A Sustainable Solution to Obtain P-K-Mn Glass Fertilizers from Cheap and Readily Available Wastes
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
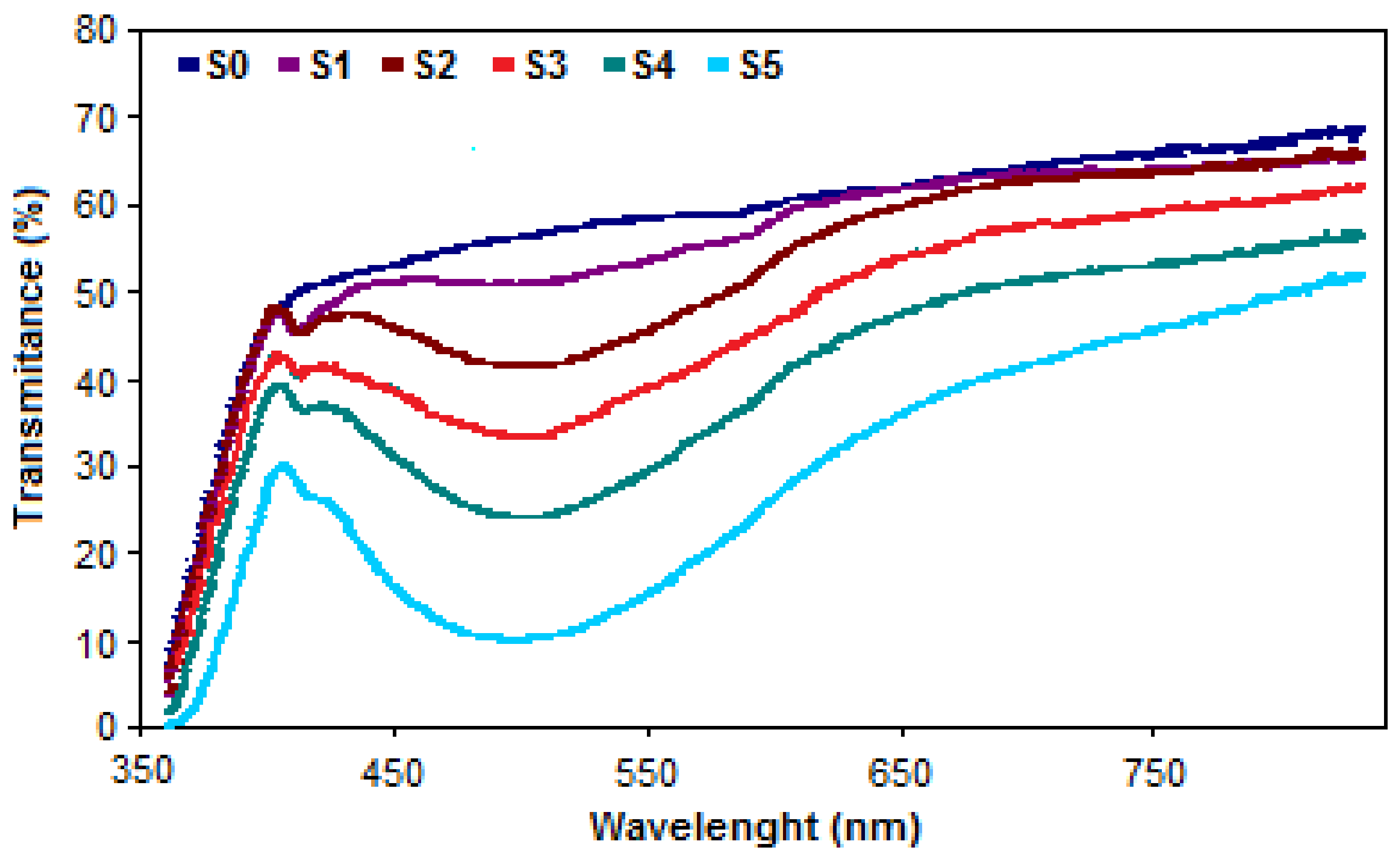
3.1. UV–VIS Agriglass Characterization
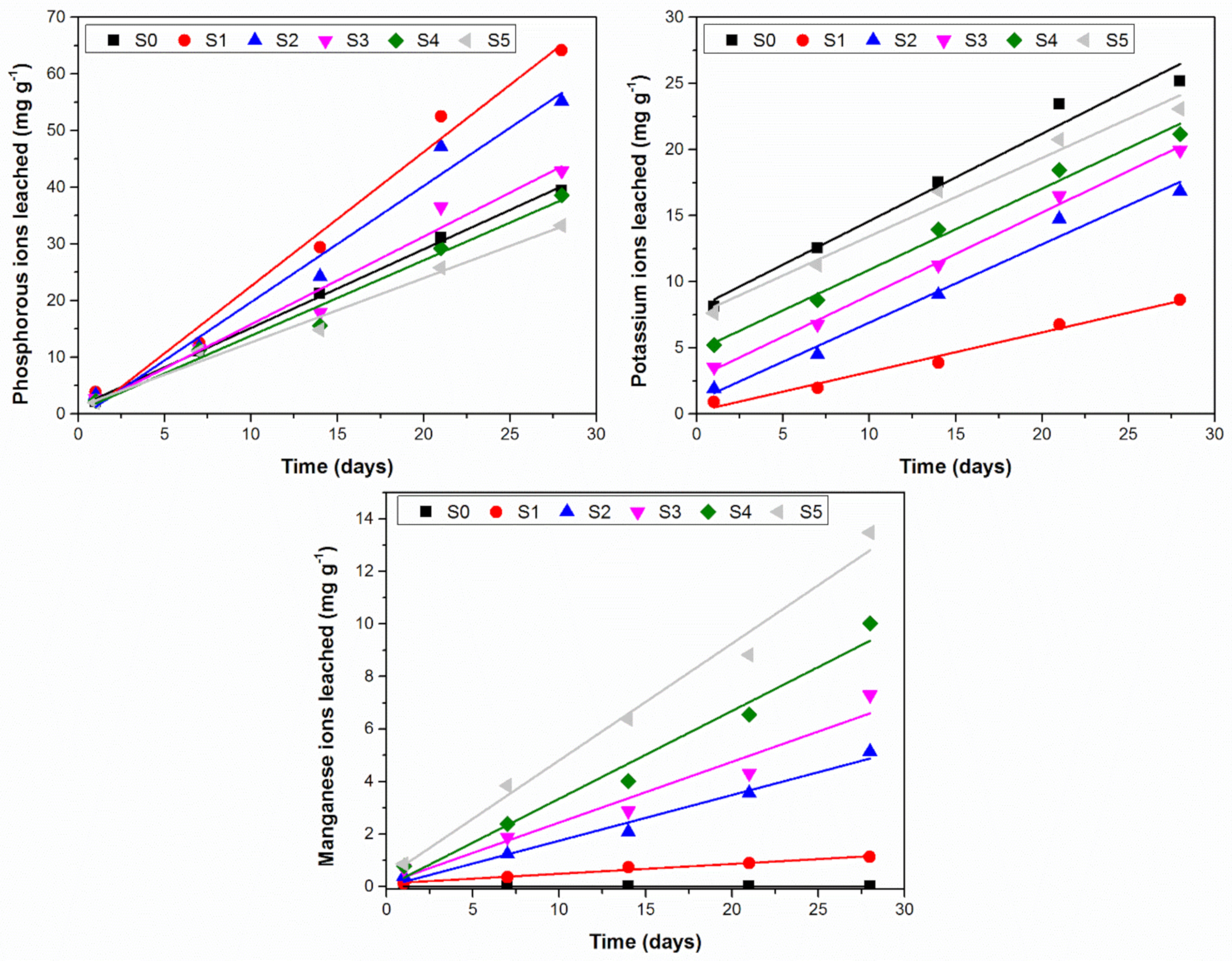
3.2. Chemical Activity
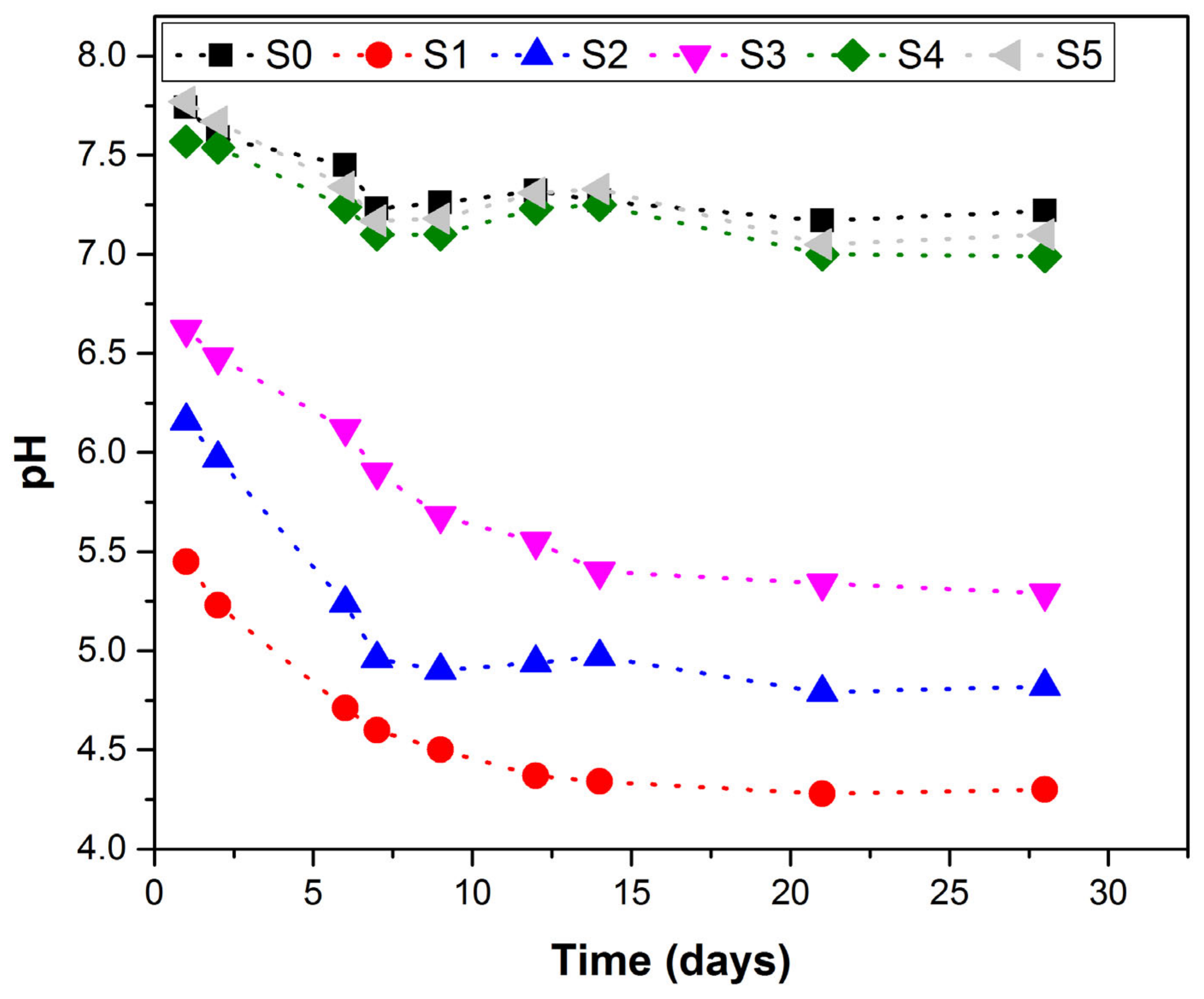
3.3. pH Evolution
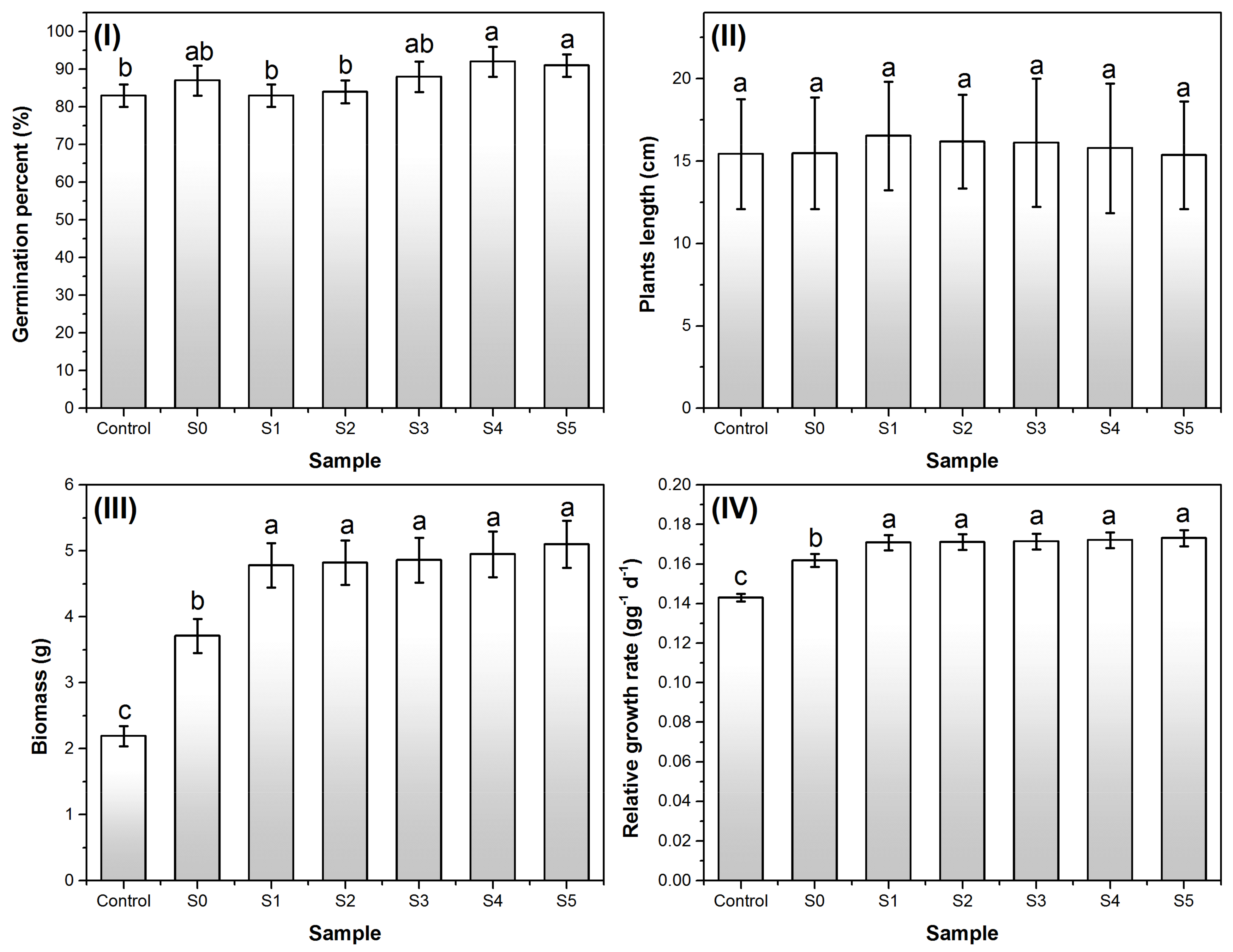
3.4. Effects on Plants Growth
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Debrah, J.K.; Vidal, D.G.; Dinis, M.A.P. Raising awareness on solid waste management through formal education for sustainability: A developing countries evidence—Review. Recycling 2021, 6, 6. [Google Scholar] [CrossRef]
- Vallero, D.A.; Shulman, V. Introduction to waste management. In Waste, 2nd ed.; Letcher, T., Vallero, D.A., Eds.; Academic Press: London, UK, 2019; pp. 3–14. [Google Scholar]
- Vancea, C.; Jurca, R.M.; Gheju, M.; Mosoarca, G. Eco-friendly solution for wastes resulted from the removal of Cr(VI) with Fe0 immobilization in glass based stoneware matrix. Rev. Rom. Mater. 2018, 48, 308–314. [Google Scholar]
- Dai, Y.; Sun, Q.; Wang, W.; Lu, L.; Liu, M.; Li, J.; Yang, S.; Sun, Y.; Zhang, K.; Xu, J.; et al. Utilizations of agricultural waste as adsorbent for the removal of contaminants: A review. Chemosphere 2018, 211, 235–253. [Google Scholar] [CrossRef]
- Das, S.; Lee, S.H.; Kumar, P.; Kim, K.H.; Lee, S.S.; Bhattacharya, S.S. Solid waste management: Scope and the challenge of sustainability. J. Clean. Prod. 2019, 228, 658–678. [Google Scholar] [CrossRef]
- Gaur, V.K.; Sharma, P.; Sirohi, R.; Awasthi, M.K.; Dussap, C.G.; Pandey, A. Assessing the impact of industrial waste on environment and mitigation strategies: A comprehensive review. J. Hazard. Mater. 2020, 398, 123019. [Google Scholar] [CrossRef]
- Usmani, Z.; Sharma, M.; Karpichev, Y.; Pandey, A.; Kuhad, R.C.; Bhat, R.; Punia, R.; Aghbashlo, M.; Tabatabaei, M.; Gupta, V.K. Advancement in valorization technologies to improve utilization of bio-based waste in bioeconomy context. Renew. Sust. Energ. Rev. 2020, 131, 109965. [Google Scholar] [CrossRef]
- Fava, G.; Naik, T.R.; Pierpaoli, M. Compressive strength and leaching behavior of mortars with biomass ash. Recycling 2018, 3, 46. [Google Scholar] [CrossRef]
- Faraca, G.; Boldrin, A.; Astrup, T. Resource quality of wood waste: The importance of physical and chemical impurities in wood waste for recycling. Waste Manag. 2019, 87, 135–147. [Google Scholar] [CrossRef]
- Siddique, R. Utilization of wood ash in concrete manufacturing. Resour. Conserv. Recycl. 2012, 67, 27–33. [Google Scholar] [CrossRef]
- Romero, E.; Quirantes, M.; Nogales, R. Characterization of biomass ashes produced at different temperatures from olive-oil-industry and greenhouse vegetable wastes. Fuel 2017, 208, 1–9. [Google Scholar] [CrossRef]
- Zajac, G.; Szyszlak-Barglowicz, J.; Golebiowski, W.; Szczepanik, M. Chemical characteristics of biomass ashes. Energies 2018, 11, 2885. [Google Scholar] [CrossRef]
- Oburger, E.; Jager, A.; Pasch, A.; Dellantonio, A.; Stampfer, K.; Wenzel, W.W. Environmental impact assessment of wood ash utilization in forest road construction and maintenance—A field study. Sci. Total Environ. 2016, 544, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Asquer, C.; Cappai, G.; De Gioannis, G.; Muntoni, A.; Piredda, M.; Spiga, D. Biomass ash reutilisation as an additive in the composting process of organic fraction of municipal solid waste. Waste Manag. 2017, 69, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, T.P.; Quinteiro, P.; Tarelho, L.A.C.; Arroja, L.; Dias, A.C. Environmental assessment of valorization alternatives for woody biomass ash in construction materials. Resour. Conserv. Recycl. 2019, 148, 67–79. [Google Scholar] [CrossRef]
- Da Costa, T.P.; Quinteiro, P.; Tarelho, L.A.C.; Arroja, L.; Dias, A.C. Life cycle assessment of woody biomass ash for soil amelioration. Waste Manag. 2020, 101, 126–140. [Google Scholar] [CrossRef]
- Fernandez-Delgado Juarez, M.; Prahauser, B.; Walter, A.; Insam, H.; Franke-Whittle, I.H. Co-composting of biowaste and wood ash, influence on a microbially driven-process. Waste Manag. 2015, 46, 155–164. [Google Scholar] [CrossRef]
- Fort, J.; Sal, J.; Zak, J.; Cerny, R. Assessment of wood-based fly ash as alternative cement replacement. Sustainability 2020, 12, 9580. [Google Scholar] [CrossRef]
- Guo, Y.; Zhao, C.; Chen, X.; Li, C. CO2 capture and sorbent regeneration performances of some wood ash materials. Appl. Energy 2015, 137, 26–36. [Google Scholar] [CrossRef]
- Kizinievic, O.; Kizinievic, V. Utilisation of wood ash from biomass for the production of ceramic products. Construct. Build. Mater. 2016, 127, 264–273. [Google Scholar] [CrossRef]
- Gaudreault, C.; Lama, I.; Sain, D. Is the beneficial use of wood ash environmentally beneficial? A screening-level life cycle assessment and uncertainty analysis. J. Ind. Ecol. 2020, 24, 1187–1392. [Google Scholar] [CrossRef]
- Mosoarca, G.; Vancea, C.; Popa, S.; Boran, S.; Tanasie, C. A green approach for treatment of wastewater with manganese using wood ash. J. Chem. Technol. Biotechnol. 2020, 95, 1781–1789. [Google Scholar] [CrossRef]
- Nordmark, D.; Vestin, J.; Lagerkvist, A.; Lind, B.B.; Arm, M.; Hallgren, P. Geochemical behavior of a gravel road upgraded with wood fly ash. J. Environ. Eng. 2014, 140, 05014002. [Google Scholar] [CrossRef]
- Pehlivan, E.; Kahraman, H.; Pehlivan, E. Sorption equilibrium of Cr(VI) ions on oak wood charcoal (Carbo Ligni) and charcoal ash as low-cost adsorbents. Fuel Process. Technol. 2011, 92, 65–70. [Google Scholar] [CrossRef]
- Tosti, L.; van Zomeren, A.; Pels, J.R.; Damgaard, A.; Comans, R.N.J. Life cycle assessment of the reuse of fly ash from biomass combustion as secondary cementitious material in cement products. J. Clean. Prod. 2019, 245, 118937. [Google Scholar] [CrossRef]
- Logsdon, G.; Hess, A.; Horsley, M. Guide to selection of water treatment processes. In Water Quality and Treatment—A Handbook of Community Water Supplies, 5th ed.; Letterman, R.D., Ed.; McGraw-Hill Inc.: New York, NY, USA, 1999; pp. 3.1–3.26. [Google Scholar]
- Singer, P.C.; Reckhow, D.A. Chemical oxidation. In Water Quality and Treatment—A Handbook of Community Water Supplies, 5th ed.; Letterman, R.D., Ed.; McGraw-Hill Inc.: New York, NY, USA, 1999; pp. 12.1–12.51. [Google Scholar]
- Negrea, A.; Lupa, L.; Negrea, P.; Mosoarca, G.; Ciopec, M. Studies concerning minimization and recycling of sludge resulted during hot-dip galvanization. In Proceedings of the 1st International Conference “Hazardous Waste Management”, Chania, Greece, 1–3 October 2008; pp. 71–72. [Google Scholar]
- Hazra, G.; Das, T. A review on controlled release advanced glassy fertilizer. GJSFR 2014, 14, 33–44. [Google Scholar]
- Labbilta, T.; Ait-El-Mokhtar, M.; Abouliatim, Y.; Khouloud, M.; Meddich, A.; Mesnaoui, M. Elaboration and characterization of vitreous fertilizers and study of their impact on the growth, photosynthesis, and yield of wheat (Triticum durum L.). Materials 2021, 14, 1295. [Google Scholar] [CrossRef] [PubMed]
- Ouis, M.A.; Ghoneim, N.A.; El Batal, H.A.; Shedeed, S.I. Evaluation of the suitability of agriglasses containing ZnO for plant fertilization. Silicon 2012, 4, 61–71. [Google Scholar] [CrossRef]
- Mandal, B.; Das, T.; Hazra, G. Advanced controlled release glass fertilizer: An inner view. J. Emerg. Technol. Innov. Res. 2018, 5, 698–717. [Google Scholar]
- Ivanenko, V.; Karapetyan, G.; Lipovskii, A.; Maksimov, L.; Rusan, V.; Tagantsev, D.; Tatarintsev, B.; Fleckenstein, J.; Schnug, E. Principal studies on phosphate glasses for fertilizers. Landbauforschung Völkenrode 2007, 57, 323–332. [Google Scholar]
- Ma, L.; Brow, R.K.; Schlesinger, M.E. Dissolution behavior of Na2O–FeO–Fe2O3–P2O5 glasses. J. Non. Cryst. Solids 2017, 463, 90–101. [Google Scholar] [CrossRef]
- Hazra, G. Different types of eco-friendly fertilizers: An overview. Sustain. Environ. 2016, 1, 54–70. [Google Scholar] [CrossRef]
- Kiwsakunkran, N.; Chanthima, N.; Kaewkhao, J.; Sangwaranateec, N. Composition and structural studies of glass fertilizer. In Proceedings of the 8th International Conference on Theoretical and Applied Physics, Medan, Indonesia, 20–21 September 2018; Journal of Physics: Conference Series. Volume 1120, p. 012016. [Google Scholar]
- Sava, B.A.; Boroica, L.; Sava, M.; Elisa, M.; Vasiliu, C.I.; Nastase, F.; Nastase, C.; Medianu, R. Potassium phosphate glasses used as agro-fertilizers with controlled solubility. J. Optoelectron. Adv. Mater. 2011, 13, 1534–1541. [Google Scholar]
- Sulowska, J.; Waclawska, I. Structural role of Cu in the soil active glasses. Process. Appl. Ceram. 2012, 6, 77–82. [Google Scholar] [CrossRef]
- Gheju, M.; Balcu, I. Assisted green remediation of chromium pollution. J. Environ. Manag. 2017, 203, 920–924. [Google Scholar] [CrossRef]
- Saha, P.; Shinde, O.; Sarkar, S. Phytoremediation of industrial mines wastewater using water hyacinth. Int. J. Phytorem. 2017, 19, 87–96. [Google Scholar] [CrossRef]
- Babu, K.V.; Rao, A.S.; Kumar, K.N.; Rao, M.V. Spectral and luminescence properties of manganese doped sodium lead alumino borosilicate glass system. J. Aircr. Spacecr. Technol. 2019, 3, 248–255. [Google Scholar] [CrossRef]
- Czaja, M.; Lisiecki, R.; Chrobak, A.; Sitko, R.; Mazurak, Z. The absorption and luminescence spectra of Mn3+ in beryl and vesuvianite. Phys. Chem. Minerals 2018, 45, 475–488. [Google Scholar] [CrossRef]
- Winterstein, A.; Akamatsu, H.; Moncke, D.; Tanaka, K.; Schmidt, M.A.; Wondraczek, L. Magnetic and magneto-optical quenching in (Mn2+, Sr2+) metaphosphate glasses. Opt. Mater. Express 2013, 3, 184–193. [Google Scholar] [CrossRef]
- Volpi, V.; Montesso, M.; Ribeiro, S.J.L.; Viali, W.R.; Magon, C.J.; Silva, I.D.A.; Donoso, J.P.; Nalin, M. Optical and structural properties of Mn2+ doped PbGeO3–SbPO4 glasses and glass–ceramics. J. Non. Cryst. Solids 2016, 431, 135–139. [Google Scholar] [CrossRef]
- Gao, H.; Tan, T.; Wang, D. Dissolution mechanism and release kinetics of phosphate controlled release glasses in aqueous medium. J. Control Release. 2004, 96, 29–36. [Google Scholar] [CrossRef]
- Simonin, J.P.; Boute, J. Intraparticle diffusion-adsorption model to describe liquid/solid adsorption kinetics. Rev. Mex. Ing. Quim. 2016, 15, 161–173. [Google Scholar]
- Nethaji, S.; Sivasamy, A.; Mandal, A.B. Adsorption isotherms, kinetics and mechanism for the adsorption of cationic and anionic dyes onto carbonaceous particles prepared from Juglans regia shell biomass. Int. J. Environ. Sci. Technol. 2013, 10, 231–242. [Google Scholar] [CrossRef]
- Han, X.; Niu, X.; Ma, X. Adsorption characteristics of methylene blue on poplar leaf in batch mode: Equilibrium, kinetics and thermodynamics. Korean J. Chem. Eng. 2012, 29, 494–502. [Google Scholar] [CrossRef]
- An, B. Cu(II) and As(V) adsorption kinetic characteristic of the multifunctional amino groups in chitosan. Processes 2020, 8, 1194. [Google Scholar] [CrossRef]
- Mekonnen, D.T.; Alemayehu, E.; Lennartz, B. Adsorptive removal of phosphate from aqueous solutions using low-cost volcanic rocks: Kinetics and equilibrium approaches. Materials 2021, 14, 1312. [Google Scholar] [CrossRef]
- Munoz, F.; Rocherulle, J.; Ahmed, I.; Hu, L. Phosphate glasses. In Springer Handbook of Glass; Musgraves, J.D., Hu, J., Calvez, L., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 553–594. [Google Scholar]
- Aras, S.; Aydin, S.S.; Korpe, D.A.; Donmez, C. Comparative genotoxicity analysis of heavy metal contamination in higher plants. In Ecotoxicology; Begum, G., Ed.; InTechOpen: Rijeka, Croatia, 2012; pp. 107–124. [Google Scholar]
- Fontanetti, C.S.; Nogarol, L.R.; de Souza, R.B.; Perez, D.G.; Maziviero, G.T. Bioindicators and biomarkers in the assessment of soil toxicity. In Soil Contamination; Pascucci, S., Ed.; InTechOpen: Rijeka, Croatia, 2011; pp. 143–168. [Google Scholar]
- Liu, W.; Li, P.J.; Qi, X.M.; Zhou, Q.X.; Zheng, L.; Sun, T.H.; Yang, Y.S. DNA changes in barley (Hordeum vulgare) seedlings induced by cadmium pollution using RAPD analysis. Chemosphere 2005, 61, 158–167. [Google Scholar] [CrossRef]
- White, P.A.; Claxton, L.D. Mutagens in contaminated soil: A review. Mutat. Res. 2004, 567, 227–245. [Google Scholar] [CrossRef]


| Oxide | SiO2 | Al2O3 | Fe2O3 | MnO2 | Na2O | K2O | CaO | MgO | P2O5 |
|---|---|---|---|---|---|---|---|---|---|
| (%) | 38.52 | 2.27 | 1.74 | 2.67 | 0.27 | 15.57 | 26.63 | 7.40 | 4.93 |
| Sample | S0 | S1 | S2 | S3 | S4 | S5 |
|---|---|---|---|---|---|---|
| Wood ash amount (mg g−1) | 0 | 20 | 40 | 60 | 80 | 100 |
| Corresponding agriglasses’ molar oxide composition | ||||||
| SiO2 (%) | 3.72 | 5.43 | 8.23 | 10.59 | 12.00 | 13.05 |
| Al2O3 (%) | 0.00 | 2.49 | 3.78 | 4.87 | 5.51 | 6.00 |
| Fe2O3 (%) | 0.00 | 0.13 | 0.19 | 0.24 | 0.28 | 0.30 |
| MnO (%) | 0.00 | 2.84 | 6.13 | 7.66 | 8.28 | 8.91 |
| Na2O (%) | 16.55 | 22.91 | 18.72 | 16.42 | 14.22 | 12.40 |
| K2O (%) | 18.99 | 1.53 | 2.32 | 3.63 | 8.81 | 10.97 |
| CaO (%) | 15.94 | 1.95 | 2.98 | 3.82 | 4.34 | 6.77 |
| MgO (%) | 13.39 | 2.26 | 11.36 | 12.81 | 12.37 | 11.68 |
| P2O5 (%) | 31.42 | 60.46 | 46.29 | 39.95 | 34.18 | 29.93 |
| Sample | S0 | S1 | S2 | S3 | S4 | S5 |
|---|---|---|---|---|---|---|
| Ion Leached | Phosphorous | |||||
| Equation | y = 1.3888x + 1.2904 | y = 2.3681x − 1.1212 | y = 2.0519x − 0.7908 | y = 1.5497x + 0.3149 | y = 1.3305x + 0.5071 | y = 1.1357x + 1.2584 |
| R² | 0.9982 | 0.9853 | 0.9765 | 0.9722 | 0.9802 | 0.9845 |
| Ion leached | Potassium | |||||
| Equation | y = 0.6598x + 7.9997 | y = 0.2985x + 0.1971 | y = 0.5917x + 0.994 | y = 0.5917x + 0.994 | y = 0.6137x + 4.7697 | y = 0.5928x + 7.5082 |
| R² | 0.978 | 0.9846 | 0.9827 | 0.9827 | 0.9896 | 0.9805 |
| Ion leached | Manganese | |||||
| Equation | y = 0.0373x + 0.1163 | y = 0.1738x + 0.0092 | y = 0.231x + 0.1296 | y = 0.3341x + 0.0054 | y = 0.4444x + 0.3635 | |
| R² | 0.9807 | 0.9826 | 0.9499 | 0.9758 | 0.9849 | |
| Sample | S0 | S1 | S2 | S3 | S4 | S5 |
|---|---|---|---|---|---|---|
| Ion leached | Phosphorous | |||||
| Step 1 | ||||||
| kt | 0.1393 | 0.1837 | 0.1521 | 0.1125 | 0.0993 | 0.0971 |
| C | 22.361 | 36.916 | 34.140 | 31.057 | 29.159 | 27.754 |
| R² | 0.9758 | 0.9841 | 0.9611 | 0.9995 | 0.9924 | 0.9864 |
| Step 2 | ||||||
| kt | 0.2391 | 0.4613 | 0.4122 | 0.333 | 0.3044 | 0.2431 |
| C | 24.087 | 41.611 | 38.559 | 34.934 | 32.925 | 30.452 |
| R² | 0.9997 | 0.9806 | 0.9712 | 0.965 | 0.9968 | 0.9964 |
| Ion leached | Potassium | |||||
| Step 1 | ||||||
| kt | 0.0685 | 0.0212 | 0.0515 | 0.056 | 0.0633 | 0.0671 |
| C | 16.148 | 1.1004 | 1.9337 | 2.9437 | 7.2967 | 9.1585 |
| R² | 0.9780 | 0.9745 | 0.9692 | 0.9864 | 0.9814 | 0.9746 |
| Step 2 | ||||||
| kt | 0.1023 | 0.0632 | 0.1042 | 0.1148 | 0.0954 | 0.0819 |
| C | 16.665 | 1.8335 | 2.7895 | 3.9504 | 7.7943 | 9.3389 |
| R² | 0.9358 | 0.9944 | 0.9753 | 0.9952 | 0.9918 | 0.9915 |
| Ion leached | Manganese | |||||
| Step 1 | ||||||
| kt | 0.0045 | 0.0124 | 0.0163 | 0.0239 | 0.0407 | |
| C | 1.4159 | 3.4269 | 4.4605 | 4.9908 | 5.5832 | |
| R² | 0.9887 | 0.9871 | 0.996 | 0.9865 | 0.9955 | |
| Step 2 | ||||||
| kt | 0.005 | 0.04 | 0.0575 | 0.0785 | 0.0926 | |
| C | 1.4224 | 3.9313 | 5.2431 | 6.0011 | 6.5639 | |
| R² | 0.9887 | 0.9954 | 0.9393 | 0.981 | 0.9496 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vancea, C.; Mosoarca, G.; Popa, S. A Sustainable Solution to Obtain P-K-Mn Glass Fertilizers from Cheap and Readily Available Wastes. Int. J. Environ. Res. Public Health 2021, 18, 6585. https://doi.org/10.3390/ijerph18126585
Vancea C, Mosoarca G, Popa S. A Sustainable Solution to Obtain P-K-Mn Glass Fertilizers from Cheap and Readily Available Wastes. International Journal of Environmental Research and Public Health. 2021; 18(12):6585. https://doi.org/10.3390/ijerph18126585
Chicago/Turabian StyleVancea, Cosmin, Giannin Mosoarca, and Simona Popa. 2021. "A Sustainable Solution to Obtain P-K-Mn Glass Fertilizers from Cheap and Readily Available Wastes" International Journal of Environmental Research and Public Health 18, no. 12: 6585. https://doi.org/10.3390/ijerph18126585
APA StyleVancea, C., Mosoarca, G., & Popa, S. (2021). A Sustainable Solution to Obtain P-K-Mn Glass Fertilizers from Cheap and Readily Available Wastes. International Journal of Environmental Research and Public Health, 18(12), 6585. https://doi.org/10.3390/ijerph18126585








