAS). J. Exp. Anal. Environ. Epidemiol. 1999, 9, 402–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ysart, G.; Miller, P.; Croasdale, M.; Crews, H.; Robb, P.; Baxter, M.; De L’Argy, C.; Harrison, N. 1997 UK Total Diet Study dietary exposures to aluminium, arsenic, cadmium, chromium, copper, lead, mercury, nickel, selenium, tin and zinc. Food Addit. Contam. 2000, 17, 775–786. [Google Scholar] [CrossRef]
- Elsenhans, B.; Schüller, N.; Schümann, K.; Forth, W. Oral and subcutaneous administration of cadmium chloride and the distribution of metallothionein and cadmium along the villus-crypt axis in rat jejunum. Biol. Trace Elem. Res. 1994, 42, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Ryan, P.B.; Scanlon, K.A.; MacIntosh, D.L. Analysis of dietary intake of selected metals in the NHEXAS-Maryland investigation. Environ. Health Perspect. 2001, 109, 121–128. [Google Scholar] [CrossRef]
- Wilhelm, M.; Wittsiepe, J.; Schrey, P.; Budde, U.; Idel, H. Dietary intake of cadmium by children and adults from Germany using duplicate portion sampling. Sci. Total Environ. 2002, 285, 11–19. [Google Scholar] [CrossRef]
- Baer, K.N.; Benson, W.H. Influence of chemical and environmental stressors on acute cadmium toxicity. J. Toxicol. Environ. Heal. Part A Curr. Issues 1987, 22, 35–44. [Google Scholar] [CrossRef]
- Basinger, M.A.; Jones, M.M.; Holscher, M.A.; Vaughn, W.K. Antagonists for acute oral cadmium chloride intoxication. J. Toxicol. Environ. Heal. Part A Curr. Issues 1988, 23, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Kostial, K.; Kello, D.; Jugo, S.; Rabar, I.; Maljkovic, T. Influence of age on metal metabolism and toxicity. Environ. Health Perspect. 1978, 25, 81–86. [Google Scholar] [CrossRef]
- Sciences, H.; City, K.; Laboratories, S. The Relationship of Metallothidnein to the Toxicity of Cadmium after Prolonged Oral Administration to Rats. Toxicol. Appl. Pharmacol. 1978, 54, 39–54. [Google Scholar]
- Shimizu, M.; Morita, S. Effects of fasting on cadmium toxicity, glutathione metabolism, and metallothionein synthesis in rats. Toxicol. Appl. Pharmacol. 1990, 103, 28–39. [Google Scholar] [CrossRef]
- Borzelleca, J.F.; Clarke, E.C.; Condie Jr, L.W. Short-term toxicity (1 and 10 days) of cadmium chloride in male and female rats: Gavage and drinking water. J. Am. Coll. Toxicol. 1989, 8, 377–404. [Google Scholar] [CrossRef]
- Kostial, K.; Kargačin, B.; Landeka, M. Gut retention of metals in rats. Biol. Trace Elem. Res. 1989, 21, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Çilenk, K.T.; Öztürk, İ.; Sönmez, M.F. Ameliorative effect of propolis on the cadmium-induced reproductive toxicity in male albino rats. Exp. Mol. Pathol. 2016, 101, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Robb, G.W.; Amann, R.P.; Killian, G.J. Daily sperm production and epididymal sperm reserves of pubertal and adult rats. Reproduction 1978, 54, 103–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jahan, S.; Rehman, S.; Ullah, H.; Munawar, A.; Ain, Q.U. Ameliorative effect of quercetin against arsenic-induced sperm DNA damage and daily sperm production in adult male rats. Drug Chem. Toxicol. 2015, 39, 290–296. [Google Scholar] [CrossRef]
- Ahmad, L.; Jalali, S.; Shami, S.A.; Akram, Z. Sperm preparation: DNA damage by comet assay in normo- and teratozoospermics. Arch. Androl. 2007, 53, 325–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asia, E.; Ikeda, M.; Nakatsuka, H.; Watanabe, T.; Shimbo, S. Journal of Trace Elements in Medicine and Biology Estimation of dietary intake of cadmium from cadmium in blood or urine in. J. Trace Elem. Med. Biol. 2018, 50, 24–27. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Wang, Y.; Fan, R.; Qiu, C.; Zhong, S.; Wei, L.; Luo, D. Effect of Cadmium on Cellular Ultrastructure in Mouse Ovary. Ultrastruct. Pathol. 2015, 39, 324–328. [Google Scholar] [CrossRef]
- Bertin, G.; Averbeck, D. Cadmium: Cellular effects, modifications of biomolecules, modulation of DNA repair and genotoxic consequences (a review). Biochimie 2006, 88, 1549–1559. [Google Scholar] [CrossRef]
- Waisberg, M.; Joseph, P.; Hale, B.; Beyersmann, D. Molecular and cellular mechanisms of cadmium carcinogenesis. Toxicology 2003, 192, 95–117. [Google Scholar] [CrossRef]
- Fang, M.; Mar, W.; Cho, M. Cadmium affects genes involved in growth regulation during two-stage transformation of Balb/3T3 cells. Toxicology 2002, 177, 253–265. [Google Scholar] [CrossRef]
- Oh, S.; Lim, S. A rapid and transient ROS generation by cadmium triggers apoptosis via caspase-dependent pathway in HepG2 cells and this is inhibited through N -acetylcysteine-mediated catalase upregulation. Toxicol. Appl. Pharmacol. 2006, 212, 212–223. [Google Scholar] [CrossRef]
- Yang, P.; Chiu, S.; Lin, K.; Lin, L. Effect of cadmium on cell cycle progression in chinese hamster ovary cells. Chem. Biol. Interact. 2004, 149, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Shao, W.; Zuo, L.; Zhao, W.E.I.; Qin, H. Mechanism of cadmium poisoning on testicular injury in mice. Oncol. Lett. 2019, 18, 1035–1042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sagba, S.O.A.; Daikpoh, M.A.A.; Adiri, H.K.; Bi, F.O.O. Influence of Aqueous Extract of Hibiscus sabdariffa L. Petal on Cadmium Toxicity in Rats. Biol. Trace Element Res. 2007, 115, 47–57. [Google Scholar]
- Lynch, G.P.; Smith, D.F.I.; Fisher, M.; Pike, T.L.; Weinland, B.T. Physiological responses of calves to cadmium and lead. J. Anim. Sci. 1976, 42, 410–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bebe, F.N.; Panemangalore, M. Modulation of tissue trace metal concentration in weanling rats fed different level of zinc and exposed to oral lead and cadmium. Nutr. Res. 1996, 16, 1368–1380. [Google Scholar] [CrossRef]
- Santos, F.W.; Grac, D.L.; Zeni, G.; Weis, S.N.; Favero, A.M.; Nogueira, C.W. Sub-chronic administration of diphenyl diselenide potentiates cadmium-induced testicular damage in mice. Reproduct. Toxicol. 2006, 22, 546–550. [Google Scholar] [CrossRef]
- El-demerdash, F.M.; Yousef, M.I.; Kedwany, F.S.; Baghdadi, H.H. Cadmium-induced changes in lipid peroxidation, blood hematology, biochemical parameters and semen quality of male rats: Protective role of Vitamin E and b-carotene. Food Chem. Toxicol. 2004, 42, 1563–1571. [Google Scholar] [CrossRef]
- Predes, F.D.S.; Diamante, M.A.S.; Dolder, H. Testis response to low doses of cadmium in Wistar rats. Int. J. Exp. Pathol. 2010, 91, 125–131. [Google Scholar] [CrossRef]
- Wade, M.G.; Foster, W.G.; Younglai, E.V.; Mcmahon, A.; Leingartner, K.; Yagminas, A.; Blakey, D.; Fournier, M.; Desaulniers, D.; Hughes, C.L.; et al. Effects of Subchronic Exposure to a Complex Mixture of Persistent Contaminants in Male Rats: Systemic, Immune, and Reproductive Effects. Toxicol. Sci. 2002, 143, 131–143. [Google Scholar] [CrossRef] [Green Version]
- Sinha Hikim, A.P.; Bartke, A.; Russell, L.D. Morphometric Studies on Hamster Testes in Gonadally Active and Inactive States: Light Microscope Findings1. Biol. Reprod. 1988, 39, 1225–1237. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, H.; Spanò, M.; Santos, C.; de Lourdes Pereira, M. Adverse effects of cadmium exposure on mouse sperm. Reprod. Toxicol. 2009, 28, 550–555. [Google Scholar] [CrossRef]
- Saïd, L.; Banni, M.; Kerkeni, A.; Saïd, K.; Messaoudi, I. Influence of combined treatment with zinc and selenium on cadmium induced testicular pathophysiology in rat. Food Chem. Toxicol. 2010, 48, 2759–2765. [Google Scholar] [CrossRef] [PubMed]
- Sundukov, Y.N. First record of the ground beetle Trechoblemus postilenatus (Coleoptera, Carabidae) in Primorskii krai. Far East. Entomol. 2006, 165, 16. [Google Scholar] [CrossRef]
- Adamkovicova, M.; Toman, R.; Cabaj, M.; Massanyi, P.; Martiniakova, M.; Omelka, R.; Krajcovicova, V.; Duranova, H. Effects of subchronic exposure to cadmium and diazinon on testis and epididymis in rats. Sci. World J. 2014, 2014, 632581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afsar, T.; Razak, S.; Almajwal, A.; Khan, M.R. Acacia hydaspica R. Parker ameliorates cisplatin induced oxidative stress, DNA damage and morphological alterations in rat pulmonary tissue. BMC Complement Altern. Med. 2018, 18, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakr, S.A.; Nooh, H.Z. Effect of Ocimum basilicum extract on cadmium-induced testicular histomorphometric and immunohistochemical alterations in albino rats. Anat. Cell Biol. 2013, 46, 122. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Zhang, R.; Song, Y.; Li, T.; Ge, M. Protective Effect of Ganoderma Triterpenoids on Cadmium-Induced Testicular Toxicity in Chickens. Biol. Trace Elem. Res. 2019, 187, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Al-azemi, M.; Omu, F.E. Lithium protects against toxic effects of cadmium in the rat testes. J. Assisted Reproduc. Genet. 2010, 27, 469–476. [Google Scholar] [CrossRef] [Green Version]
- Wong, E.W.P.; Cheng, C.Y. Impacts of environmental toxicants on male reproductive dysfunction. Trends Pharmacol. Sci. 2011, 32, 290–299. [Google Scholar] [CrossRef] [Green Version]
- Barali, K.; Javorac, D.; Djordjevic, A.B.; Bulat, Z. ScienceDirect Toxicology An overview of molecular mechanisms in cadmium toxicity. Curr. Opin. Toxicol. 2020, 12, 56–62. [Google Scholar] [CrossRef]
- Joseph, P. Mechanisms of cadmium carcinogenesis. Toxicol. Appl. Pharmacol. 2009, 238, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xu, Y.; Cheng, Z.; Su, H.; Liu, X. Low-dose cadmium activates the JNK signaling pathway in human renal podocytes. Int. J. Mol. Med. 2018, 41, 2359–2365. [Google Scholar] [CrossRef] [Green Version]
- Rani, A.; Kumar, A.; Lal, A.; Pant, M. Cellular mechanisms of cadmium-induced toxicity: A review. Int. J. Environ. Health Res. 2014, 24, 378–399. [Google Scholar] [CrossRef] [PubMed]

| Groups (n = 4) | Control (6 Animals) | 5 mg/kg (6 Animals) | 10 mg/kg (6 Animals) | 15 mg/kg (6 Animals) | |
|---|---|---|---|---|---|
| DSP (×106/100 mg) | 14.53 ± 1.17 | 12.07 ± 12.84 | 11.51 ± 0.17 | 13.05 ± 0.7 | |
| Body weight (grams) | Initial | 238.25 ± 6.46 | 254 ± 4.97 | 252.5 ± 14.26 | 287.5 ± 14.26 |
| Final | 266.75 ± 6.69 | 289.75 ± 6.41 | 235.5 ± 9.5 | 253.67 ± 6.89 | |
| Weight of testis (grams) | Right | 1.47 ± 0.05 | 1.92 ± 0.075 | 1.85 ± 0.05 | 1.73 ± 0.03 |
| Left | 1.47 ± 0.05 | 1.9 ± 1.22 | 1.85 ± 0.15 | 1.76 ± 0.03 | |
| Relative weight of testis (mg/g) | 1.75 ± 0.02 | 1.85 ± 0.19 | 1.68 ± 0.12 | 2.06 ± 0.02 a*,c** | |
| Weight of epididymis (g) | Right | 0.63 ± 0.09 | 0.97 ± 0.02 | 0.71 ± 0.01 | 0.83 ± 0.32 |
| Left | 0..63 ± 0.09 | 0.89 ± 0.03 | 0.69 ± 0.011 | 0.70 ± 0.04 | |
| Groups | Interstitial Space | Thickness of the Tunica Albuginea (µm) | Diameter of the Seminiferous Tubules (µm) | Height of the Epithelium (µm) | Diameter of the Tubular Lumen (µm) |
|---|---|---|---|---|---|
| Control | 63.58 ± 4.61 | 36.45 ± 1.5 | 235.34 ± 5.95 | 70.98 ± 2.02 | 92.51 ± 3.50 |
| 5 mg/kg | 60.45 ± 4.54 | 33.33 ± 1.53 | 244.04 ± 4.73 | 76.13 ± 2.71 | 95.30 ± 5.81 |
| 10 mg/kg | 23.96 ± 2.67 a***, b*** | 23.26 ± 0.67 a***, b*** | 273.6 ± 7.17 a***, b*** | 89.72 ± 3.53 a***, b** | 90.59 ± 3.33 |
| 15 mg/kg | 24.58 ± 2.84 a***, b*** | 36.23 ± 3.2 c*** | 258.14 ± 7.67 | 92.58 ± 3.08 a***, b** | 53.02 ± 2.44 a***, b***,c*** |
| Parameter | Control | 5 mg/kg | 10 mg/kg | 15 mg/kg |
|---|---|---|---|---|
| Head Length (µm) | 231.86 ± 34.47 | 122.23 ± 9.38 *** | 156.93± 15.73 * | 131.0 ± 11.97 ** |
| Tail Length (µm) | 7.29 ± 1.70 | 11.08 ± 1.55 | 9.8 ± 1.96 | 9.59 ± 1.15 |
| Comet Length (µm) | 239.14 ± 34.32 | 133.30 ± 8.85 ** | 166.8 ± 15.11 * | 166.06 ± 13.57 * |
| % DNA in Head | 97.93 ± 0.95 | 96.58 ± 0.62 | 96.58 ± 0.62 | 95.23 ± 0.62 |
| % DNA in Tail | 2.07 ± 0.95 | 3.42 ± 0.62 | 3.32 ± 0.90 | 4.76 ± 0.62 |
| Tail Moment | 0.39 ± 0.15 | 0.48 ± 0.15 | 0.53 ± 0.25 | 0.53 ± 0.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iqbal, T.; Cao, M.; Zhao, Z.; Zhao, Y.; Chen, L.; Chen, T.; Li, C.; Zhou, X. Damage to the Testicular Structure of Rats by Acute Oral Exposure of Cadmium. Int. J. Environ. Res. Public Health 2021, 18, 6038. https://doi.org/10.3390/ijerph18116038
Iqbal T, Cao M, Zhao Z, Zhao Y, Chen L, Chen T, Li C, Zhou X. Damage to the Testicular Structure of Rats by Acute Oral Exposure of Cadmium. International Journal of Environmental Research and Public Health. 2021; 18(11):6038. https://doi.org/10.3390/ijerph18116038
Chicago/Turabian StyleIqbal, Tariq, Maosheng Cao, Zijiao Zhao, Yun Zhao, Lu Chen, Tong Chen, Chunjin Li, and Xu Zhou. 2021. "Damage to the Testicular Structure of Rats by Acute Oral Exposure of Cadmium" International Journal of Environmental Research and Public Health 18, no. 11: 6038. https://doi.org/10.3390/ijerph18116038






