Association of Mercury Exposure and Maternal Sociodemographics on Birth Outcomes of Indigenous and Tribal Women in Suriname
Abstract
:1. Introduction
2. Materials and Methods
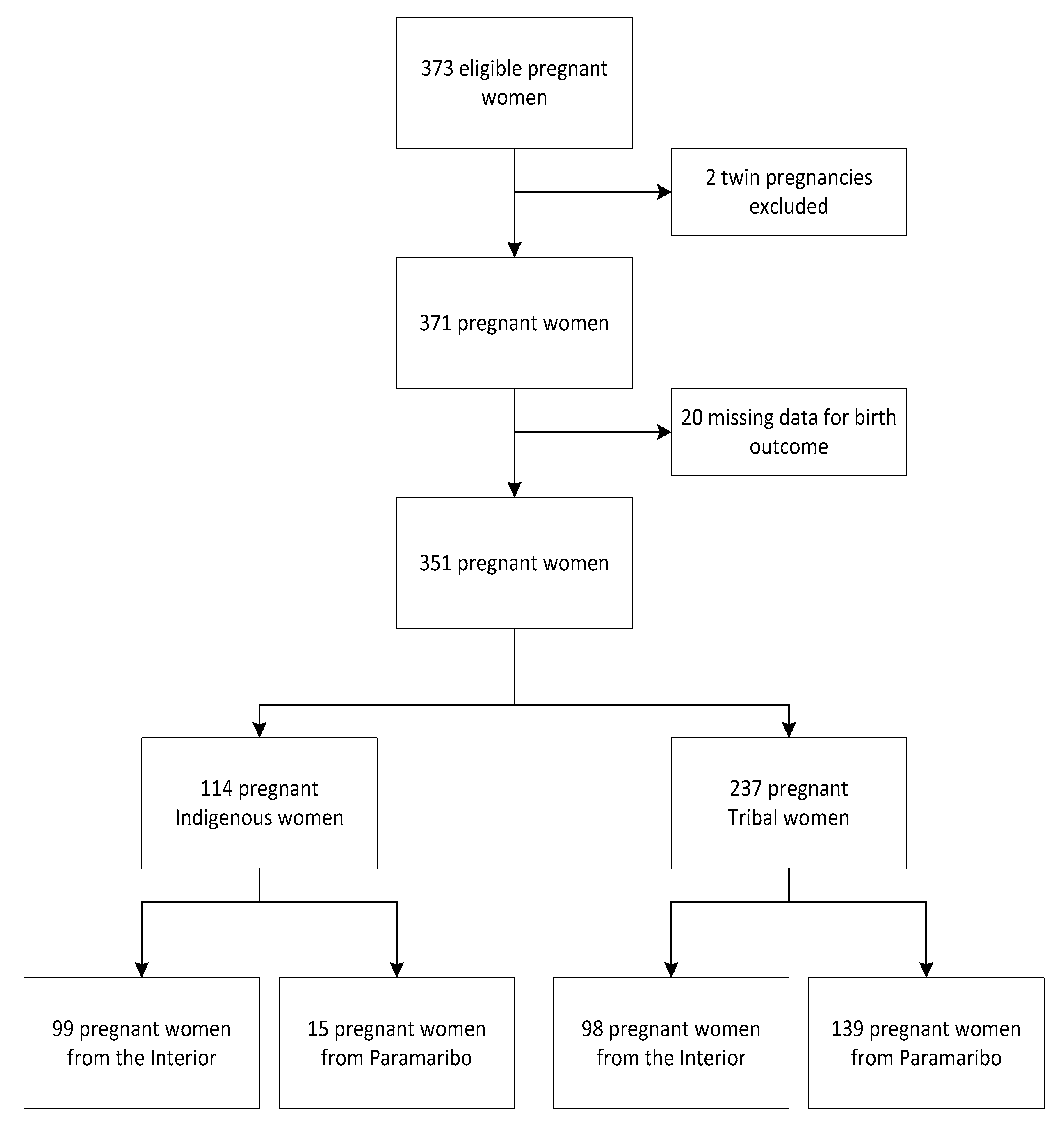
2.1. Study Population
2.2. Mercury Levels
2.3. Outcome Variables
2.4. Covariates
2.5. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Verschueren, K.J.C.; Prüst, Z.D.; Paidin, R.R.; Kodan, L.R.; Bloemenkamp, K.W.M.; Rijken, M.J.; Browne, J.L. Childbirth outcomes and ethnic disparities in Suriname: A nationwide registry-based study in a middle-income country. Reprod. Health 2020, 17, 62. [Google Scholar] [CrossRef]
- Mersky, J.P.; Lee, C.P. Adverse childhood experiences and poor birth outcomes in a diverse, low-income sample. BMC Pregnancy Childbirth 2019, 19, 387. [Google Scholar] [CrossRef]
- Ngandu, C.B.; Momberg, D.; Magan, A.; Chola, L.; Norris, S.A.; Said-Mohamed, R. The association between household socio-economic status, maternal socio-demographic characteristics and adverse birth and infant growth outcomes in sub-Saharan Africa: A systematic review. J. Dev. Orig. Health Dis. 2020, 11, 317–334. [Google Scholar] [CrossRef] [PubMed]
- Poets, C.F.; Wallwiener, D.; Vetter, K. Risks associated with delivering infants 2 to 6 weeks before term--a review of recent data. Dtsch. Arztebl. Int. 2012, 109, 721–726. [Google Scholar] [PubMed]
- Gedefaw, G.; Alemnew, B.; Demis, A. Adverse fetal outcomes and its associated factors in Ethiopia: A systematic review and meta-analysis. BMC Pediatr. 2020, 20, 269. [Google Scholar] [CrossRef] [PubMed]
- Stylianou-Riga, P.; Kouis, P.; Kinni, P.; Rigas, A.; Papadouri, T.; Yiallouros, P.K.; Theodorou, M. Maternal socioeconomic factors and the risk of premature birth and low birth weight in Cyprus: A case–control study. Reprod. Health 2018, 15, 157. [Google Scholar] [CrossRef]
- Chaibva, B.V.; Olorunju, S.; Nyadundu, S.; Beke, A. Adverse pregnancy outcomes, “stillbirths and early neonatal deaths” in Mutare district, Zimbabwe (2014): A descriptive study. BMC Pregnancy Childbirth 2019, 19, 86. [Google Scholar] [CrossRef] [Green Version]
- Prüst, Z.D.; Verschueren, K.J.C.; Bhikha-Kori, G.A.A.; Kodan, L.R.; Bloemenkamp, K.W.M.; Browne, J.L.; Rijken, M.J. Investigation of stillbirth causes in Suriname: Application of the WHO ICD-PM tool to national-level hospital data. Glob. Health Action 2020, 13, 1794105. [Google Scholar] [CrossRef]
- Gokoel, A.R.; Zijlmans, W.C.W.R.; Covert, H.H.; Abdoel Wahid, F.; Shankar, A.; MacDonald-Ottevanger, M.S.; Hindori-Mohangoo, A.D.; Wickliffe, J.K.; Lichtveld, M.Y.; Harville, E.W. Influence of Prenatal Exposure to Mercury, Perceived Stress, and Depression on Birth Outcomes in Suriname: Results from the MeKiTamara Study. Int. J. Environ. Res. Public. Health 2020, 17, 4444. [Google Scholar] [CrossRef]
- Li, F.; Wu, T.; Lei, X.; Zhang, H.; Mao, M.; Zhang, J. The apgar score and infant mortality. PLoS ONE 2013, 8, e69072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gebregzabiherher, Y.; Haftu, A.; Weldemariam, S.; Gebrehiwet, H. The Prevalence and Risk Factors for Low Birth Weight among Term Newborns in Adwa General Hospital, Northern Ethiopia. Obstet. Gynecol. Int. 2017, 2017, 2149156. [Google Scholar] [CrossRef] [Green Version]
- Assunção Salustiano, E.M.; DuarteBonini Campos, J.A.; Ibidi, S.M.; Ruano, R.; Zugaib, M. Low Apgar scores at 5 minutes in a low risk population: Maternal and obstetrical factors and postnatal outcome. Rev. Assoc. Médica Bras. 2012, 58, 587–593. [Google Scholar] [CrossRef]
- Organisation for Economic Co-operation and Development. The World Bank Health at a Glance: Latin America and the Caribbean 2020; Organisation for Economic Co-Operation and Development: Paris, France, 2020; ISBN 978-92-64-69289-3. [Google Scholar]
- Ministry of Foreign Affairs, Suriname. General Bureau of Statistics Suriname 2015–2018. Available online: https://statistics-suriname.org/en/population-statistics-2/ (accessed on 15 February 2021).
- Baldewsingh, G.K.; Jubitana, B.C.; van Eer, E.D.; Shankar, A.; Hindori-Mohangoo, A.D.; Covert, H.H.; Shi, L.; Lichtveld, M.Y.; Zijlmans, C.W.R. Adequate antenatal care and ethnicity affect preterm birth in pregnant women living in the tropical rainforest of Suriname. BMC Pregnancy Childbirth 2020, 20, 683. [Google Scholar] [CrossRef]
- Baldewsingh, G.K.; Wickliffe, J.K.; van Eer, E.D.; Shankar, A.; Hindori-Mohangoo, A.D.; Harville, E.W.; Covert, H.H.; Shi, L.; Lichtveld, M.Y.; Zijlmans, W.C.W.R. Prenatal Mercury Exposure in Pregnant Women from Suriname’s Interior and Its Effects on Birth Outcomes. Int. J. Environ. Res. Public. Health 2020, 17, 4032. [Google Scholar] [CrossRef] [PubMed]
- Zijlmans, W.; Wickliffe, J.; Hindori-Mohangoo, A.; MacDonald-Ottevanger, S.; Ouboter, P.; Landburg, G.; Codrington, J.; Roosblad, J.; Baldewsingh, G.; Ramjatan, R.; et al. Caribbean Consortium for Research in Environmental and Occupational Health (CCREOH) Cohort Study: Influences of complex environmental exposures on maternal and child health in Suriname. BMJ Open 2020, 10, e034702. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. World Health Organization Internation Programme on Chemical safety (IPCS); World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- Murcia, M.; Ballester, F.; Enning, A.M.; Iñiguez, C.; Valvi, D.; Basterrechea, M.; Rebagliato, M.; Vioque, J.; Maruri, M.; Tardon, A.; et al. Prenatal mercury exposure and birth outcomes. Environ. Res. 2016, 151, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, W.; Li, H.; Cao, L.; Wu, M.; Liu, J.; Gao, Z.; Zhou, C.; Liu, J.; Yan, C. Relation of prenatal low-level mercury exposure with early child neurobehavioral development and exploration of the effects of sex and DHA on it. Environ. Int. 2019, 126, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Ouboter, P.E.; Landburg, G.A.; Quik, J.H.M.; Mol, J.H.A.; van der Lugt, F. Mercury Levels in Pristine and Gold Mining Impacted Aquatic Ecosystems of Suriname, South America. AMBIO 2012, 41, 873–882. [Google Scholar] [CrossRef]
- Camacho, A.; Brussel, E.V.; Carrizales, L.; Flores-Ramírez, R.; Verduzco, B.; Huerta, S.R.-A.; Leon, M.; Díaz-Barriga, F. Mercury Mining in Mexico: I. Community Engagement to Improve Health Outcomes from Artisanal Mining. Ann. Glob. Health 2016, 82, 149. [Google Scholar] [CrossRef]
- Van Brussel, E.; Carrizales, L.; Flores-Ramirez, R.; Camacho, A.; Leon-Arce, M.; Diaz-Barriga, F. The “CHILD” framework for the study of artisanal mercury mining communities. Rev. Environ. Health 2016, 31, 43–45. [Google Scholar] [CrossRef]
- Wickliffe, J.K.; Lichtveld, M.Y.; Zijlmans, C.W.; MacDonald-Ottevanger, S.; Shafer, M.; Dahman, C.; Harville, E.W.; Drury, S.; Landburg, G.; Ouboter, P. Exposure to total and methylmercury among pregnant women in Suriname: Sources and public health implications. J. Expo. Sci. Environ. Epidemiol. 2020, 31, 117–125. [Google Scholar] [CrossRef]
- Ouboter, P.; Landburg, G.; Satnarain, G.; Starke, S.; Nanden, I.; Simon-Friedt, B.; Hawkins, W.; Taylor, R.; Lichtveld, M.; Harville, E.; et al. Mercury Levels in Women and Children from Interior Villages in Suriname, South America. Int. J. Environ. Res. Public. Health 2018, 15, 1007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Research Council. Toxicological Effects of Methylmercury; National Academy Press: Washington, DC, USA, 2000; ISBN 978-0-309-07140-6. [Google Scholar]
- United Nations. UNFPA, UNICEF, UN WOMEN Fact Sheet: Indigenous Women’s Maternal Health and Maternal Mortality; UN Permanent Forum on Indigenous Women, 15th Session; United Nations: Neww York, NY, USA, 2018. [Google Scholar]
- Eersel, M.G.; Vreden, S.G.; van Eer, E.D.; Mans, D.R. Fifty years of primary health care in the rainforest: Temporal trends in morbidity and mortality in indigenous Amerindian populations of Suriname. J. Glob. Health 2018, 8, 020403. [Google Scholar] [CrossRef]
- Heemskerk, M.; Delvoye, K.; Noordam, D.; Teunissen, P. Wayana Baseline Study; Amazone Conservation Team-Suriname: Hoop, Paramaribo, Suriname, 2007. [Google Scholar]
- Palacios, J.; Kennedy, H.P. Reflections of Native American teen mothers. J. Obstet. Gynecol. Neonatal Nurs. JOGNN 2010, 39, 425–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crittenden, C.P.; Boris, N.W.; Rice, J.C.; Taylor, C.A.; Olds, D.L. The Role of Mental Health Factors, Behavioral Factors, and Past Experiences in the Prediction of Rapid Repeat Pregnancy in Adolescence. J. Adolesc. Health 2009, 44, 25–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hogan, D.P.; Sun, R.; Cornwell, G.T. Sexual and fertility behaviors of American females aged 15–19 years: 1985, 1990, and 1995. Am. J. Public Health 2000, 90, 1421–1425. [Google Scholar] [PubMed] [Green Version]
- Manlove, J.; Ikramullah, E.; Mincieli, L.; Holcombe, E.; Danish, S. Trends in Sexual Experience, Contraceptive Use, and Teenage Childbearing: 1992–2002. J. Adolesc. Health 2009, 44, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Raneri, L.G.; Wiemann, C.M. Social Ecological Predictors of Repeat Adolescent Pregnancy. Perspect. Sex. Reprod. Health 2007, 39, 39–47. [Google Scholar] [CrossRef] [Green Version]
- Bakker, R.; Steegers, E.A.P.; Hofman, A.; Jaddoe, V.W.V. Blood Pressure in Different Gestational Trimesters, Fetal Growth, and the Risk of Adverse Birth Outcomes. Am. J. Epidemiol. 2011, 174, 797–806. [Google Scholar] [CrossRef]
- Elphinstone, R.E.; Weckman, A.M.; McDonald, C.R.; Tran, V.; Zhong, K.; Madanitsa, M.; Kalilani-Phiri, L.; Khairallah, C.; Taylor, S.M.; Meshnick, S.R.; et al. Early malaria infection, dysregulation of angiogenesis, metabolism and inflammation across pregnancy, and risk of preterm birth in Malawi: A cohort study. PLoS Med. 2019, 16, e1002914. [Google Scholar] [CrossRef] [Green Version]
- Ncube, C.N.; Enquobahrie, D.A.; Burke, J.G.; Ye, F.; Marx, J.; Albert, S.M. Transgenerational Transmission of Preterm Birth Risk: The Role of Race and Generational Socio-Economic Neighborhood Context. Matern. Child Health J. 2017, 21, 1616–1626. [Google Scholar] [CrossRef]
- Liu, L.; Ma, Y.; Wang, N.; Lin, W.; Liu, Y.; Wen, D. Maternal body mass index and risk of neonatal adverse outcomes in China: A systematic review and meta-analysis. BMC Pregnancy Childbirth 2019, 19, 105. [Google Scholar] [CrossRef]
- Rahman, M.M.; Abe, S.K.; Kanda, M.; Narita, S.; Rahman, M.S.; Bilano, V.; Ota, E.; Gilmour, S.; Shibuya, K. Maternal body mass index and risk of birth and maternal health outcomes in low- and middle-income countries: A systematic review and meta-analysis: Body mass index and pregnancy and health outcomes. Obes. Rev. 2015, 16, 758–770. [Google Scholar] [CrossRef]
- Liu, P.; Xu, L.; Wang, Y.; Zhang, Y.; Du, Y.; Sun, Y.; Wang, Z. Association between perinatal outcomes and maternal pre-pregnancy body mass index: Perinatal Outcomes and Maternal BMI. Obes. Rev. 2016, 17, 1091–1102. [Google Scholar] [CrossRef]
- Villar, J.; Bergsjo, P. WHO Antenatal Care Randomized Trial: Manual for the Implementation of the New Model 2002; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- World Health Organization, Department of Reproductive Health. Antenatal Care in Developing Countries: Promises, Achievements and Missed Opportunities; An Analysis of Trends, Levels and Differentials, 1990–2001; Abou-Zahr, C.L., Weltgesundheitsorganisation UNICEF, Eds.; World Health Organization: Geneva, Switzerland, 2003; ISBN 978-92-4-159094-5. [Google Scholar]
- Hanson, J.D. Understanding prenatal health care for American Indian women in a Northern Plains tribe. J. Transcult. Nurs. Off. J. Transcult. Nurs. Soc. 2012, 23, 29–37. [Google Scholar] [CrossRef] [Green Version]
- Ministry of Public Health, Guyana. UNICEF Situation Analysis of Adolescent Pregnancy in Guyana; UNICEF: Georgetown, Guyana, 2018.
- Neal, S.; Harvey, C.; Chandra-Mouli, V.; Caffe, S.; Camacho, A.V. Trends in adolescent first births in five countries in Latin America and the Caribbean: Disaggregated data from demographic and health surveys. Reprod. Health 2018, 15, 146. [Google Scholar] [CrossRef] [Green Version]
- Bramante, C.T.; Spiller, P.; Landa, M. Fish Consumption during Pregnancy: An Opportunity, Not a Risk. JAMA Pediatr. 2018, 172, 801. [Google Scholar] [CrossRef] [PubMed]
- Burch, J.B.; Wagner Robb, S.; Puett, R.; Cai, B.; Wilkerson, R.; Karmaus, W.; Vena, J.; Svendsen, E. Mercury in fish and adverse reproductive outcomes: Results from South Carolina. Int. J. Health Geogr. 2014, 13, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, C.; Golding, J.; Emond, A. Blood mercury levels and fish consumption in pregnancy: Risks and benefits for birth outcomes in a prospective observational birth cohort. Int. J. Hyg. Environ. Health 2016, 219, 513–520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Starling, P.; Charlton, K.; McMahon, A.; Lucas, C. Fish Intake during Pregnancy and Foetal Neurodevelopment—A Systematic Review of the Evidence. Nutrients 2015, 7, 2001–2014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Passos, C.J.S.; Mergler, D. Human mercury exposure and adverse health effects in the Amazon: A review. Cad. Saúde Pública 2008, 24, s503–s520. [Google Scholar] [CrossRef] [Green Version]
- Ogawa, K.; Matsushima, S.; Urayama, K.Y.; Kikuchi, N.; Nakamura, N.; Tanigaki, S.; Sago, H.; Satoh, S.; Saito, S.; Morisaki, N. Association between adolescent pregnancy and adverse birth outcomes, a multicenter cross sectional Japanese study. Sci. Rep. 2019, 9, 2365. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.-K.; Wen, S.W.; Fleming, N.; Demissie, K.; Rhoads, G.G.; Walker, M. Teenage pregnancy and adverse birth outcomes: A large population based retrospective cohort study. Int. J. Epidemiol. 2007, 36, 368–373. [Google Scholar] [CrossRef] [Green Version]
- Shrim, A.; Ates, S.; Mallozzi, A.; Brown, R.; Ponette, V.; Levin, I.; Shehata, F.; Almog, B. Is Young Maternal Age Really a Risk Factor for Adverse Pregnancy Outcome in a Canadian Tertiary Referral Hospital? J. Pediatr. Adolesc. Gynecol. 2011, 24, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Ganchimeg, T.; Ota, E.; Morisaki, N.; Laopaiboon, M.; Lumbiganon, P.; Zhang, J.; Yamdamsuren, B.; Temmerman, M.; Say, L.; Tunçalp, Ö.; et al. Pregnancy and childbirth outcomes among adolescent mothers: A World Health Organization multicountry study. BJOG Int. J. Obstet. Gynaecol. 2014, 121, 40–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- da Silva, A.A.M.; Simoes, V.M.F.; Barbieri, M.A.; Bettiol, H.; Lamy-Filho, F.; Coimbra, L.C.; Alves, M.T.S.S.B. Young maternal age and preterm birth. Paediatr. Perinat. Epidemiol. 2003, 17, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, R.E.; Ahmed, A.N.U.; Ahmed, S.; Saha, S.K.; Chowdhury, M.A.; Black, R.E.; Santosham, M.; Darmstadt, G.L. Determining Gestational Age in a Low-resource Setting: Validity of Last Menstrual Period. J. Health Popul. Nutr. 2009, 27, 332–338. [Google Scholar]
- Mayo, J.A.; Shachar, B.Z.; Stevenson, D.K.; Shaw, G.M. Nulliparous teenagers and preterm birth in California. J. Perinat. Med. 2017, 45, 959–967. [Google Scholar] [CrossRef]
- Ananth, C.V.; Peltier, M.R.; Getahun, D.; Kirby, R.S.; Vintzileos, A.M. Primiparity: An ‘intermediate’ risk group for spontaneous and medically indicated preterm birth. J. Matern. Fetal Neonatal Med. 2007, 20, 605–611. [Google Scholar] [CrossRef]
- Koullali, B.; van Zijl, M.D.; Kazemier, B.M.; Oudijk, M.A.; Mol, B.W.J.; Pajkrt, E.; Ravelli, A.C.J. The association between parity and spontaneous preterm birth: A population based study. BMC Pregnancy Childbirth 2020, 20, 233. [Google Scholar] [CrossRef] [Green Version]
- Goldenberg, R.L.; Culhane, J.F.; Iams, J.D.; Romero, R. Epidemiology and causes of preterm birth. Lancet 2008, 371, 75–84. [Google Scholar] [CrossRef]
- Hailemichael, H.T.; Debelew, G.T.; Alema, H.B.; Weldu, M.G.; Misgina, K.H. Determinants of adverse birth outcome in Tigrai region, North Ethiopia: Hospital-based case-control study. BMC Pediatr. 2020, 20, 10. [Google Scholar] [CrossRef] [PubMed]
- McGrady, G.A.; Sung, J.F.C.; Rowley, D.L.; Hogue, C.J.R. Preterm Delivery and Low Birth Weight among First-Born Infants of Black and White College Graduates. Am. J. Epidemiol. 1992, 136, 266–276. [Google Scholar] [CrossRef]
- Manuck, T.A. Racial and ethnic differences in preterm birth: A complex, multifactorial problem. Semin. Perinatol. 2017, 41, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Schaaf, J.; Liem, S.; Mol, B.; Abu-Hanna, A.; Ravelli, A. Ethnic and Racial Disparities in the Risk of Preterm Birth: A Systematic Review and Meta-Analysis. Am. J. Perinatol. 2012, 30, 433–450. [Google Scholar]

| Variables | Indigenous (n = 114) | Tribal (n = 237) | p-Value | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Hair Hg | |||||
| Median [IQR] | 105 | 7.9 [3.7–11.0] | 199 | 1.4 [0.7–2.3] | <0.001 |
| Hg exposure | |||||
| Low + medium | 24 | 22.9% | 179 | 89.9% | <0.001 |
| High | 81 | 77.1% | 20 | 10.1% | |
| Age | |||||
| Median [IQR] | 114 | 25.1 [20.0–30.7] | 237 | 29.1 [22.5–34.5] | 0.001 |
| Age | |||||
| 16–19 | 31 | 27.2% | 32 | 13.5% | 0.002 |
| 20–34 | 70 | 61.4% | 156 | 65.8% | |
| 35+ | 13 | 11.4% | 49 | 20.7% | |
| Parity | |||||
| no previous live births | 30 | 26.3% | 51 | 21.7% | 0.338 |
| 1+ previous live births | 84 | 73.7% | 184 | 78.3% | |
| Educational level | |||||
| primary or not | 90 | 79.6% | 106 | 45.3% | <0.001 |
| secondary and up | 23 | 20.4% | 128 | 54.7% | |
| Household income in SRD | |||||
| <1500 | 94 | 83.9% | 117 | 53.9% | <0.001 |
| 1500+ | 18 | 16.1% | 100 | 46.1% | |
| Timing of first antenatal visit | |||||
| Median [IQR] | 111 | 15 [9–21] | 224 | 12 [7–16] | 0.002 |
| Timing of first antenatal visit | |||||
| <12 weeks | 34 | 30.6% | 99 | 44.2% | 0.017 |
| 12+ weeks | 77 | 69.4% | 125 | 55.8% | |
| Variables | Interior (n = 197) | Paramaribo (n = 154) | p-Value | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Hair Hg | |||||
| Median [IQR] | 185 | 3.6 [2.1–8.7] | 119 | 0.8 [0.4–1.4] | <0.001 |
| Hg exposure | |||||
| Low + medium | 87 | 47.0% | 116 | 97.5% | <0.001 |
| High | 98 | 53.0% | 3 | 2.5% | |
| Age | |||||
| Median [IQR] | 197 | 25.8 [20.5–32.0] | 154 | 29.4 [23.8–34.9] | 0.001 |
| Age | |||||
| 16–19 | 45 | 22.8% | 18 | 11.7% | 0.010 |
| 20–34 | 124 | 62.9% | 102 | 66.2% | |
| 35+ | 28 | 14.2% | 34 | 22.1% | |
| Parity | |||||
| no previous live births | 39 | 20.0% | 42 | 27.3% | 0.110 |
| 1+ previous live births | 156 | 80.0% | 112 | 72.7% | |
| Ethnic background | |||||
| Indigenous | 99 | 50.3% | 15 | 9.7% | <0.001 |
| Tribal | 98 | 49.7% | 139 | 90.3% | |
| Educational level | |||||
| primary or not | 164 | 85.0% | 32 | 20.8% | <0.001 |
| secondary and up | 29 | 15.0% | 122 | 79.2% | |
| Household income in SRD | |||||
| <1500 | 165 | 88.7% | 46 | 32.2% | <0.001 |
| 1500+ | 21 | 11.3% | 97 | 67.8% | |
| Timing of first antenatal visit | |||||
| Median [IQR] | 187 | 16 [12–21] | 148 | 8 [5–14] | <0.001 |
| Timing of first antenatal visit | |||||
| <12 weeks | 35 | 18.7% | 98 | 66.2% | <0.001 |
| 12+ weeks | 152 | 81.3% | 50 | 33.8% | |
| Variables | ABO Yes (n = 81) | ABO No (n = 270) | p-Value | PTB Yes (n = 52) | PTB No (n = 287) | p-Value | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ethnic background | ||||||||||
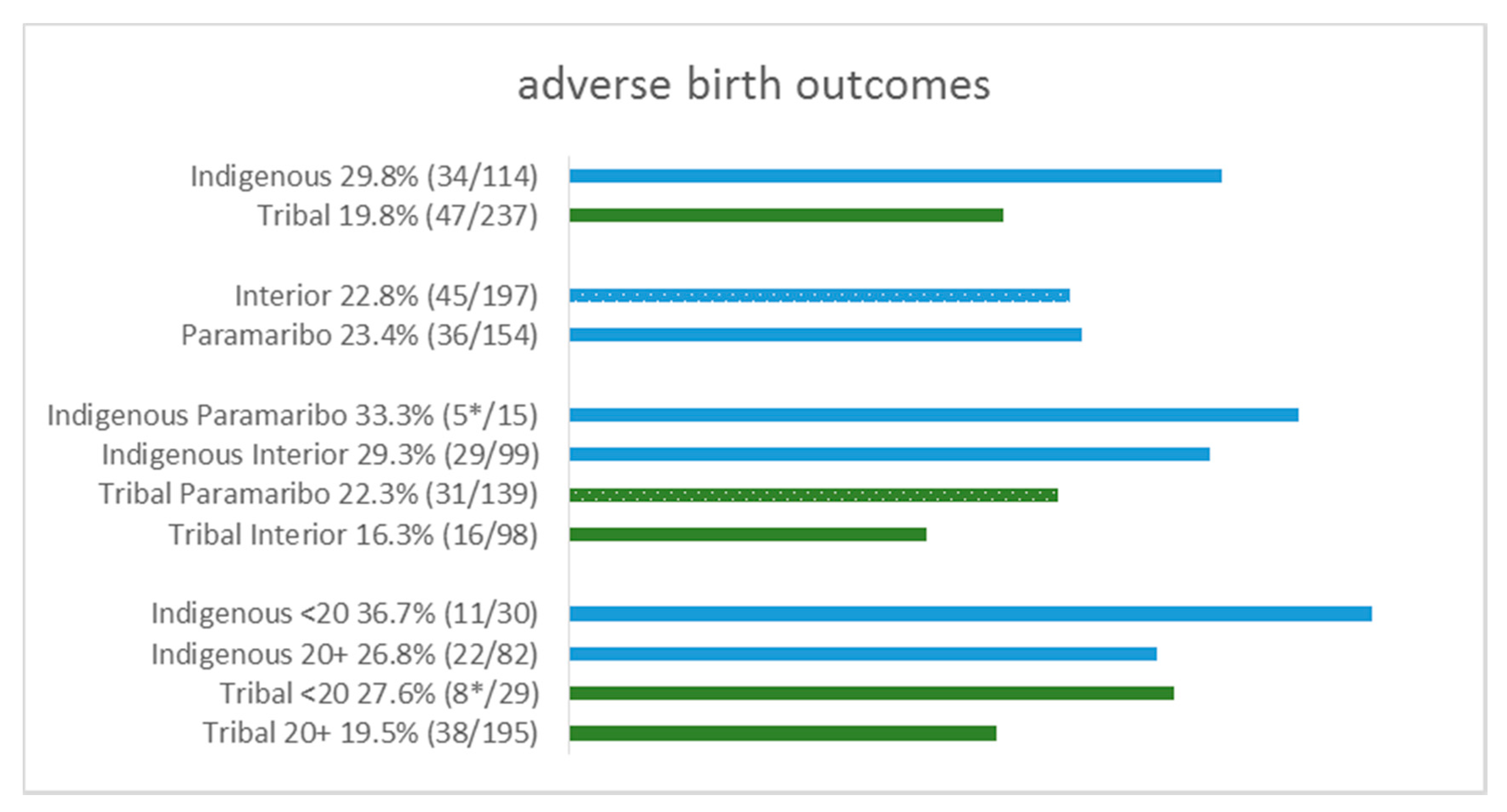
| Indigenous | 34 | 29.8% | 80 | 70.2% | 0.037 | 24 | 21.2% | 89 | 78.8% | 0.033 |
| Tribal | 47 | 19.8% | 190 | 80.2% | 28 | 12.4% | 198 | 87.6% | ||
| Geographic area | ||||||||||
| Interior | 45 | 22.8% | 152 | 77.2% | 0.906 | 31 | 15.9% | 164 | 84.1% | 0.740 |
| Paramaribo | 36 | 23.4% | 118 | 76.6% | 21 | 14.6% | 123 | 85.4% | ||
| Hair Hg | ||||||||||
| Median [IQR] | 69 | 1.7 [0.7–6.5] | 235 | 2.1 [1.0–5.8] | 0.460 | 47 | 1.8 [0.7–6.8] | 250 | 2.1 [1.0–5.8] | 0.454 |
| Hg exposure | ||||||||||
| Low + medium | 46 | 22.7% | 157 | 77.3% | 0.982 | 30 | 15.3% | 166 | 84.7% | 0.733 |
| high | 23 | 22.8% | 78 | 77.2% | 17 | 16.8% | 84 | 83.2% | ||
| Age | ||||||||||
| Median [IQR] | 81 | 27.0 [19.8–33.8] | 270 | 27.7 [22.2–32.9] | 0.385 | 52 | 24.0 [19.1–31.3] | 287 | 27.8 [22.2–33.2] | 0.016 |
| Age | ||||||||||
| 16–19 | 21 | 33.3% | 42 | 66.7% | 0.080 | 16 | 25.8% | 46 | 74.2% | 0.034 |
| 20–34 | 45 | 19.9% | 181 | 80.1% | 30 | 13.6% | 190 | 86.4% | ||
| 35+ | 15 | 24.2% | 47 | 75.8% | 6 | 10.5% | 51 | 89.5% | ||
| Parity | ||||||||||
| no previous live births | 26 | 32.1% | 55 | 67.9% | 0.031 | 14 | 18.7% | 61 | 81.3% | 0.379 |
| 1+ previous live births | 55 | 20.5% | 213 | 79.5% | 38 | 14.5% | 224 | 85.5% | ||
| Educational level | ||||||||||
| primary or not | 41 | 20.9% | 155 | 79.1% | 0.428 | 27 | 14.1% | 165 | 85.9% | 0.735 |
| secondary and up | 37 | 24.5% | 114 | 75.5% | 22 | 15.4% | 121 | 84.6% | ||
| Household income in SRD | ||||||||||
| <1500 | 45 | 21.3% | 166 | 78.7% | 0.396 | 28 | 13.5% | 179 | 86.5% | 0.092 |
| 1500+ | 30 | 25.4% | 88 | 74.6% | 19 | 17.0% | 93 | 83.0% | ||
| Timing of first antenatal visit | ||||||||||
| Median [IQR] | 113 | 12.5 [5.75–18] | 246 | 13 [8.5–18] | 0.103 | 47 | 12 [5–18] | 276 | 13.5 [9–18] | 0.227 |
| Timing of first antenatal visit | ||||||||||
| <12 weeks | 33 | 24.8% | 100 | 75.2% | 0.330 | 22 | 17.7% | 102 | 82.3% | 0.199 |
| 12+ weeks | 41 | 20.3% | 161 | 79.7% | 25 | 12.6% | 174 | 87.4% | ||
| Adverse Birth Outcomes (Yes vs. No) | Outcome Preterm Birth (Yes vs. No) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Crude model | p-Value | Crude OR | 95% CI | Crude model | p-Value | Crude OR | 95% CI | ||
| LB | UB | LB | UB | ||||||
| Indigenous vs. Tribal | 0.039 | 1.72 | 1.03 | 2.87 | Indigenous vs. Tribal | 0.035 | 1.91 | 1.05 | 3.47 |
| Adjusted model with all covariates | p-value | Adjusted OR | 95% CI | Adjusted model with all covariates | p-value | Adjusted OR | 95% CI | ||
| LB | UB | LB | UB | ||||||
| Indigenous vs. Tribal | 0.007 | 3.11 | 1.36 | 7.08 | Indigenous vs. Tribal | 0.017 | 3.23 | 1.24 | 8.44 |
| Paramaribo vs. Interior | 0.820 | 1.12 | 0.44 | 2.86 | Paramaribo vs. Interior | 0.366 | 1.67 | 0.55 | 5.07 |
| Age in years | 0.517 | 0.99 | 0.94 | 1.03 | Age in years | 0.004 | 0.92 | 0.87 | 0.97 |
| Parity no vs. 1+ previous live births | 0.319 | 1.44 | 0.70 | 2.97 | Parity no vs. 1+ previous live births | 0.220 | 0.58 | 0.24 | 1.39 |
| Education primary or not vs. secondary and up | 0.649 | 0.82 | 0.35 | 1.92 | Education primary or not vs. other | 0.793 | 0.88 | 0.33 | 2.35 |
| Household income <1500 vs. other | 0.767 | 0.88 | 0.39 | 2.02 | Household income <1500 vs. other | 0.414 | 0.67 | 0.25 | 1.76 |
| First antenatal visit <12 vs. 12+ weeks | 0.541 | 0.81 | 0.41 | 1.61 | First antenatal visit <12 vs. 12+ weeks | 0.533 | 0.78 | 0.36 | 1.71 |
| Hg exposure | 0.119 | 0.92 | 0.83 | 1.02 | Hg exposure | 0.357 | 0.95 | 0.84 | 1.06 |
| Final adjusted model (stepwise procedure) | p-value | Adjusted OR | 95% CI | Final adjusted model (stepwise procedure) | p-value | Adjusted OR | 95% CI | ||
| LB | UB | LB | UB | ||||||
| Indigenous vs. Tribal | 0.001 | 3.60 | 1.70 | 7.63 | Indigenous vs. Tribal | 0.004 | 3.43 | 1.48 | 7.96 |
| Hg exposure | 0.011 | 0.88 | 0.80 | 0.97 | Hg exposure | 0.027 | 0.88 | 0.79 | 0.99 |
| Age in years | 0.040 | 0.95 | 0.91 | 1.00 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baldewsingh, G.K.; Hindori-Mohangoo, A.D.; van Eer, E.D.; Covert, H.H.; Shankar, A.; Wickliffe, J.K.; Shi, L.; Lichtveld, M.Y.; Zijlmans, W.C.W.R. Association of Mercury Exposure and Maternal Sociodemographics on Birth Outcomes of Indigenous and Tribal Women in Suriname. Int. J. Environ. Res. Public Health 2021, 18, 6370. https://doi.org/10.3390/ijerph18126370
Baldewsingh GK, Hindori-Mohangoo AD, van Eer ED, Covert HH, Shankar A, Wickliffe JK, Shi L, Lichtveld MY, Zijlmans WCWR. Association of Mercury Exposure and Maternal Sociodemographics on Birth Outcomes of Indigenous and Tribal Women in Suriname. International Journal of Environmental Research and Public Health. 2021; 18(12):6370. https://doi.org/10.3390/ijerph18126370
Chicago/Turabian StyleBaldewsingh, Gaitree K., Ashna D. Hindori-Mohangoo, Edward D. van Eer, Hannah H. Covert, Arti Shankar, Jeffrey K. Wickliffe, Lizheng Shi, Maureen Y. Lichtveld, and Wilco C. W. R. Zijlmans. 2021. "Association of Mercury Exposure and Maternal Sociodemographics on Birth Outcomes of Indigenous and Tribal Women in Suriname" International Journal of Environmental Research and Public Health 18, no. 12: 6370. https://doi.org/10.3390/ijerph18126370
APA StyleBaldewsingh, G. K., Hindori-Mohangoo, A. D., van Eer, E. D., Covert, H. H., Shankar, A., Wickliffe, J. K., Shi, L., Lichtveld, M. Y., & Zijlmans, W. C. W. R. (2021). Association of Mercury Exposure and Maternal Sociodemographics on Birth Outcomes of Indigenous and Tribal Women in Suriname. International Journal of Environmental Research and Public Health, 18(12), 6370. https://doi.org/10.3390/ijerph18126370








