Abstract
Total hip replacement (THR) and total knee replacement (TKR) are among the most common elective surgical procedures. There is a large consensus on the importance of physical activity promotion for an active lifestyle in persons who underwent THR or TKR to prevent or mitigate disability and improve the quality of life (QoL) in the long term. However, there is no best practice in exercise and physical activity specifically designed for these persons. The present protocol aims to evaluate the efficacy and safety of an exercise program (6 month duration) designed for improving quality of life in people who had undergone THR or TKR. This paper describes a randomized controlled trial protocol that involves persons with THR or TKR. The participant will be randomly assigned to an intervention group or a control group. The intervention group will perform post-rehabilitation supervised training; the control group will be requested to follow the usual care. The primary outcome is QoL, measured with the Short-Form Health Survey (SF-36); Secondary outcomes are clinical, functional and lifestyle measures that may influence QoL. The results of this study could provide evidence for clinicians, exercise trainers, and policymakers toward a strategy that ensures safe and effective exercise physical activity after surgery.
1. Introduction
Total hip replacement (THR) and total knee replacement (TKR) are among the most common elective surgical procedures [1,2,3,4]. Osteoarthritis is the most common underlying condition for both THR and TKR. A large body of published evidence has consistently shown that these surgical procedures successfully decrease pain and improve mobility and quality of life [5,6].
The utilization rates of THR and TKR procedures have been increasing in the last 2–3 decades due to technical surgical improvements, significant benefits obtained, and the populations increased longevity. This volume growth poses an increasing economic burden in healthcare systems in terms of hospitalization costs and subsequent rehabilitation [7,8]. This trend is similar in all developed countries, although the difference in these surgical procedures’ incidence may be due to variations in economic status, health care delivery systems, patient preferences, or osteoarthritis prevalence [9].
As THR and TKR relieve joint pain, this represents an opportunity for these individuals to become more physically active [10]. Regular physical activity is one of the most effective interventions to improve the prevalent chronic comorbid conditions, including obesity, diabetes, hypertension, and cardiovascular deconditioning that commonly coexist with hip and knee osteoarthritis [11]. Furthermore, as physical activity induces quadriceps hypertrophy and improves strength, it is one of the main modifiable factors in patients with knee and hip osteoarthrosis who often exhibit weakness and atrophy [12] Therefore, THR and TKR could benefit not only the overall health of the individual undergoing surgery [13,14], but also relieve symptoms by increasing physical activity.
However, individuals who have completed THR or TKR treatment (surgery plus subsequent rehabilitation phase) do not seem to increase physical activity from pre-to-post-surgery. American [15,16] and Dutch [17,18] studies, show that individuals who undergo THR or TKR tend to be older sedentary adults, and it is estimated that around 49% of them are over-weight or obese, 16% have diabetes, and about half have high blood pressure. Studies on physical activity changes due to THR or TKR are challenged by wide variability in demographics, methods used to assess physical activity, and different care pathways used across studies [13,14]. However, the studies that assessed PA from pre- to post-THR and -TKR have reported that physical activity does not change the first three months post-surgery. The results of studies with follow-up assessments longer than three months but shorter than 12 months are contradictory, and the results of follow-ups longer than 12 months provide weak evidence of increased physical activity [13,14,17,18,19]. Vissers et al. [20] reported that 4 year post-surgery patients continued to improve their perceived physical functioning, capacity, and performance of daily life activities. However, Hootman et al. [21] and Wallis et al. [22] found that persons who received THR or TKR are significantly less likely to meet WHO physical activity guidelines [23]. In addition, only 48% individuals after hip and 60% after knee replacement perform more than 7000 steps per day, respectively [21,22].
To date, there are limited data to guide orthopedic surgeons’ recommendations regarding leisure time, exercises, and sports activities after THR or TKR. Moreover, there is little evidence on the strategies to improve and maintain physical function after rehabilitation in the long term. However, it is well known that these patients have significant muscular atrophy and weakness in the affected limb that can persist for months after surgery [24], with mobility deficits remaining for many years [13,14]. Exercise programs beyond the initial post-operative rehabilitation period have been shown to reduce pain and joint stiffness, improve physical function and lessen the chance of accidental falls after surgery [25,26,27,28,29,30]. These programs have generally used strengthening exercises and functional tasks such as stair climbing to improve muscular strength and power, walking speed, and mobility. Participating in these exercise programs, persons can be physically prepared to become active again in physical activity and sport. However, a disadvantage of these programs is that persons need to exercise under clinicians’ supervision at a hospital or rehabilitation center. This makes the delivery of these programs expensive due to the high costs associated with supervised treatment and transport. In addition, some persons are excluded because of difficulties with mobility and transport to a center preclude participation [27].
Current recommendations of health-enhancing sports activities in the long term are very generic and are based on the orthopedic surgeons’ opinions [31,32,33]. The following points for exercise prescription have been evidenced. Patients should be encouraged to remain physically active to improve general health, and bone mineral density. There is evidence that increased bone quality will improve prosthesis fixation and decrease the incidence of early loosening [34,35]. Factors such as wear, joint load, intensity, and type of prosthesis must be taken into account for each subject and sport to safely recommend a specific activity after THR or TKR. It has been shown that the wear decrease is one of the main factors in improving long-term results after total joint replacement. Wear is dependent on the load, the number of steps and the material properties of joint replacements [34,35]. It is unwise to start technically demanding activities after THR or TKR, as the joint loads and the risk for injuries are generally higher for these activities in unskilled individuals [32]. Finally, it is essential to distinguish among suitable activities following THR or TKR. In order to recommend suitable physical activity after total knee replacement, it is important to consider both the load and the knee flexion angle of the peak load, while for total hip replacement, which involves a ball and socket joint, the flexion angle does not play an important role. Thus, it is prudent to be more conservative after total knee arthroplasty than after total hip arthroplasty for activities that exhibit high joint loads in knee flexion [32].
In conclusion, although there is a large consensus on the importance of physical function improvement to prevent or mitigate disability in persons who underwent THR/TKR in the long term, there is no best practice specifically designed to these persons. Therefore, taking into account previously published experiences [25,26,27,28,29,30] and experts’ opinion [31,32,33], we designed an exercise protocol aimed at improving quality of life (QoL) after THR and TKR for primary osteoarthritis. This goal is intended to be achieved by improving a variety of factors which influence QoL, including muscle strength, joint mobility, gait and balance function, and pain [13,14,24,25,26,27,28,29,30]. This paper presents the protocol of a single-blinded randomized trial aimed at evaluating the efficacy and safety of this exercise program. We hypothesize that after THR/TKR treatment those participating in this exercise program will have better outcomes in terms of quality of life and functional and clinical scores than persons following the usual care.
2. Materials and Methods
This study is carried out within the project “Physical ActIvity after hip and knee Replacement” (PAIR) and funded within the Erasmus Plus Sport program (Grant Agreement 613008-EPP-1-2019-1-IT-SPO-SCP). The study was approved by the Local Ethics Committee (Comitato Etico Indipendente di Area Vasta Emilia Centro, CE-AVEC) of the Emilia-Romagna Region (reference number AVEC: 1005/2020/Sper/IOR) and registered in ClinicalTrial.Gov (NCT04761367).
2.1. Study Design
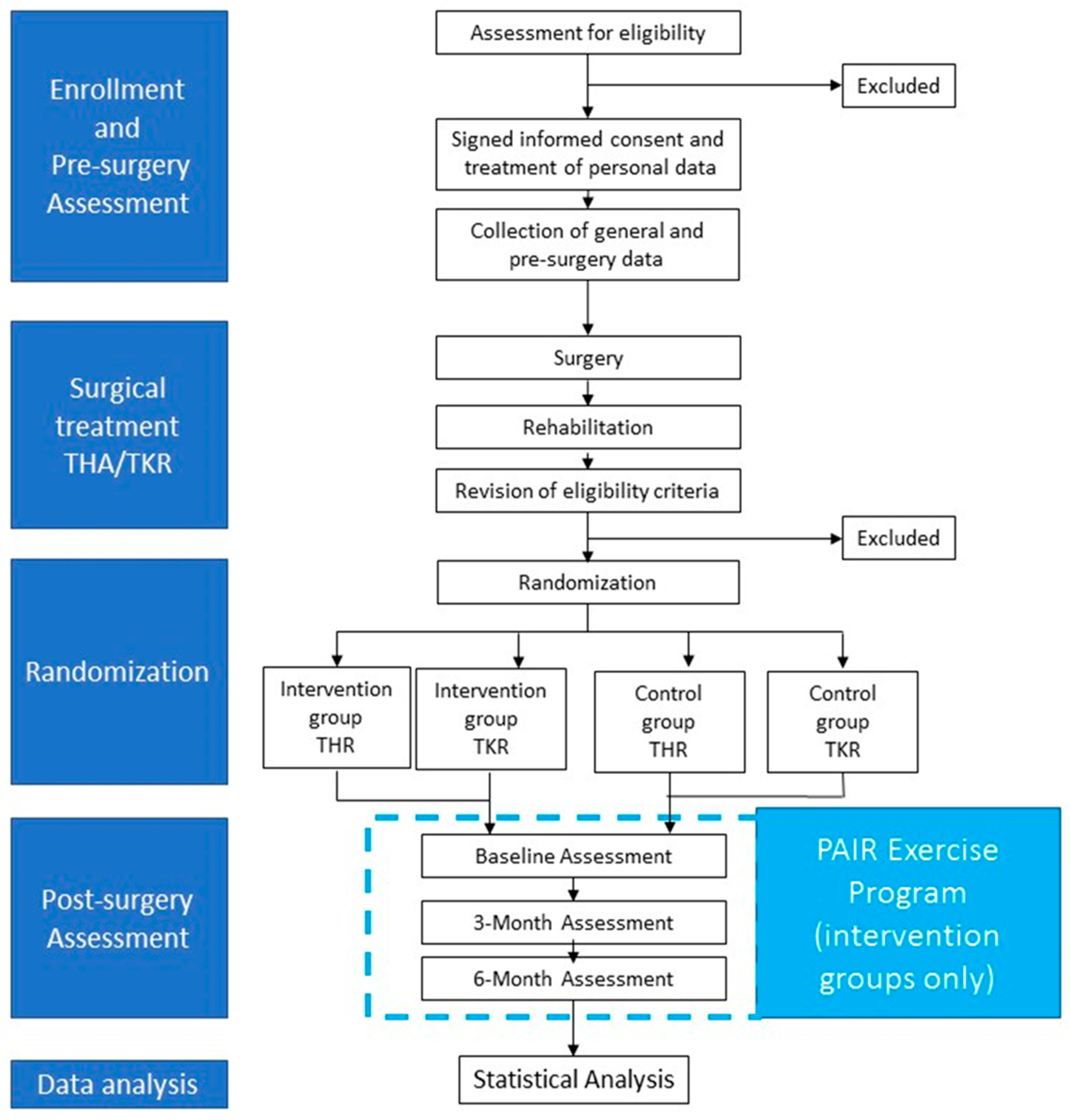
The study is a randomized controlled trial. At the moment of the pre-surgery medical check-up, persons will be enrolled. After surgical treatment (THR or TKR) and subsequent rehabilitation treatment, persons will be randomly assigned to an intervention group or a control group. The intervention group will participate in a 6-month exercise program based on the PAIR exercise protocol and will receive educational sessions on the importance of maintaining an active lifestyle after THR or TKR. The control group will follow usual-care and will receive recommendations by surgeons and physiotherapist on the importance of maintaining an active lifestyle after THR or TKR. Six months after surgery, participants of both intervention and control group will be assessed before randomization (post-surgery baseline) and, subsequently, after 3 and 6 months (Figure 1).
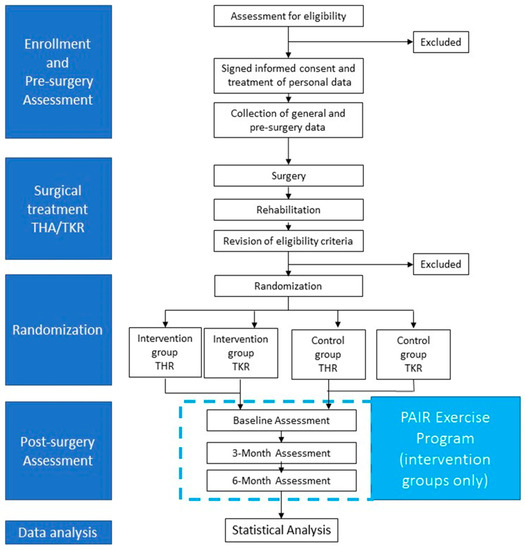
Figure 1.
Study design.
2.2. Participant Recruitment
Study participation will be proposed to all patients during the pre-surgery clinical assessment (within 15 days before the scheduled day of surgical intervention) by the medical personnel of the Clinical Units Chirurgia Ortopedica Ricostruttiva Tecniche Innovative and Clinica Ortopedica e Traumatologica II of the Istituto Ortopedico Rizzoli of Bologna after verifying the presence of inclusion/exclusion criteria. Subsequently, patients will receive information about the study and will be requested to sign the informed consent form. Patients will be enrolled after signing the informed consent form.
2.3. Inclusion and Exclusion Procedures
During the pre-surgery assessment, the inclusion/exclusion criteria associated with age, diagnosis and comorbid conditions will be verified by a medical doctor, while criteria linked to motor function and physical activity by a professional trainer. Subsequently, criteria will be verified again at the post-surgery assessment. In this latter assessment will also be verified the presence of two additional inclusion criteria, i.e., post-surgery functional performance and pain (Table 1).

Table 1.
Inclusion and exclusion criteria.
If eligible for the study, personal data (first and family name, address, telephone number(s)) will be recorded only on the informed consent form together with a study code. In all the other forms used in the study, patients will be identified with the patient’s study code.
2.4. Sample Size
The sample size was calculated through an a priori power analysis calculated on the study’s primary outcome, the physical health domain of the 36-item Short Form Health Survey (SF-36), assessed at post-surgery baseline and six-month assessments in both intervention and control groups. A randomized controlled pilot study in the literature with a similar rationale [36] was considered as a reference. Starting from this study, in which the SF-36 was used before and three months after the start of the intervention, was found a size effect of 1.06 (considered “increased”) with a standard deviation of 10 for each considered group. The power analysis was performed separately for THR and TKR, using a two-code test for independent groups with an error α = 0.05 and a sampling power (1-β) of 0.8, and a drop-out percentage of 30%. The minimum number required was estimated in 20 patients per group. Considering the four groups evaluated in the present study, two intervention groups (THR or TKR) and the two respective control groups, the final sample size was identified in a total of 80 patients.
2.5. Description of Procedure and Randomization
Both THR and TKR patients will be randomly assigned to the intervention groups and control groups after the post-surgery rehabilitation after having revised inclusion/exclusion criteria again. A randomization list, defined by a generator of random numbers available on the Emilia-Romagna Region website (http://wwwservizi.regione.emilia-romagna.it/generatore/, accessed on 11 April 2021) surgical intervention, 40 numbers within the interval (1,40) will be generated.
2.6. Allocation, Concealment and Blinding
This is a single blind RCT study. Random assignment to intervention groups or control groups will be performed by personnel other than those who will perform clinical and functional assessments. The list of allocation of the patients to the groups will be kept locked and separated from the rest of the material used to collect patients’ information. At any time, professionals who perform motor assessments will not be aware of which group participants have been assigned. Participants will be clearly instructed not to reveal the trainer who performs the assessment to which exercise group they have been assigned.
2.6.1. Intervention Groups (IGs)
The intervention groups for THR and TKR will be trained under the direct supervision of a graduate trainer in Sports Science. The protocol aims to reach fitness by improving muscular strength, range of motion (ROM), and muscular elasticity, as well as gait, balance and aerobic capacity. The exercise program is structured in 2 days per week 1 h sessions, and lasts six months. Each session is divided into warm-up, strength, balance, flexibility and cool-down sections. The protocol defines strategies to instruct patients and check the correct and safe execution of the motor tasks for both THR and TKR.
The exercises are chosen to avoid potentially harmful or painful movements or positions according to the participants’ conditions. For instance, exercises in quadrupedal position are excluded for persons with TKR. Exercises with leg adduction movements and in lateral decubitus positions are excluded for persons with THR. In order to follow the progressive workload adapting criteria, frequency, type, time and duration of the training will be modified based on the functional capacity reached by participants. Only common-use low-cost tools, such as elastic bands, yoga mats, dumbbells and soft dumbbells, are used within the exercise sessions.
In addition, all persons of the intervention groups will be requested to choose an additional third day of the week to carry out at least one of the following activities: brisk walking, cycling or swimming. The duration of these additional activities will be at least 30 min in order to reach the minimum amount of exercise per week of 150/300 min recommended by WHO [23]. Participants to the intervention group will be monthly request how many hours of additional activities has been done during the last month.
2.6.2. Control Groups (CGs)
The THR and TKR control groups will be requested to follow the usual care. The usual care is defined as the full spectrum of patient care practices in which clinicians have the opportunity to individualize care [37]. In this study, after having completed the rehabilitation treatment after surgery, patients will receive recommendations by surgeons and physiotherapists during follow-up visits on the importance and the appropriate quantity of weekly exercise in accordance with WHO recommendations [23]. Participants to the control group will be monthly contacted to monitor the compliance to the WHO’s recommendations. However, no structured long-term exercise training program will be provided.
2.7. Data Collection and Measures
All recruited participants will undergo multidimensional assessments before surgery and, subsequently, after completing the rehabilitation treatment (post-surgery baseline) and, 3 and 6 months later. Measures/records collected in each assessment session is summarized in Table 2 and pertain the following domains: general characteristics (age, gender, body mass index, comorbidity, education, social and professional), QoL, impairments and functional status, lifestyle, and safety. In addition, adherence to the exercise program, home-gym distance, and patient’s satisfaction will be investigated only in the participants of the THR and TKR intervention groups.

Table 2.
Variables collected during the study and time of recording.
2.7.1. General Characteristics
The factors that are considered relevant for the outcome of surgical treatment and/or physical activity programs are: age, gender, comorbidity, body mass index, educational level [38,39,40,41], social status [38,39,40], professional status [38,39,40], and home gym-distance [42], the possibility to reach the gym autonomously or with someone’s help, duration and number of sessions of rehabilitation treatment before and after surgery [43].
2.7.2. Primary Outcome
The primary outcome is QoL’s modification, measured with the SF-36 [44,45,46]. SF-36 is one of the most widely used questionnaires to measure the health-related QoL in total hip and total knee arthroplasty patients [47]. SF-36 is composed of 36 items and is focused on measures of two main domains: physical and mental health. It is a multi-item scale that assesses eight health concepts [44]: (1) limitations in physical activities caused by health problems; (2) limitations in social activities because of physical or emotional problems; (3) limitations in usual role activities because of physical health problems; (4) body pain; (5) general mental health (psychological distress and well-being); (6) limitations in usual role activities because of emotional problems; (7) vitality (energy and fatigue); (8) general health perceptions. Validity and reliability of SF-36 have been previously proved also for the Italian version of the questionnaire [48].
2.7.3. Secondary Outcomes
Secondary outcomes are measures which may influence QoL.
Impairments, functional and clinical status.
Maximal strength of the lower limbs can be reliably measured with a hand-held dynamometer [49,50,51,52]. It measures the peak isometric force generated from a muscle group. It is widely used to evaluate the strength of knee [49,50,51] and hip muscles [52,53,54] in healthy elderly and people with osteoarthritis.
Hip and knee joints mobility [55] are measured by goniometry.
The Hand Grip test will be measured by Hydraulic Hand Jamar Dynamometer. This measure has been strongly associated with frailty in the elderly population [56,57].
Pain is measured by visual analogue scale (VAS) [58,59,60]. VAS is a psychometric response scale widely used for measuring subjective characteristics that cannot be directly measured such as pain. It is widely used to measure pain in orthopedic studies.
Time Up and Go is a valid and reliable test used to examine physical performance and lower extremity [61]. It has been used in several clinical trials concerning different conditions [62,63,64], including arthroplasty [65,66,67,68]. It measures the time to stand from a standard chair, walk to a 3 m distance, turn around and return to sit on the same chair.
Single Stance test [69] is a useful tool for estimating standing balance and discriminating from low to high functional ability in individuals of various ages and functional levels. It is reliable, swift and easy to administer. It measures time subjects can balance on one leg up to a maximum of 30 s.
The 30 s Chair-Stand Test [70,71] is a valid and reliable single-item performance test for assessing lower limb function. It is performed by counting the number of stands completed in 30 s with hands crossed on the chest.
The Harris Hip Score (HHS) [72] is a disease-specific, instrument widely used as outcome measure after THR. The domains covered are pain, function, absence of deformity and ROM. It has been translated and validated in the Italian language [73].
The Hip Disability and Osteoarthritis Score (HOOS) was developed in 2003 and showed to be effective in measuring patient-relevant outcomes in osteoarthritis patients even after THR [74]. It is a 39-item self-administered questionnaire with five separate sub-scales: pain, symptoms, stiffness, daily living activities, sport and recreation function, and hip-related quality of life. It has been translated and validated in several languages, including Italian [75].
The American Knee Society Scoring (KSS) [76] is a disease-specific scoring system developed to assess patients’ clinical outcomes and functional ability before and after total knee arthroplasty. Knees are examined for ROM, flexion contractures, extension leg, alignment and stability; functional score rates the patient’s ability to walk and climb stairs.
The Knee Injury and Osteoarthritis Outcome Score (KOOS) is a 42-item self-administered questionnaire that proved to be a reliable and valid instrument for evaluating surgery outcomes physical therapy [77,78] for knee impairments. It assesses five outcomes: pain, symptoms, daily living activities, sport and recreation function, and knee-related QoL. It has been translated and validated in Italian [79].
The Western Ontario and McMasters Universities Osteoarthritis Index (WOMAC) [80,81] is a valid and reliable instrument designed to provide a disease-specific measure for patients with osteoarthritis of hip and knee. It includes 24 items in three dimensions: pain (5 items), function (17 items) and stiffness (2 items). Validity and reliability of the instrument have also been demonstrated for the Italian version [82].
The High-Activity Arthroplasty Score (HAAS) [83,84] is focused on lower limb functions. It was specifically developed to assess functional ability variations after lower limb arthroplasty with particular regard to high functioning individuals. The score is a four-item self-assessment measure covering the four domains of walking, running, stair climbing, and general activities. The Italian version of the instrument has been previously validated [85].
Life Style
The Recent Physical Activity Questionnaire (RPAQ) [86] is a self-administered instrument. It is divided into three sections (home activities, activity at work, recreation) during the last four weeks. It has been translated and validated in different languages, including Italian [87]. Weekly activity questionnaires will also be used to monitor the physical activity performed by participants of the intervention group outside the gym and by those of the control group. Each participant will be requested to record the amount (minutes) of moderate/intense activity performed each week.
The PAIR questionnaire for patients’ attitudes toward physical activity is an instrument generated within the PAIR project. It investigates following domains: QoL, practice of physical activity, attitude towards physical activity, function, kinesiophobia.
Safety
Adverse clinical events (ACEs) that will occur to participants during the study will be carefully recorded. For the intervention groups, the trainer will record the ACEs occurred during the exercise sessions and outside the gym at the end of each session. In the case of three consecutive absences, the coordinating center will contact the participant by telephone to investigate whether the cause of non-attendance at the gym sessions was an ACE. For participants in control group, the ACEs will be registered by the study staff during follow-up. Based on the records, ACEs will be classified for severity (severe: if the ACE involved hospitalization/access to the emergency room; moderate: if the ACE required the intervention of a doctor and/or modification of the usual pharmacological therapy; mild: if the ACE did not require medical intervention and/or modification of the usual pharmacological therapy), place (home: ACE occurred at home; outside: ACE exercise occurred outside the home; gym: ACE occurred during the exercise session), and apparatus (apparatus/system involved).
Adherence
The adherence of each participant to the exercise program will be monitored in the intervention groups. The adherence will be measured as the per cent of exercise sessions actually performed/total number of scheduled exercise sessions. Moreover, also the third additional activity performed will be recorded.
Participants’ Satisfaction
Finally, the intervention group participants’ perception of the quality of the exercise program will be investigated, as it strongly influences the adherence to an exercise program [42]. It will be verified by a dedicated questionnaire with structured responses based on a 7-point Likert scale [88,89,90].
2.8. Statistical Analysis
The qualitative variables will be summarized in terms of frequency, and the quantitative ones in terms of mean and standard deviation for both intervention groups and control groups and for the three times of assessment. To compare characteristics between and within groups the principle of intention to treat will be used, adjusting for adherence to the exercise program. In order to compare the baseline characteristics between the two groups, the following tests will be used: Student’s t-test for parametric quantitative variables; Mann–Whitney test for non-parametric variables; Chi-square test for qualitative dichotomous variables. To compare the changes between and within intervention groups and control groups among baseline and follow-up assessments the following tests will be used: for the normally distributed variables, analyses of variance for repeated measures followed by t-test with Sidak post hoc corrections; for non-parametric variables, the Friedman test followed by the Wilcoxon test with Bonferroni correction.
3. Discussion
This protocol presents a randomized trial aimed at evaluating the efficacy and safety of an exercise program designed for improving the quality of life in people who had undergone THR or TKR. Most patients report successful long-term outcomes and reduced or cured pain after surgery [8,9,91], but recovery is variable, and the majority of patients continue to demonstrate lower extremity muscle weakness and functional deficits compared to age-matched control persons [92,93,94]. The exercise program could improve these limitations and prepared the persons to become active again in physical activity and sports.
Many studies have been focused on exercise after THR/TKR, but their goals differed from those of the present study. Indeed, the literature often focused on the correction of sarcopenia, neuromuscular function, and pain-induced disuse of the affected leg before and in the first weeks or months immediately after surgery [95,96,97]. Other studies focused on which type of athletic activities (e.g., golf, swimming, tennis, walking, mountaineering, jogging, tennis, gym activities) deemed acceptable in the long-term in order to avoid damages of the implanted arthroplasty [98,99,100]. Nevertheless, the study published regarding sport activity after THR and TKR, there are no recognized criteria for exercise prescription for those who have completed surgical and rehabilitative treatment after THR/TKR, and the workout characteristics are simply based on allowed and not recommended sports activities [31,32]. Thus, this study will investigate the safety and efficacy of an exercise program specifically designed for persons who have completed surgical and rehabilitative treatment after THR/TKR.
Physical activity has a primary role in preventing chronic diseases and their consequences associated with the aging process. Arthrosis is an aging process that leads to pain and, consequently, a state of inactivity. This problem has already been studied in the literature by programs tested in different countries, which aimed to increase physical activity, functional status and improve the response to the surgery [101]. In the case of severe osteoarthrosis, hip or knee replacement reduce pain and improve function; therefore, we would expect a significant change of motor behavior and hypothesis an active lifestyle by these persons [10] after surgery. However, most persons do not increase physical activity from pre-to-post-surgery but remain sedentary and present a high prevalence of diabetes, hypertension [13,17,18,100], and overweight or obesity [102]. Indeed, despite the total knee and hip prostheses lead to global benefits independently of body weight, the risk of disability increases among persons with high BMI following THR and TKR [103,104]; in particular, a higher obesity level correlates with lower post-surgery functional scores [105,106].
Adherence to an exercise program is a crucial aspect of successful training. This protocol includes gym group training because there is evidence that adherence is higher in supervised programs than in those unsupervised, as proved by systematic reviews [107,108,109,110,111]. Group training facilitates participation by reducing costs and promoting social interactions, which lead to improved social, mental, and emotional health [107,108].
Mobility and transport difficulties often preclude regular participation in an exercise program in senior citizens [27,42]. To solve this issue, we selected exercises that can be performed using easily available cheap equipment (elastic bands, yoga mats, dumbbells and soft dumbbells) instead of gym machines. This may facilitate the scaling up of the training program, if proved effective and safe, as it will be possible to replicate it not only in well-equipped gyms but also at home or in suitable spaces close to home. The use of community environments, where gyms are not available, has been reported to be very effective for sustains exercise adherence in previous research [42].
Health related QoL is considered of paramount importance to evaluate the impact of chronic diseases and treatment outcomes [112]. As a primary outcome measure, we selected the SF-36 because it is a multidimensional instrument widely used to evaluate the THR and TKR outcomes (see, e.g., References [113,114]). However, this is a generic instrument which has not been designed to approach QoL aspects specifically related to the evaluation of the outcomes of these surgical procedures. Thus, we included as secondary outcomes a multidimensional system of measures of impairments, functional and clinical status specifically related to the expected effects of the exercise program on the type of persons who are the focus of this study. This could allow a better understanding the results and, if necessary, further optimization of the exercise protocol.
Limitation
The final goal of this study is to increase regular physical activity among the persons who underwent THR/TKR. Thus, to detect modifications of lifestyle activity represents an important goal of our project. However, to measure lifestyle activity is not an easy matter and several methods have been proposed including self-report questionnaires, smart phone technology, motion sensors, and pedometers [115]. All these methods have strengths and limitations in terms of methodological effectiveness and feasibility. The use of wearable devices or smart phones can be accurate but not easy to use in long-term study by aged participants. Thus, we decided to use a self-report questionnaire of physical activity (RPAQ) and weekly logs although social desirability may result in over-reporting of PA among participants keen to comply with the intervention aims [116]. These factors will require careful consideration in the interpretation of the results.
The protocol takes into account the duration and number of sessions of rehabilitation which participants have undergone before and after surgery, but not the type and intensity of this treatment. This may weaken the results of the study, as large and consistent evidence has proved the importance of pre- and post-surgery rehabilitation [43]. However, we deemed it unfeasible to reliably control this variable, as rehabilitation treatment will be performed not within the premises of our hospital but at the community level within private or public facilities. Nonetheless, differences in rehabilitation treatment outcomes observed at the post-surgery baseline will be used in the results’ analysis and interpretation.
We excluded people from participation in the study who are unable to walk unassisted for 500 m or have significant pain at rest. This may limit the participation of people who need the exercise the most and weaken the study conclusions. However, people with important functional limitations and/or severe pain are eligible for medical and rehabilitation care or aquatic exercise programs while land-based group exercise could be very problematic or even unsafe.
4. Conclusions
Exercise is fundamental to maintain or improve health and prevent disability in subjects who undergo THR/TKR. However, no specific indications on the appropriate exercise program are available for these subjects after the rehabilitation phase. The results of this study could add evidence for clinicians, exercise trainers, and policy makers toward the best strategy to ensure safe and effective exercise after surgery.
Author Contributions
G.B. conceived the study; L.B., F.B., G.B., E.P., R.Z. and Group PS. contributed to the study design; L.B., G.B., E.P., R.Z. and F.B. drafted the manuscript; all authors provided feedback and approved of the final document. All authors have read and agreed to the published version of the manuscript.
Funding
The research was funded within the PAIR project by Erasmus + Sport (grant agreement N. 613008-EPP-1-2019-1-IT-SPO-SCP).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and ap-proved by Ethics Committee of Comitato Etico Indipendente di Area Vasta Emilia Centro, CE-AVEC (AVEC: 1005/2020/Sper/IOR; 30 November 2020).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Kurtz, S.M.; Ong, K.L.; Lau, E.; Bozic, K.J. Impact of the economic downturn on total joint replacement demand in the United States: Updated projections to 2021. J. Bone Jt. Surg. Am. 2014, 96, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, S.; Mowat, F.; Ong, K.; Chan, N.; Lau, E.; Halpern, M. Prevalence of primary and revision total hip and knee arthroplasty in the United States from 1990 through 2002. J. Bone Jt. Surg. Am. 2005, 87, 1487–1497. [Google Scholar]
- Kurtz, S.; Ong, K.; Lau, E.; Mowat, F.; Halpern, M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J. Bone Jt. Surg. Am. 2007, 89, 780–785. [Google Scholar] [CrossRef] [PubMed]
- Lubbeke, A.; Silman, A.J.; Prieto-Alhambra, D.; Adler, A.I.; Barea, C.; Carr, A.J. The role of national registries in improving patient safety for hip and knee replacements. BMC Musculoskelet. Disord. 2017, 18, 414. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, A.; Kennedy, L.G.; Baxter, K.; Donovan, J.; Wilkinson, M.; Bevan, G. Effectiveness of hip prostheses in primary total hip replacement: A critical review of evidence and an economic model. Health Technol. Assess. 1998, 2, 1–133. [Google Scholar] [CrossRef]
- Meira, E.P.; Zeni, J., Jr. Sports participation following total hip arthroplasty. Int. J. Sports Phys. Ther. 2014, 9, 839–850. [Google Scholar]
- Piscitelli, P.; Iolascon, G.; Di Tanna, G.; Bizzi, E.; Chitano, G.; Argentiero, A.; Neglia, C.; Giolli, L.; Distante, A.; Gimigliano, R.; et al. Socioeconomic burden of total joint arthroplasty for symptomatic hip and knee osteoarthritis in the Italian population: A 5-year analysis based on hospitalization records. Arthritis Care Res. 2012, 64, 1320–1327. [Google Scholar] [CrossRef]
- Maravic, M.; Landais, P. Usefulness of a national hospital database to evaluate the burden of primary joint replacement for coxarthrosis and gonarthrosis in patients aged over 40 years. Osteoarthr. Cartil. 2006, 14, 612–615. [Google Scholar] [CrossRef][Green Version]
- Singh, J.A. Epidemiology of knee and hip arthroplasty: A systematic review. Open Orthop. J. 2011, 5, 80–85. [Google Scholar] [CrossRef]
- Meessen, J.M.; Peter, W.F.; Wolterbeek, R.; Cannegieter, S.C.; Tilbury, C.; Benard, M.R.; van der Linden, H.M.; Onstenk, R.; Tordoir, R.; Vehmeijer, S.B.; et al. Patients who underwent total hip or knee arthroplasty are more physically active than the general Dutch population. Rheumatol. Int. 2017, 37, 219–227. [Google Scholar] [CrossRef]
- Singh, M.A. Exercise comes of age: Rationale and recommendations for a geriatric exercise prescription. J. Gerontol. A Biol. Sci. Med. Sci. 2002, 57, M262–M282. [Google Scholar] [CrossRef]
- Georgiev, T.; Angelov, A.K. Modifiable risk factors in knee osteoarthritis: Treatment implications. Rheumatol. Int. 2019, 39, 1145–1157. [Google Scholar] [CrossRef]
- Almeida, G.J.; Khoja, S.S.; Piva, S.R. Physical activity after total joint arthroplasty: A narrative review. Open Access J. Sports Med. 2018, 9, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Arnold, J.B.; Walters, J.L.; Ferrar, K.E. Does Physical Activity Increase After Total Hip or Knee Arthroplasty for Osteoarthritis? A Systematic Review. J. Orthop. Sports Phys. Ther. 2016, 46, 431–442. [Google Scholar] [CrossRef]
- Murphy, L.; Helmick, C.G. The impact of osteoarthritis in the United States: A population-health perspective: A population-based review of the fourth most common cause of hospitalization in U.S. adults. Orthop. Nurs. 2012, 31, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Murphy, L.; Helmick, C.G. The impact of osteoarthritis in the United States: A population-health perspective. Am. J. Nurs. 2012, 112 (Suppl. 1), S13–S19. [Google Scholar] [CrossRef]
- Wagenmakers, R.; Stevens, M.; Groothoff, J.W.; Zijlstra, W.; Bulstra, S.K.; van Beveren, J.; van Raaij, J.J.; van den Akker-Scheek, I. Physical activity behavior of patients 1 year after primary total hip arthroplasty: A prospective multicenter cohort study. Phys. Ther. 2011, 91, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Wagenmakers, R.; Stevens, M.; Zijlstra, W.; Jacobs, M.L.; van den Akker-Scheek, I.; Groothoff, J.W.; Bulstra, S.K. Habitual physical activity behavior of patients after primary total hip arthroplasty. Phys. Ther. 2008, 88, 1039–1048. [Google Scholar] [CrossRef] [PubMed]
- Paxton, E.W.; Torres, A.; Love, R.M.; Barber, T.C.; Sheth, D.S.; Inacio, M.C. Total joint replacement: A multiple risk factor analysis of physical activity level 1-2 years postoperatively. Acta Orthop. 2016, 87 (Suppl. 1), 44–49. [Google Scholar] [CrossRef]
- Vissers, M.M.; Bussmann, J.B.; de Groot, I.B.; Verhaar, J.A.; Reijman, M. Physical functioning four years after total hip and knee arthroplasty. Gait Posture 2013, 38, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Hootman, J.M.; Macera, C.A.; Ham, S.A.; Helmick, C.G.; Sniezek, J.E. Physical activity levels among the general US adult population and in adults with and without arthritis. Arthritis Rheum. 2003, 49, 129–135. [Google Scholar] [CrossRef]
- Wallis, J.A.; Webster, K.E.; Levinger, P.; Taylor, N.F. What proportion of people with hip and knee osteoarthritis meet physical activity guidelines? A systematic review and meta-analysis. Osteoarthr. Cartil. 2013, 21, 1648–1659. [Google Scholar] [CrossRef]
- WHO. WHO Guidelines on Physical Activity and Sedentary Behaviour; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Reardon, K.; Galea, M.; Dennett, X.; Choong, P.; Byrne, E. Quadriceps muscle wasting persists 5 months after total hip arthroplasty for osteoarthritis of the hip: A pilot study. Intern. Med. J. 2001, 31, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Gilbey, H.J.; Ackland, T.R.; Wang, A.W.; Morton, A.R.; Trouchet, T.; Tapper, J. Exercise improves early functional recovery after total hip arthroplasty. Clin. Orthop. Relat. Res. 2003, 408, 193–200. [Google Scholar] [CrossRef]
- Hauer, K.; Specht, N.; Schuler, M.; Bartsch, P.; Oster, P. Intensive physical training in geriatric patients after severe falls and hip surgery. Age Ageing 2002, 31, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Galea, M.P.; Levinger, P.; Lythgo, N.; Cimoli, C.; Weller, R.; Tully, E.; McMeeken, J.; Westh, R. A targeted home- and center-based exercise program for people after total hip replacement: A randomized clinical trial. Arch. Phys. Med. Rehabil. 2008, 89, 1442–1447. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, M.; Sawano, S.; Kugo, M.; Maegawa, S.; Kawasaki, T.; Ichihashi, N. Physical Activity Promotes Gait Improvement in Patients With Total Knee Arthroplasty. J. Arthroplast. 2016, 31, 984–988. [Google Scholar] [CrossRef]
- Wylde, V.; Dennis, J.; Gooberman-Hill, R.; Beswick, A.D. Effectiveness of postdischarge interventions for reducing the severity of chronic pain after total knee replacement: Systematic review of randomised controlled trials. BMJ Open 2018, 8, e020368. [Google Scholar] [CrossRef]
- Zaffagnini, S.; Di Paolo, S.; Meena, A.; Alesi, D.; Zinno, R.; Barone, G.; Pizza, N.; Bragonzoni, L. Causes of stiffness after total knee arthroplasty: A systematic review. Int. Orthop. 2021. [Google Scholar] [CrossRef]
- Mayer, F.; Dickhuth, H.-H. FIMS Position Statement. Sport Präv. 2010, 40, 10–13. [Google Scholar] [CrossRef]
- Kuster, M.S. Exercise recommendations after total joint replacement: A review of the current literature and proposal of scientifically based guidelines. Sports Med. 2002, 32, 433–445. [Google Scholar] [CrossRef]
- Stevens, M.; Reininga, I.H.; Bulstra, S.K.; Wagenmakers, R.; van den Akker-Scheek, I. Physical activity participation among patients after total hip and knee arthroplasty. Clin. Geriatr Med. 2012, 28, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Dubs, L.; Gschwend, N.; Munzinger, U. Sport after total hip arthroplasty. Arch. Orthop. Trauma. Surg. 1983, 101, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Widhalm, R.; Hofer, G.; Krugluger, J.; Bartalsky, L. Is there greater danger of sports injury or osteoporosis caused by inactivity in patients with hip prosthesis? Sequelae for long-term stability of prosthesis anchorage. Z. Orthop. Ihre Grenzgeb. 1990, 128, 139–143. [Google Scholar] [CrossRef] [PubMed]
- McKay, C.; Prapavessis, H.; Doherty, T. The effect of a prehabilitation exercise program on quadriceps strength for patients undergoing total knee arthroplasty: A randomized controlled pilot study. PM R 2012, 4, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Thompson, B.T.; Schoenfeld, D. Usual care as the control group in clinical trials of nonpharmacologic interventions. Proc. Am. Thorac. Soc. 2007, 4, 577–582. [Google Scholar] [CrossRef]
- Thomas, S.; Halbert, J.; Mackintosh, S.; Quinn, S.; Crotty, M. Sociodemographic factors associated with self-reported exercise and physical activity behaviors and attitudes of South Australians: Results of a population-based survey. J. Aging Health 2012, 24, 287–306. [Google Scholar] [CrossRef]
- Resnick, B.; D’Adamo, C. Factors associated with exercise among older adults in a continuing care retirement community. Rehabil. Nurs. 2011, 36, 47–53. [Google Scholar] [CrossRef]
- O’Dougherty, M.; Dallman, A.; Turcotte, L.; Patterson, J.; Napolitano, M.A.; Schmitz, K.H. Barriers and motivators for strength training among women of color and Caucasian women. Women Health 2008, 47, 41–62. [Google Scholar] [CrossRef]
- Rhodes, R.E.; Martin, A.D.; Taunton, J.E.; Rhodes, E.C.; Donnelly, M.; Elliot, J. Factors associated with exercise adherence among older adults. An individual perspective. Sports Med. 1999, 28, 397–411. [Google Scholar] [CrossRef]
- Hicks, G.E.; Benvenuti, F.; Fiaschi, V.; Lombardi, B.; Segenni, L.; Stuart, M.; Pretzer-Aboff, I.; Gianfranco, G.; Macchi, C. Adherence to a community-based exercise program is a strong predictor of improved back pain status in older adults: An observational study. Clin. J. Pain 2012, 28, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Ng, L.; Gonzalez, S.; Hale, T.; Turner-Stokes, L. Multidisciplinary rehabilitation programmes following joint replacement at the hip and knee in chronic arthropathy. Cochrane Database Syst. Rev. 2008, CD004957. [Google Scholar] [CrossRef]
- Ware, J.E., Jr.; Sherbourne, C.D. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med. Care 1992, 30, 473–483. [Google Scholar] [CrossRef] [PubMed]
- McHorney, C.A.; Ware, J.E., Jr.; Lu, J.F.; Sherbourne, C.D. The MOS 36-item Short-Form Health Survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med. Care 1994, 32, 40–66. [Google Scholar] [CrossRef] [PubMed]
- McHorney, C.A.; Ware, J.E., Jr.; Raczek, A.E. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med. Care 1993, 31, 247–263. [Google Scholar] [CrossRef] [PubMed]
- Ethgen, O.; Bruyère, O.; Richy, F.; Dardennes, C.; Reginster, J.Y. Health-related quality of life in total hip and total knee arthroplasty. A qualitative and systematic review of the literature. J. Bone Jt. Surg. Am. 2004, 86, 963–974. [Google Scholar] [CrossRef]
- Apolone, G.; Mosconi, P. The Italian SF-36 Health Survey: Translation, validation and norming. J. Clin. Epidemiol. 1998, 51, 1025–1036. [Google Scholar] [CrossRef]
- Mentiplay, B.F.; Perraton, L.G.; Bower, K.J.; Adair, B.; Pua, Y.H.; Williams, G.P.; McGaw, R.; Clark, R.A. Assessment of Lower Limb Muscle Strength and Power Using Hand-Held and Fixed Dynamometry: A Reliability and Validity Study. PLoS ONE 2015, 10, e0140822. [Google Scholar] [CrossRef]
- Bandinelli, S.; Benvenuti, E.; Del Lungo, I.; Baccini, M.; Benvenuti, F.; Di Iorio, A.; Ferrucci, L. Measuring muscular strength of the lower limbs by hand-held dynamometer: A standard protocol. Aging 1999, 11, 287–293. [Google Scholar] [CrossRef]
- Hirano, M.; Katoh, M.; Gomi, M.; Arai, S. Validity and reliability of isometric knee extension muscle strength measurements using a belt-stabilized hand-held dynamometer: A comparison with the measurement using an isokinetic dynamometer in a sitting posture. J. Phys. Ther. Sci. 2020, 32, 120–124. [Google Scholar] [CrossRef]
- Chopp-Hurley, J.N.; Wiebenga, E.G.; Gatti, A.A.; Maly, M.R. Investigating the Test-Retest Reliability and Validity of Hand-Held Dynamometry for Measuring Knee Strength in Older Women with Knee Osteoarthritis. Physiother. Can. 2019, 71, 231–238. [Google Scholar] [CrossRef]
- Bohannon, R.W. Test-retest reliability of hand-held dynamometry during a single session of strength assessment. Phys. Ther 1986, 66, 206–209. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Olson, S.L.; Protas, E.J. Test-retest strength reliability: Hand-held dynamometry in community-dwelling elderly fallers. Arch. Phys. Med. Rehabil. 2002, 83, 811–815. [Google Scholar] [CrossRef] [PubMed]
- Kendal, F.M.E.; Provance, P. Muscles: Testing and Function; Williams & Wilkins: Baltimore, MD, USA, 1993. [Google Scholar]
- Prasitsiriphon, O.; Pothisiri, W. Associations of Grip Strength and Change in Grip Strength With All-Cause and Cardiovascular Mortality in a European Older Population. Clin. Med. Insights Cardiol. 2018, 12, 1179546818771894. [Google Scholar] [CrossRef]
- Xue, Q.L.; Beamer, B.A.; Chaves, P.H.; Guralnik, J.M.; Fried, L.P. Heterogeneity in rate of decline in grip, hip, and knee strength and the risk of all-cause mortality: The Women’s Health and Aging Study II. J. Am. Geriatr. Soc. 2010, 58, 2076–2084. [Google Scholar] [CrossRef]
- Aitken, R.C. Measurement of feelings using visual analogue scales. Proc. R Soc. Med. 1969, 62, 989–993. [Google Scholar] [PubMed]
- Carpenter, J.S.; Brockopp, D. Comparison of patients’ ratings and examination of nurses’ responses to pain intensity rating scales. Cancer Nurs. 1995, 18, 292–298. [Google Scholar] [CrossRef]
- Lennard, T.W.S.; Singla, A.; Vivian, D. Pain Procedures in Clinical Practice; Elsevier Sanders: Philadelphia, PA, USA, 2011; p. 656. [Google Scholar]
- Yuksel, E.; Unver, B.; Kalkan, S.; Karatosun, V. Reliability and minimal detectable change of the 2-minute walk test and Timed Up and Go test in patients with total hip arthroplasty. Hip. Int. 2021, 31, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Huisman, M.G.; van Leeuwen, B.L.; Ugolini, G.; Montroni, I.; Spiliotis, J.; Stabilini, C.; de’Liguori Carino, N.; Farinella, E.; de Bock, G.H.; Audisio, R.A. “Timed Up & Go”: A screening tool for predicting 30-day morbidity in onco-geriatric surgical patients? A multicenter cohort study. PLoS ONE 2014, 9, e86863. [Google Scholar]
- Michelson, A.T.; Tsapepas, D.S.; Husain, S.A.; Brennan, C.; Chiles, M.C.; Runge, B.; Lione, J.; Kil, B.H.; Cohen, D.J.; Ratner, L.E.; et al. Association between the "Timed Up and Go Test" at transplant evaluation and outcomes after kidney transplantation. Clin. Transplant. 2018, 32, e13410. [Google Scholar] [CrossRef] [PubMed]
- Son, K.Y.; Shin, D.W.; Lee, J.E.; Kim, S.H.; Yun, J.M.; Cho, B. Association of timed up and go test outcomes with future incidence of cardiovascular disease and mortality in adults aged 66 years: Korean national representative longitudinal study over 5.7 years. BMC Geriatr. 2020, 20, 111. [Google Scholar] [CrossRef]
- Boonstra, M.C.; De Waal Malefijt, M.C.; Verdonschot, N. How to quantify knee function after total knee arthroplasty? Knee 2008, 15, 390–395. [Google Scholar] [CrossRef]
- Taniguchi, M.; Sawano, S.; Maegawa, S.; Ikezoe, T.; Ichihashi, N. Physical Activity Mediates the Relationship between Gait Function and Fall Incidence after Total Knee Arthroplasty. J. Knee Surg. 2020. [Google Scholar] [CrossRef]
- Oosting, E.; Jans, M.P.; Dronkers, J.J.; Naber, R.H.; Dronkers-Landman, C.M.; Appelman-de Vries, S.M.; van Meeteren, N.L. Preoperative home-based physical therapy versus usual care to improve functional health of frail older adults scheduled for elective total hip arthroplasty: A pilot randomized controlled trial. Arch. Phys. Med. Rehabil. 2012, 93, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Slaven, E.J. Prediction of functional outcome at six months following total hip arthroplasty. Phys. Ther. 2012, 92, 1386–1394. [Google Scholar] [CrossRef] [PubMed]
- Piva, S.R.; Gil, A.B.; Almeida, G.J.; DiGioia, A.M., 3rd; Levison, T.J.; Fitzgerald, G.K. A balance exercise program appears to improve function for patients with total knee arthroplasty: A randomized clinical trial. Phys. Ther. 2010, 90, 880–894. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.J.; Rikli, R.E.; Beam, W.C. A 30-s chair-stand test as a measure of lower body strength in community-residing older adults. Res. Q Exerc. Sport 1999, 70, 113–119. [Google Scholar] [CrossRef]
- Bruun, I.H.; Mogensen, C.B.; Nørgaard, B.; Schiøttz-Christensen, B.; Maribo, T. Validity and Responsiveness to Change of the 30-Second Chair-Stand Test in Older Adults Admitted to an Emergency Department. J. Geriatr. Phys. Ther. 2019, 42, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.H. Traumatic arthritis of the hip after dislocation and acetabular fractures: Treatment by mold arthroplasty. An end-result study using a new method of result evaluation. J. Bone Jt. Surg. Am. 1969, 51, 737–755. [Google Scholar] [CrossRef]
- Dettoni, F.; Pellegrino, P.; La Russa, M.R.; Bonasia, D.E.; Blonna, D.; Bruzzone, M.; Castoldi, F.; Rossi, R. Validation and cross cultural adaptation of the Italian version of the Harris Hip Score. Hip. Int. 2015, 25, 91–97. [Google Scholar] [CrossRef]
- Klässbo, M.; Larsson, E.; Mannevik, E. Hip disability and osteoarthritis outcome score. An extension of the Western Ontario and McMaster Universities Osteoarthritis Index. Scand. J. Rheumatol. 2003, 32, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Torre, M.; Luzi, I.; Mirabella, F.; Del Manso, M.; Zanoli, G.; Tucci, G.; Romanini, E. Cross-cultural adaptation and validation of the Italian version of the Hip disability and Osteoarthritis Outcome Score (HOOS). Health Qual. Life Outcomes 2018, 16, 115. [Google Scholar] [CrossRef]
- Insall, J.N.; Dorr, L.D.; Scott, R.D.; Scott, W.N. Rationale of the Knee Society clinical rating system. Clin. Orthop. Relat Res. 1989, 87, 13–14. [Google Scholar] [CrossRef]
- Roos, E.M.; Roos, H.P.; Lohmander, L.S.; Ekdahl, C.; Beynnon, B.D. Knee Injury and Osteoarthritis Outcome Score (KOOS)—Development of a self-administered outcome measure. J. Orthop. Sports Phys. Ther. 1998, 28, 88–96. [Google Scholar] [CrossRef]
- Roos, E.M.; Toksvig-Larsen, S. Knee injury and Osteoarthritis Outcome Score (KOOS)—Validation and comparison to the WOMAC in total knee replacement. Health Qual. Life Outcomes 2003, 1, 17. [Google Scholar] [CrossRef] [PubMed]
- Monticone, M.; Ferrante, S.; Salvaderi, S.; Rocca, B.; Totti, V.; Foti, C.; Roi, G.S. Development of the Italian version of the knee injury and osteoarthritis outcome score for patients with knee injuries: Cross-cultural adaptation, dimensionality, reliability, and validity. Osteoarthr. Cartil. 2012, 20, 330–335. [Google Scholar] [CrossRef]
- Bellamy, N.; Buchanan, W.W.; Goldsmith, C.H.; Campbell, J.; Stitt, L.W. Validation study of WOMAC: A health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J. Rheumatol. 1988, 15, 1833–1840. [Google Scholar]
- Bellamy, N.; Wilson, C.; Hendrikz, J.; Whitehouse, S.L.; Patel, B.; Dennison, S.; Davis, T. Osteoarthritis Index delivered by mobile phone (m-WOMAC) is valid, reliable, and responsive. J. Clin. Epidemiol. 2011, 64, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Salaffi, F.; Leardini, G.; Canesi, B.; Mannoni, A.; Fioravanti, A.; Caporali, R.; Lapadula, G.; Punzi, L. Reliability and validity of the Western Ontario and McMaster Universities (WOMAC) Osteoarthritis Index in Italian patients with osteoarthritis of the knee. Osteoarthr. Cartil. 2003, 11, 551–560. [Google Scholar] [CrossRef]
- Talbot, S.; Hooper, G.; Stokes, A.; Zordan, R. Use of a new high-activity arthroplasty score to assess function of young patients with total hip or knee arthroplasty. J. Arthroplast. 2010, 25, 268–273. [Google Scholar] [CrossRef]
- Jenny, J.Y.; Louis, P.; Diesinger, Y. High Activity Arthroplasty Score has a lower ceiling effect than standard scores after knee arthroplasty. J. Arthroplast. 2014, 29, 719–721. [Google Scholar] [CrossRef]
- Monticone, M.; Capone, A.; Frigau, L.; Marongiu, G.; Abelli, P.; Mola, F.; Maffulli, N.; Foti, C. Development of the Italian version of the High-Activity Arthroplasty Score (HAAS-I) following hip and knee total arthroplasty: Cross-cultural adaptation, reliability, validity and sensitivity to change. J. Orthop. Surg. Res. 2018, 13, 81. [Google Scholar] [CrossRef]
- Besson, H.; Brage, S.; Jakes, R.W.; Ekelund, U.; Wareham, N.J. Estimating physical activity energy expenditure, sedentary time, and physical activity intensity by self-report in adults. Am. J. Clin. Nutr. 2010, 91, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Golubic, R.; May, A.M.; Benjaminsen Borch, K.; Overvad, K.; Charles, M.A.; Diaz, M.J.; Amiano, P.; Palli, D.; Valanou, E.; Vigl, M.; et al. Validity of electronically administered Recent Physical Activity Questionnaire (RPAQ) in ten European countries. PLoS ONE 2014, 9, e92829. [Google Scholar]
- Goldsmith, C.H.; Boers, M.; Bombardier, C.; Tugwell, P. Criteria for clinically important changes in outcomes: Development, scoring and evaluation of rheumatoid arthritis patient and trial profiles. OMERACT Committee. J. Rheumatol. 1993, 20, 561–565. [Google Scholar] [PubMed]
- Jaeschke, R.; Singer, J.; Guyatt, G.H. Measurement of health status. Ascertaining the minimal clinically important difference. Control. Clin. Trials 1989, 10, 407–415. [Google Scholar] [CrossRef]
- Juniper, E.F.; Guyatt, G.H.; Willan, A.; Griffith, L.E. Determining a minimal important change in a disease-specific Quality of Life Questionnaire. J. Clin. Epidemiol. 1994, 47, 81–87. [Google Scholar] [CrossRef]
- Petis, S.M.; Howard, J.L.; Lanting, B.A.; Marsh, J.D.; Vasarhelyi, E.M. In-Hospital Cost Analysis of Total Hip Arthroplasty: Does Surgical Approach Matter? J. Arthroplast. 2016, 31, 53–58. [Google Scholar] [CrossRef]
- Mizner, R.L.; Petterson, S.C.; Stevens, J.E.; Axe, M.J.; Snyder-Mackler, L. Preoperative quadriceps strength predicts functional ability one year after total knee arthroplasty. J. Rheumatol. 2005, 32, 1533–1539. [Google Scholar]
- Meier, W.; Mizner, R.L.; Marcus, R.L.; Dibble, L.E.; Peters, C.; Lastayo, P.C. Total knee arthroplasty: Muscle impairments, functional limitations, and recommended rehabilitation approaches. J. Orthop. Sports Phys. Ther. 2008, 38, 246–256. [Google Scholar] [CrossRef]
- Suetta, C.; Aagaard, P.; Magnusson, S.P.; Andersen, L.L.; Sipila, S.; Rosted, A.; Jakobsen, A.K.; Duus, B.; Kjaer, M. Muscle size, neuromuscular activation, and rapid force characteristics in elderly men and women: Effects of unilateral long-term disuse due to hip-osteoarthritis. J. Appl. Physiol. 2007, 102, 942–948. [Google Scholar] [CrossRef] [PubMed]
- Skoffer, B.; Dalgas, U.; Mechlenburg, I. Progressive resistance training before and after total hip and knee arthroplasty: A systematic review. Clin. Rehabil. 2015, 29, 14–29. [Google Scholar] [CrossRef]
- Pozzi, F.; Snyder-Mackler, L.; Zeni, J. Physical exercise after knee arthroplasty: A systematic review of controlled trials. Eur. J. Phys. Rehabil. Med. 2013, 49, 877–892. [Google Scholar] [PubMed]
- Artz, N.; Elvers, K.T.; Lowe, C.M.; Sackley, C.; Jepson, P.; Beswick, A.D. Effectiveness of physiotherapy exercise following total knee replacement: Systematic review and meta-analysis. BMC Musculoskelet. Disord. 2015, 16, 15. [Google Scholar] [CrossRef] [PubMed]
- Vogel, L.A.; Carotenuto, G.; Basti, J.J.; Levine, W.N. Physical activity after total joint arthroplasty. Sports Health 2011, 3, 441–450. [Google Scholar] [CrossRef]
- Fawaz, W.S.; Masri, B.A. Allowed Activities After Primary Total Knee Arthroplasty and Total Hip Arthroplasty. Orthop. Clin. N. Am. 2020, 51, 441–452. [Google Scholar] [CrossRef]
- Hammett, T.; Simonian, A.; Austin, M.; Butler, R.; Allen, K.D.; Ledbetter, L.; Goode, A.P. Changes in Physical Activity After Total Hip or Knee Arthroplasty: A Systematic Review and Meta-Analysis of Six- and Twelve-Month Outcomes. Arthritis. Care Res. 2018, 70, 892–901. [Google Scholar] [CrossRef]
- Roos, E.M.; Barton, C.J.; Davis, A.M.; McGlasson, R.; Kemp, J.L.; Crossley, K.M.; Liu, Q.; Lin, J.; Skou, S.T. GLA:D to have a high-value option for patients with knee and hip arthritis across four continents: Good Life with osteoArthritis from Denmark. Br. J. Sports Med. 2018, 52, 1544–1545. [Google Scholar] [CrossRef]
- Singh, J.A.; Lewallen, D.G. Predictors of activity limitation and dependence on walking aids after primary total hip arthroplasty. J. Am. Geriatr. Soc. 2010, 58, 2387–2393. [Google Scholar] [CrossRef]
- Singh, J.A.; Lewallen, D. Age, gender, obesity, and depression are associated with patient-related pain and function outcome after revision total hip arthroplasty. Clin. Rheumatol. 2009, 28, 1419–1430. [Google Scholar] [CrossRef]
- Maniar, R.N.; Maniar, P.R.; Singhi, T.; Gangaraju, B.K. WHO Class of Obesity Influences Functional Recovery Post-TKA. Clin. Orthop. Surg. 2018, 10, 26–32. [Google Scholar] [CrossRef]
- Pua, Y.H.; Seah, F.J.; Seet, F.J.; Tan, J.W.; Liaw, J.S.; Chong, H.C. Sex Differences and Impact of Body Mass Index on the Time Course of Knee Range of Motion, Knee Strength, and Gait Speed After Total Knee Arthroplasty. Arthritis. Care Res. 2015, 67, 1397–1405. [Google Scholar] [CrossRef] [PubMed]
- Shadyab, A.H.; Li, W.; Eaton, C.B.; LaCroix, A.Z. General and Abdominal Obesity as Risk Factors for Late-Life Mobility Limitation After Total Knee or Hip Replacement for Osteoarthritis Among Women. Arthritis. Care Res. 2018, 70, 1030–1038. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Torres, S.; Fahey, T.D.; Rivera, M.A. Adherence to Exercise Programs in Older Adults: Informative Report. Gerontol Geriatr. Med. 2019, 5, 2333721418823604. [Google Scholar] [CrossRef] [PubMed]
- Picorelli, A.M.; Pereira, L.S.; Pereira, D.S.; Felicio, D.; Sherrington, C. Adherence to exercise programs for older people is influenced by program characteristics and personal factors: A systematic review. J. Physiother. 2014, 60, 151–156. [Google Scholar] [CrossRef]
- Courneya, K.S.; Karvinen, K.H.; McNeely, M.L.; Campbell, K.L.; Brar, S.; Woolcott, C.G.; McTiernan, A.; Ballard-Barbash, R.; Friedenreich, C.M. Predictors of adherence to supervised and unsupervised exercise in the Alberta Physical Activity and Breast Cancer Prevention Trial. J. Phys. Act. Health 2012, 9, 857–866. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, A.; Kressig, R.W.; Muehlbauer, T.; Gschwind, Y.J.; Pfenninger, B.; Bruegger, O.; Granacher, U. Effects of a Supervised versus an Unsupervised Combined Balance and Strength Training Program on Balance and Muscle Power in Healthy Older Adults: A Randomized Controlled Trial. Gerontology 2016, 62, 275–288. [Google Scholar] [CrossRef] [PubMed]
- Pavey, T.; Taylor, A.; Hillsdon, M.; Fox, K.; Campbell, J.; Foster, C.; Moxham, T.; Mutrie, N.; Searle, J.; Taylor, R. Levels and predictors of exercise referral scheme uptake and adherence: A systematic review. J. Epidemiol. Community Health 2012, 66, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, G.H.; Feeny, D.H.; Patrick, D.L. Measuring health-related quality of life. Ann. Intern. Med. 1993, 118, 622–629. [Google Scholar] [CrossRef]
- Keurentjes, J.C.; Van Tol, F.R.; Fiocco, M.; Schoones, J.W.; Nelissen, R.G. Minimal clinically important differences in health-related quality of life after total hip or knee replacement: A systematic review. Bone Joint Res. 2012, 1, 71–77. [Google Scholar] [CrossRef]
- Lim, J.B.; Chou, A.C.; Yeo, W.; Lo, N.N.; Chia, S.L.; Chin, P.L.; Tay, D.K.; Yeo, S.J. Comparison of patient quality of life scores and satisfaction after common orthopedic surgical interventions. Eur. J. Orthop. Surg. Traumatol. 2015, 25, 1007–1012. [Google Scholar] [CrossRef] [PubMed]
- Dowd, K.P.; Szeklicki, R.; Minetto, M.A.; Murphy, M.H.; Polito, A.; Ghigo, E.; van der Ploeg, H.; Ekelund, U.; Maciaszek, J.; Stemplewski, R.; et al. A systematic literature review of reviews on techniques for physical activity measurement in adults: A DEDIPAC study. Int. J. Behav. Nutr. Phys. Act. 2018, 15, 15. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.A.; Matthews, C.E.; Ebbeling, C.B.; Moore, C.G.; Cunningham, J.E.; Fulton, J.; Hebert, J.R. The effect of social desirability and social approval on self-reports of physical activity. Am. J. Epidemiol. 2005, 161, 389–398. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).