The Impact Assessment of CuO Nanoparticles on the Composition and Ultrastructure of Triticum aestivum L.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Plant Extract and Nanoparticle Preparation
2.2.1. Extract Preparation
2.2.2. Nanoparticle Synthesis
2.2.3. Characterization of NPs
2.3. Plant Growth Conditions
2.4. Analysis of Plant Tissues and Soil after Harvesting
2.4.1. Extraction and Characterization of Assimilating Pigments
2.4.2. Extraction and Spectrophotometric Analysis of Total Polyphenols
2.4.3. Determination of Antioxidant Capacity
2.4.4. TEM Analysis of Wheat Tissue
2.4.5. Elemental Content of the Wheat Biomass and Soil Substrate
2.5. Data Analysis
- Mobility ratio or transfer factor that expresses the ratio of element concentration in wheat to its concentration in soil; MR = Cwheat/Csoil.
- Enrichment factor (EFsoil), relative abundance of a chemical element in a soil compared to the relative abundance with respect to control sample; EFsoil = (C/Al)soil/(C/Al)soil control.
- Enrichment factor of plant (EFwheat), calculated as EFwheat = (C/Al)wheat/(C/Al)control wheat.
3. Results and Discussion
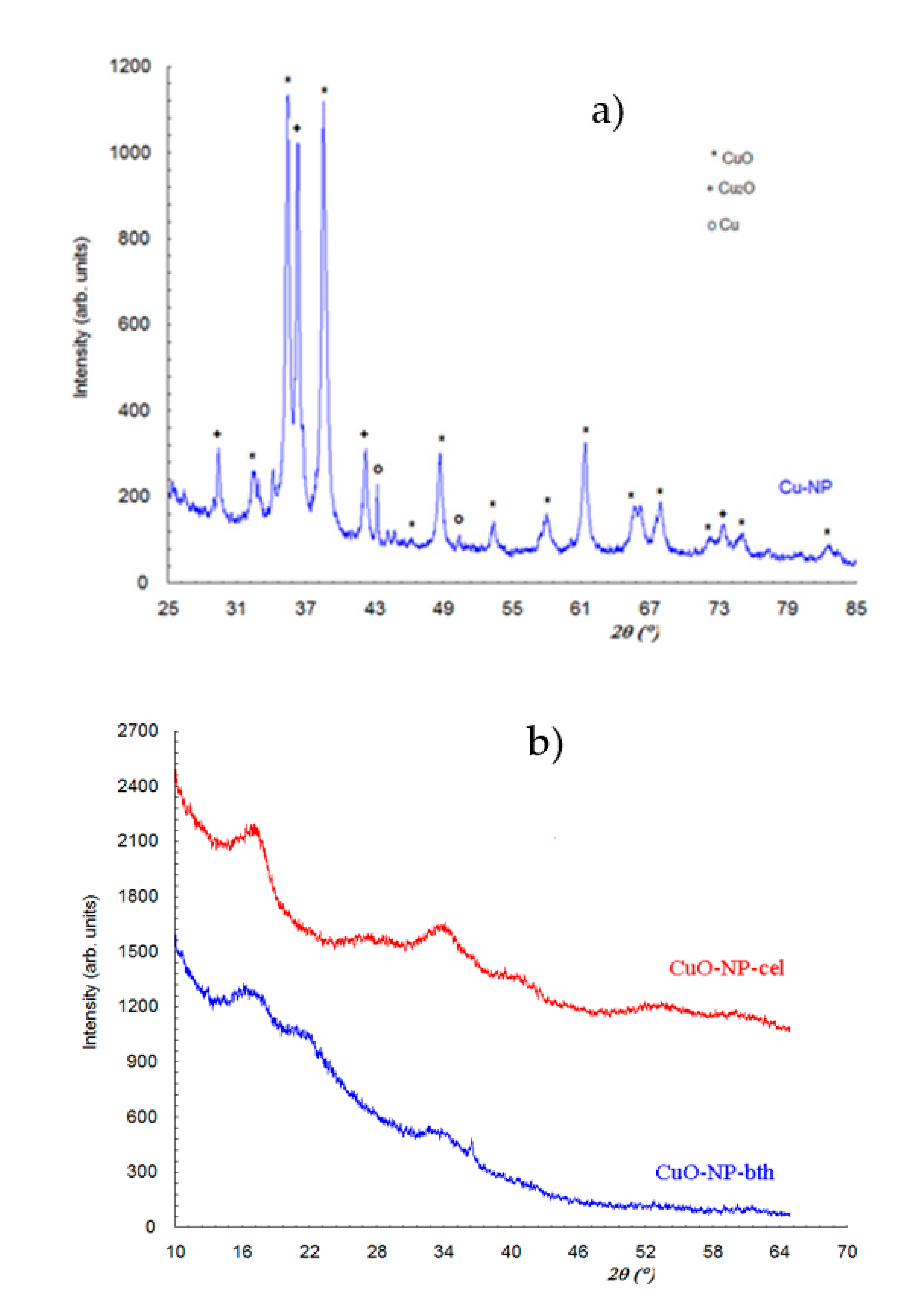
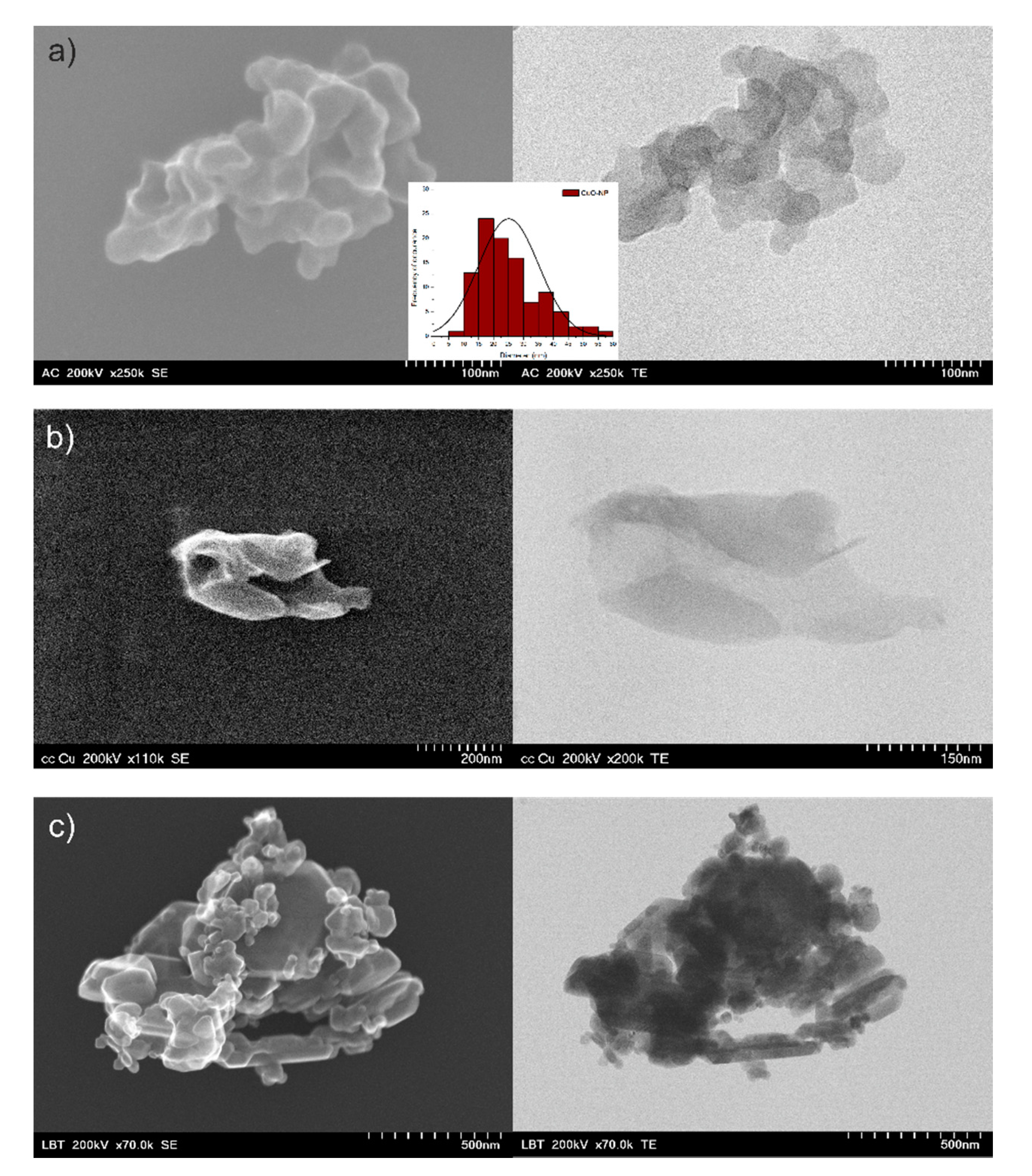
3.1. NP Characterization
3.1.1. XRD Analysis
3.1.2. Morphological Analysis
3.1.3. FTIR Analysis
3.2. Analysis of Plant Tissues
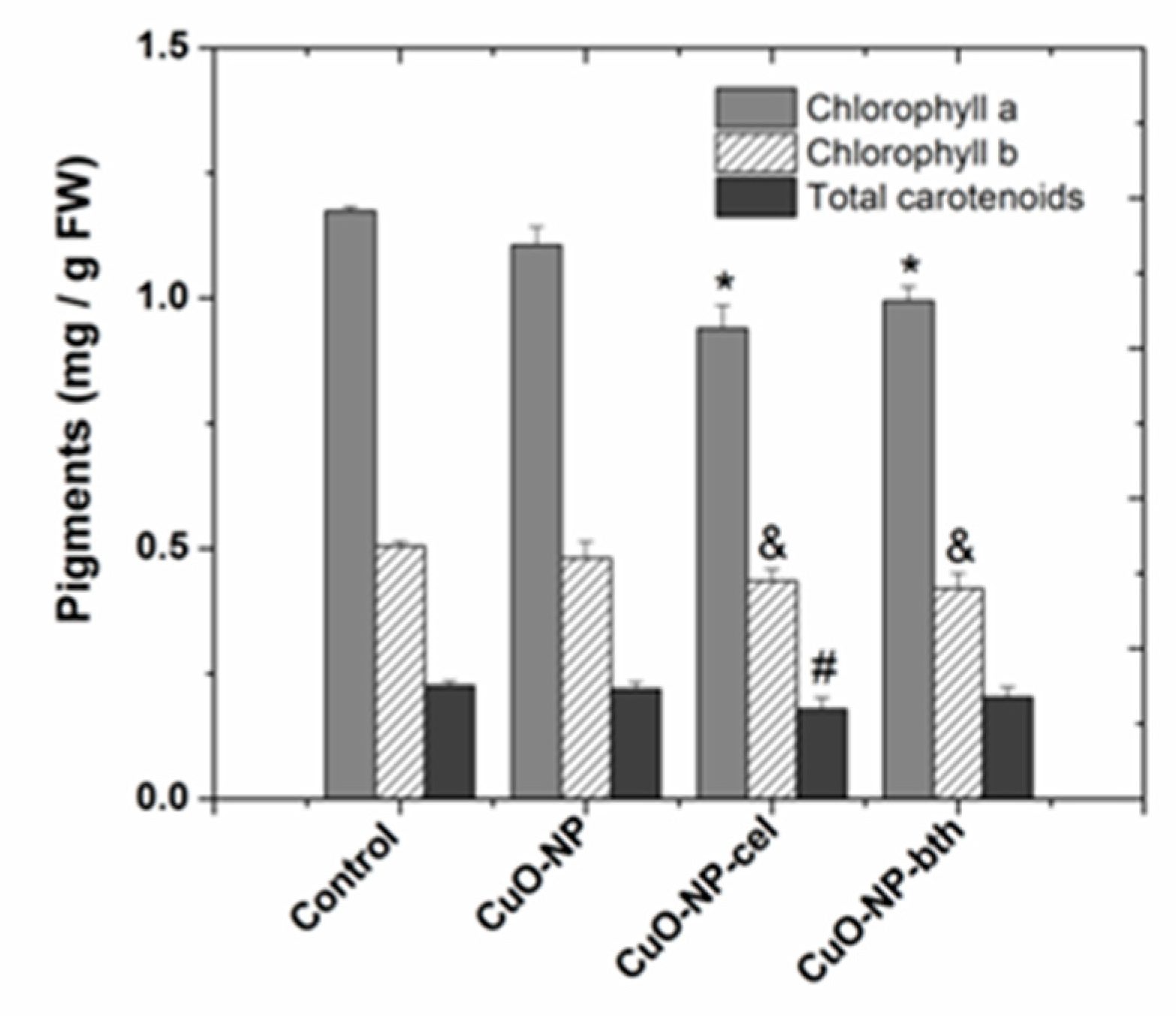
3.2.1. Characterization of the Extracts of Assimilating Pigments
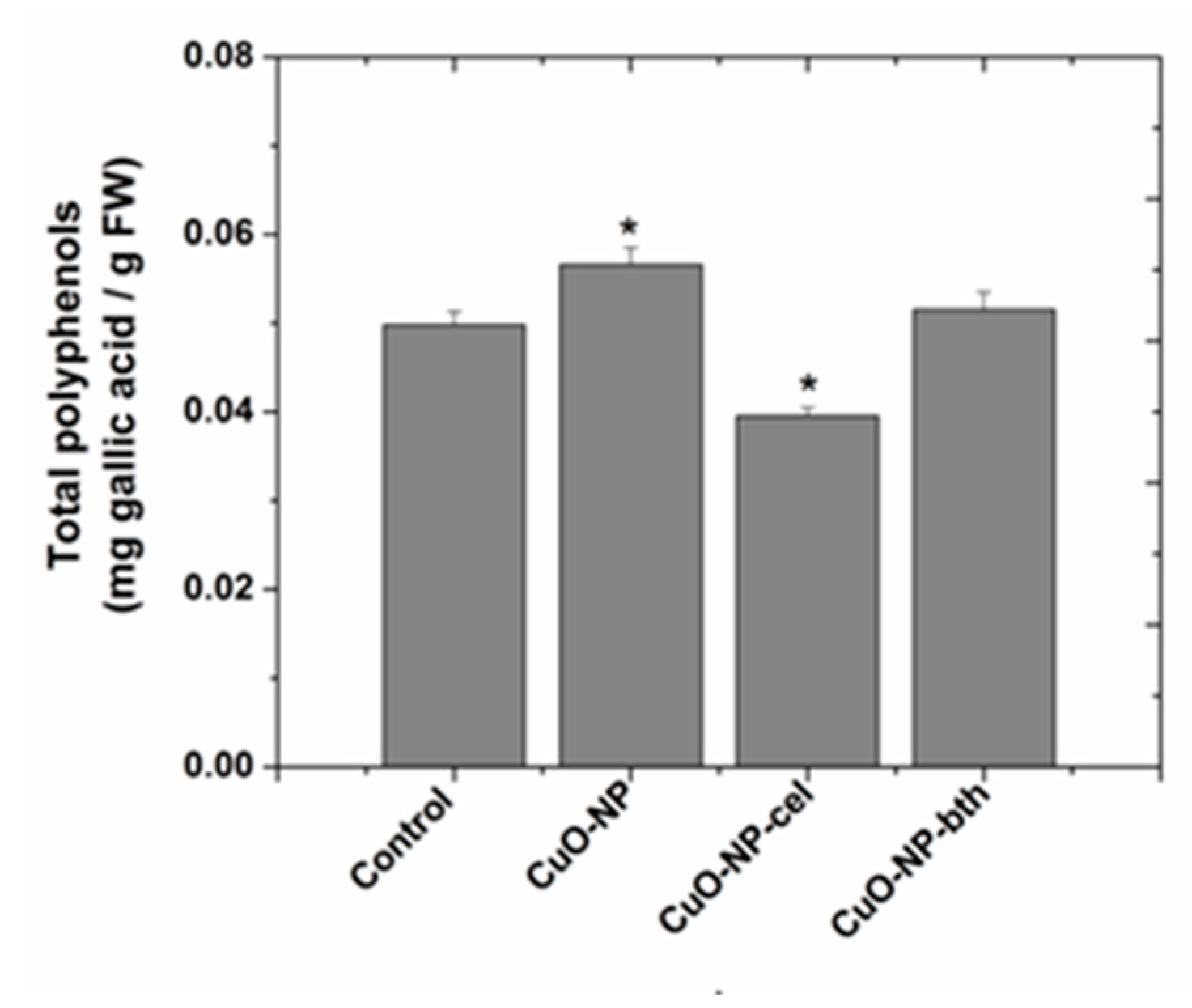
3.2.2. Determination of Total Phenolic Content
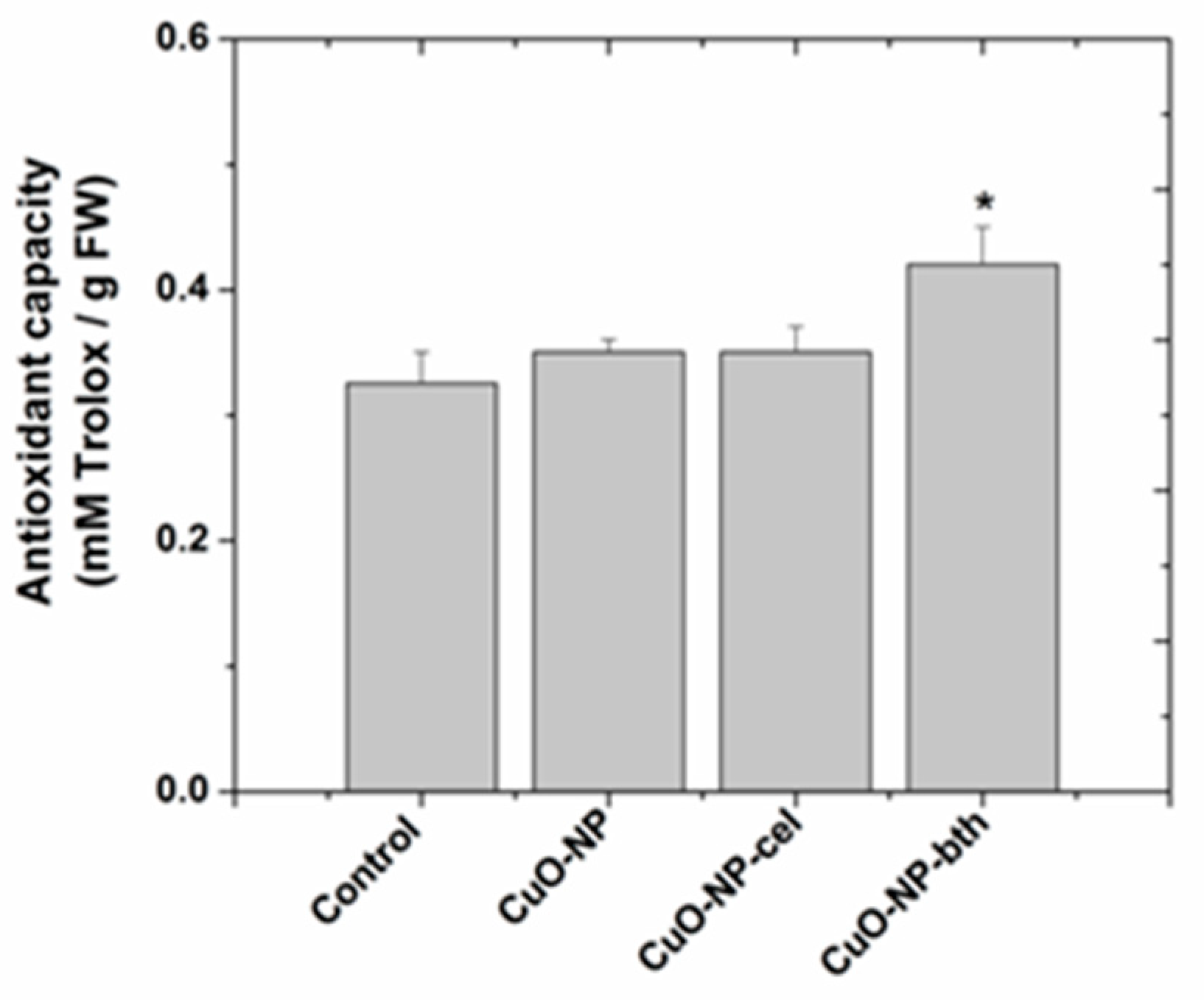
3.2.3. Determination of Antioxidant Capacity
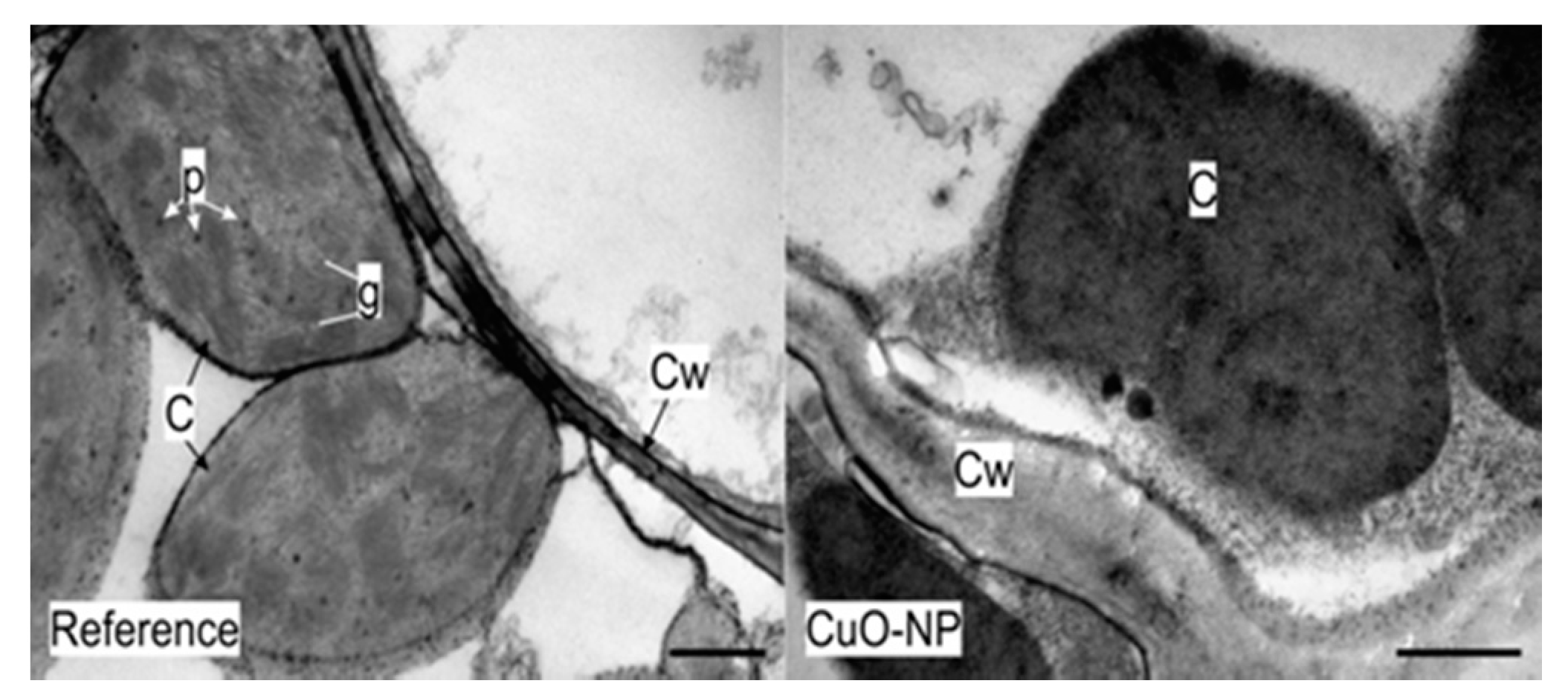
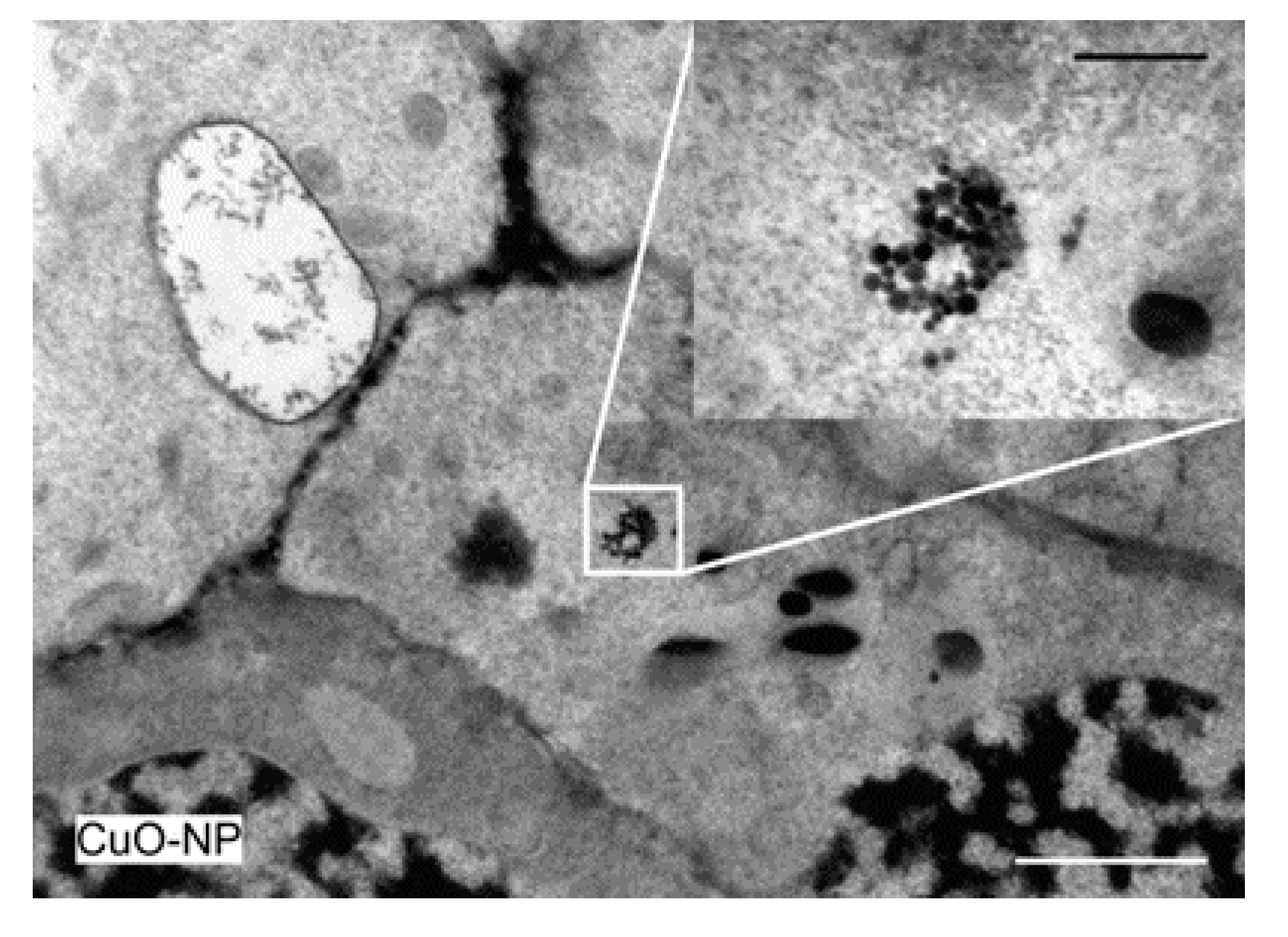
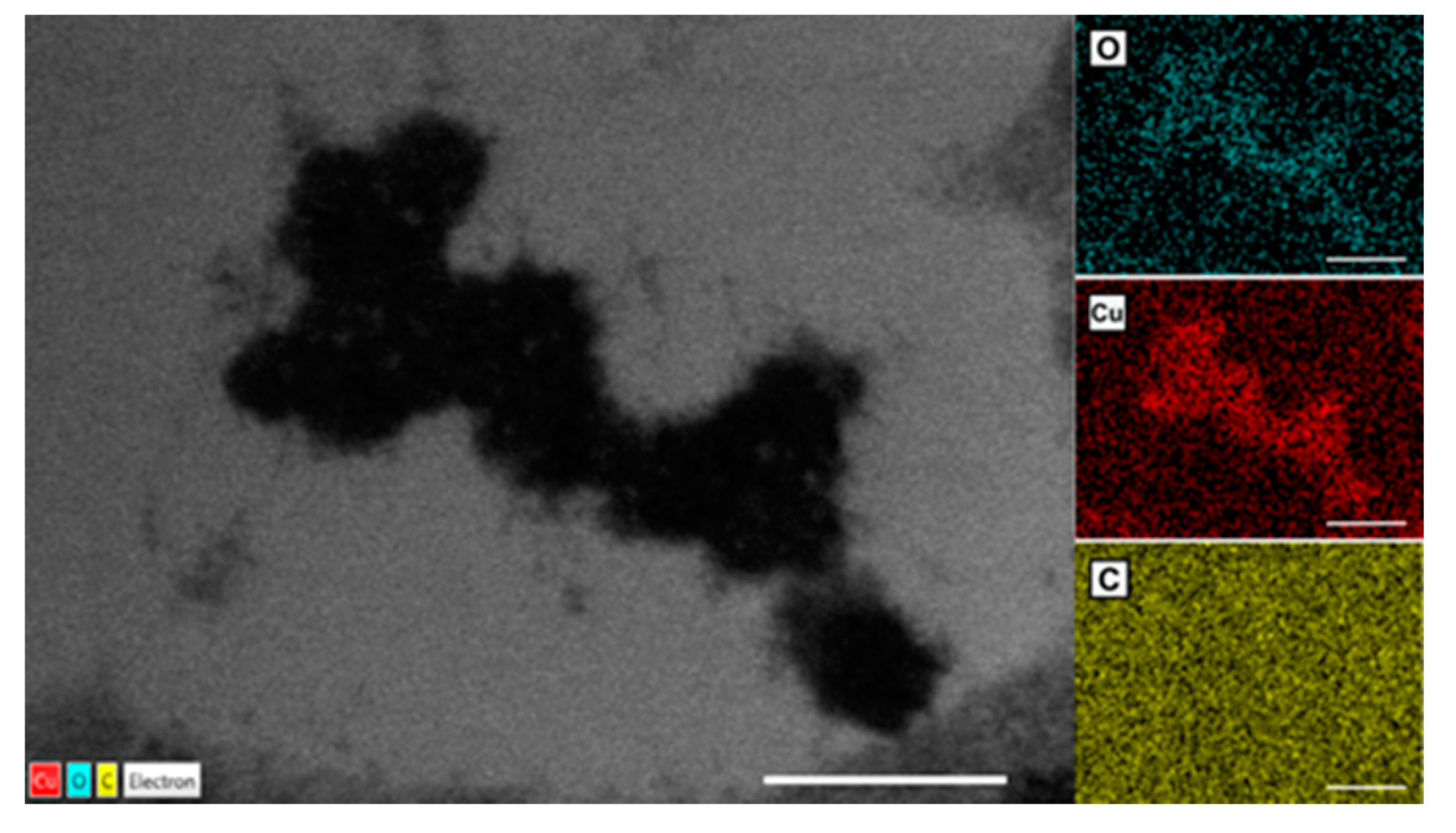
3.2.4. TEM Analysis of Wheat Tissue
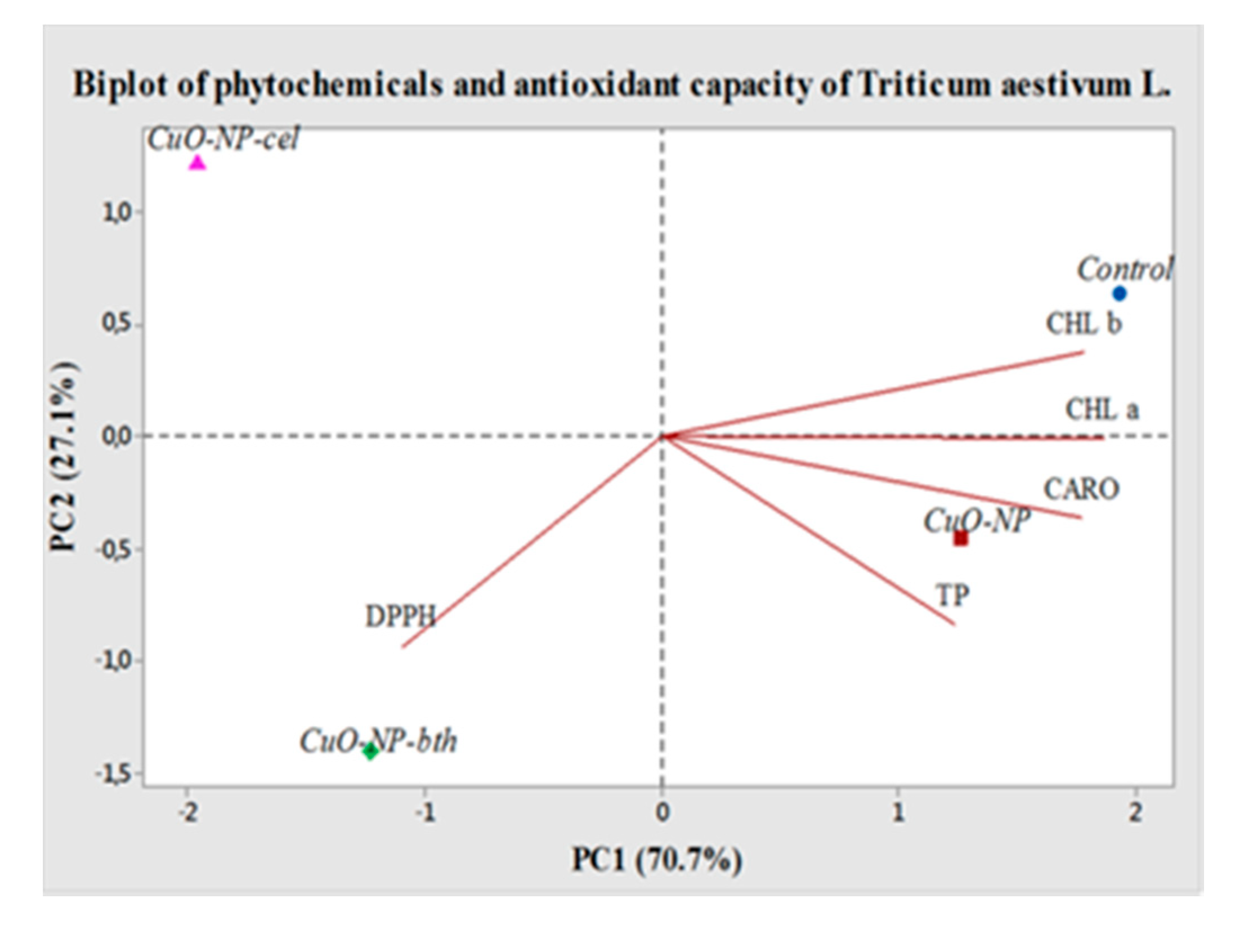
3.2.5. PCA Analysis
3.3. Soil and Plant Element Content
3.3.1. Soil Content and Enrichment Factor in Soil
3.3.2. Element Content and Enrichment Factor in Wheat
3.3.3. Soil to Plant Transfer
3.4. Correlation between the Element Content and Phenolic Compound Concentration, Assimilating Pigments Concentration, and Antioxidant Capacity in Plants
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Milewska-Hendel, A.; Gawecki, R.; Zubko, M.; Stróż, D.; Kurczyńska, E. Diverse influence of nanoparticles on plant growth with a particular emphasis on crop plants. Acta Agrobot. 2016, 69, 1694. [Google Scholar] [CrossRef] [Green Version]
- Rizwan, M.; Ali, S.; Qayyum, M.F.; Ok, Y.S.; Adrees, M.; Ibrahim, M.; Zia-ur-Rehman, M.; Farid, M.; Abbas, F. Effect of metal and metal oxide nanoparticles on growth and physiology of globally important food crops: A critical review. J. Hazard. Mater. 2017, 322, 2–16. [Google Scholar] [CrossRef]
- Safiuddin, M.; Gonzalez, M.; Cao, J.W.; Tighe, S.L. State of-the-art report on use of nanomaterials in concrete. Int. J. Pavement Eng. 2014, 15, 940–949. [Google Scholar] [CrossRef]
- Mohanty, S.R.; Rajput, P.; Kollah, B.; Chourasiya, D.; Tiwari, A.; Singh, M.; Rao, A.S. Methane oxidation and abundance of methane oxidizers in tropical agricultural soil (vertisol) in response to CuO and ZnO nanoparticles contamination. Environ. Monit. Assess. 2014, 186, 3743–3753. [Google Scholar] [CrossRef]
- Du, W.; Tan, W.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L.; Ji, R.; Yin, Y.; Guo, H. Interaction of metal oxide nanoparticles with higher terrestrial plants: Physiological and biochemical aspects. Plant Physiol. Biochem. 2017, 110, 210–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rastogi, A.; Zivcak, M.; Sytar, O.; Kalaji, H.M.; He, X.; Mbarki, S.; Brestic, M. Impact of metal and metal oxide nanoparticles on plant: A critical review. Front. Chem. 2017, 5, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Din, M.I.; Rehan, R. Synthesis, characterization, and applications of copper nanoparticles. Anal. Lett. 2017, 50, 50–62. [Google Scholar] [CrossRef]
- Bandyopadhyay, S.; Plascencia-Villa, G.; Mukherjee, A.; Rico, C.M.; José-Yacamán, M.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Comparative phytotoxicity of ZnO NPs, bulk ZnO, and ionic zinc onto the alfalfa plants symbiotically associated with Sinorhizobium meliloti in soil. Sci. Total Environ. 2015, 515, 60–69. [Google Scholar] [CrossRef]
- Cui, D.; Zhang, P.; Ma, Y.; He, X.; Li, Y.; Zhang, J.; Zhao, Y.; Zhang, Z. Effect of cerium oxide nanoparticles on asparagus lettuce cultured in an agar medium. Environ. Sci. Nano 2014, 1, 459–465. [Google Scholar] [CrossRef]
- Hong, J.; Wang, L.; Sun, Y.; Zhao, L.; Niu, G.; Tan, W.; Rico, C.M.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Foliar applied nanoscale and microscale CeO2 and CuO alter cucumber (Cucumis sativus) fruit quality. Sci. Total Environ. 2016, 563–564, 904–911. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.; Geiser-Lee, J.; Deng, Y.; Kolmakov, A. Interactions between engineered nanoparticles (ENPs) and plants: Phytotoxicity, uptake and accumulation. Sci. Total Environ. 2010, 408, 3053–3061. [Google Scholar] [CrossRef]
- Yang, J.; Cao, W.; Rui, Y. Interactions between nanoparticles and plants: Phytotoxicity and defense mechanisms. J. Plant Interact. 2017, 12, 158–169. [Google Scholar] [CrossRef]
- Monica, R.C.; Cremonini, R. Nanoparticles and higher plants. Caryologia 2009, 62, 161–165. [Google Scholar] [CrossRef] [Green Version]
- Rajput, V.D.; Minkina, T.; Suskova, S.; Mandzhieva, S.; Tsitsuashvili, V.; Chapligin, V.; Fedorenko, A. Effects of copper nanoparticles (CuO NPs) on crop plants: A Mini Review. BioNanoScience 2018, 8, 36–42. [Google Scholar] [CrossRef]
- Rico, C.M.; Majumdar, S.; Duarte-Gardea, M.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Interaction of nanoparticles with edible plants and their possible implications in the food chain. J. Agric. Food Chem. 2011, 59, 3485–3498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Z.J.; Wang, H.; Yan, B.; Zheng, H.; Jiang, Y.; Miranda, O.R.; Rotello, V.M.; Xing, B.; Vachet, R.W. Effect of surface charge on the uptake and distribution of gold nanoparticles in four plant species. Environ. Sci. Technol. 2012, 46, 12391–12398. [Google Scholar] [CrossRef] [PubMed]
- Wanga, Y.; Jianga, F.; Mab, C.; Ruia, Y.; Tsang, D.C.W.; Xing, B. Effect of metal oxide nanoparticles on amino acids in wheat grains (Triticum aestivum) in a life cycle study. J. Environ. Manag. 2019, 241, 319–327. [Google Scholar] [CrossRef]
- Adams, J.; Wright, M.; Wagner, H.; Valiente, J.; Britt, D.; Anderson, A. Cu from dissolution of CuO nanoparticles signals changes in root morphology. Plant Physiol. Biochem. 2017, 110, 108–117. [Google Scholar] [CrossRef] [Green Version]
- Zhou, D.; Jin, S.; Li, L.; Wang, Y.; Weng, N. Quantifying the adsorption and uptake of CuO nanoparticles by wheat root based on chemical extractions. J. Environ. Sci. 2011, 23, 1852–1857. [Google Scholar] [CrossRef]
- Spielman-Sun, E.; Lombi, E.; Donner, E.; Avellan, A.; Etschmann, B.; Howard, D.; Lowry, G.V. Temporal evolution of copper distribution and speciation in roots of Triticum aestivum exposed to CuO, Cu(OH)2, and CuS nanoparticles. Environ. Sci. Technol. 2018, 52, 9777–9784. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.K.; Hossain, Z. Impact of nano-CuO stress on rice (Oryza sativa L.) seedlings. Chemosphere 2013, 93, 906–915. [Google Scholar] [CrossRef]
- Singh, C.; Kumar, J.; Kumar, P.; Chauhan, B.S.; Tiwari, K.N.; Mishra, S.K.; Srikrishna, S.; Saini, R.; Nath, G.; Singh, J. Green synthesis of silver nanoparticles using aqueous leaf extract of Premna integrifolia (L.) rich in polyphenols and evaluation of their antioxidant, antibacterial and cytotoxic activity. Biotechnol. Biotechnol. Equip. 2019, 33, 359–371. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Ma, X.L.; Gu, Y.; Huang, H.; Zhang, G.W. Green synthesis of metallic nanoparticles and their potential applications to treat cancer. Front. Chem. 2020, 8, 799. [Google Scholar] [CrossRef]
- Jadoun, S.; Arif, R.; Jangid, N.K.; Meena, R.K. Green synthesis of nanoparticles using plant extracts: A review. Environ. Chem. Lett. 2021, 19, 355–374. [Google Scholar] [CrossRef]
- Tripathi, D.K.; Singh, V.P.; Prasad, S.M.; Chauhan, D.K.; Dubey, N.K. Silicon nanoparticles (SiNp) alleviate chromium (VI) phytotoxicity in Pisum sativum (L.) seedlings. Plant Physiol. Biochem. 2015, 96, 189–198. [Google Scholar] [CrossRef]
- Tripathi, D.K.; Singh, S.; Singh, V.P.; Prasad, S.M.; Chauhan, D.K.; Dubey, N.K. Silicon nanoparticles more efficiently alleviate arsenate toxicity than silicon in maize cultiver and hybrid differing in arsenate tolerance. Front. Environ. Sci. 2016, 4, 46. [Google Scholar] [CrossRef] [Green Version]
- Hayes, K.L.; Mui, J.; Song, B.; Sani, E.S.; Eisenman, S.W.; Sheffield, J.B.; Kim, B. Effects, uptake, and translocation of aluminum oxide nanoparticles in lettuce: A comparison study to phytotoxic aluminum ions. Sci. Total Environ. 2020, 719, 137393. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Wu, X.; Wu, F.; Chen, W.; Zhang, X.; White, J.C.; Li, J.; Wan, Y.; Liu, J.; Wang, X. TiO2 nanoparticle exposure on lettuce (Lactuca sativa L.): Dose-dependent deterioration of nutritional quality. Environ. Sci. Nano 2020, 7, 501–513. [Google Scholar] [CrossRef]
- Crops. Available online: http://www.fao.org/faostat/en/#data/QC/vizualize (accessed on 15 November 2020).
- Khodabande, Z.; Jafarian, V.; Sariri, R. Antioxidant activity of Chelidonium majus extract at phenological stages. Appl. Biol. Chem. 2017, 60, 497–503. [Google Scholar] [CrossRef]
- Pinacho, R.; Cavero, R.Y.; Astiasarán, I.; Ansorena, D.; Calvo, M.I. Phenolic compounds of blackthorn (Prunus spinosa L.) and influence of in vitro digestion on their antioxidant capacity. J. Funct. Foods 2015, 19, 49–62. [Google Scholar] [CrossRef]
- Parvu, M.; Vlase, L.; Fodor-Pataki, L.; Parvu, O.; Rosca-Casian, O.; Bartha, C.; Barbu-Tudoran, L.; Parvu, A.E. Chemical composition of celandine (Chelidonium majus L.) extract and its effects on Botrytis tulipae (Lib.) lind fungus and the tulip. Not. Bot. Horti Agrobot. Cluj Napoca 2013, 41, 414–426. [Google Scholar] [CrossRef] [Green Version]
- Kathad, U.; Gajera, H.P. Synthesis of copper nanoparticles by two different methods and size comparision. Int. J. Pharma Bio Sci. 2014, 5, 533–540. [Google Scholar]
- Kopecká, I.; Svobodová, E. Methodology for infrared spectroscopy analysis of sandwich multilayer samples of historical materials. Herit. Sci. 2014, 2, 22. [Google Scholar] [CrossRef] [Green Version]
- Opriș, O.; Lung, I.; Soran, M.L.; Ciorîță, A.; Copolovici, L. Investigating the effects of non-steroidal anti-inflammatory drugs (NSAIDs) on the composition and ultrastructure of green leafy vegetables with important nutritional values. Plant Phys. Biochem. 2020, 151, 342–351. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Current Protocols in Food Analytical Chemistry (Units: F4.3.1–F4.3.8); John Wiley & Sons Inc.: New York, NY, USA, 2001. [Google Scholar]
- Ivanova, V.; Stefova, M.; Chinnici, F. Determination of the polyphenol contents in Macedonian grapes and wines by standardized spectrophotometric methods. J. Serb. Chem. Soc. 2010, 75, 45–59. [Google Scholar] [CrossRef]
- Bancuta, O.R.; Chilian, A.; Bancuta, I.; Ion, R.M.; Setnescu, R.; Setnescu, T.; Gheboianu, A.; Lungulescu, M. Ft-Ir and Uv-Vis Characterization of Grape Extracts Used as Antioxidants in Polymers. Rev. Roum. Chim. 2015, 60, 571–577. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Ciorîță, A.; Tripon, S.C.; Mircea, I.G.; Podar, D.; Barbu-Tudoran, L.; Mircea, C.; Pârvu, M. The Morphological and Anatomical Traits of the Leaf in Representative Vinca Species Observed on Indoor- and Outdoor-Grown Plants. Plants 2021, 10, 622. [Google Scholar] [CrossRef]
- Frontasyeva, M.V. Neutron activation analysis in the life sciences. A review. Phys. Part. Nuclei 2011, 42, 332–378. [Google Scholar] [CrossRef]
- Culicov, O.A.; Yurukova, L.; Duliu, O.G.; Zinicovscaia, I. Elemental content of mosses and lichens from Livingston Island (Antarctica) as determined by instrumental neutron activation analysis (INAA). Environ. Sci. Pollut. Res. 2017, 24, 5717–5732. [Google Scholar] [CrossRef] [PubMed]
- Pavlov, S.S.; Dmitriev, A.Y.; Frontasyeva, M.V. Automation system for neutron activation analysis at the reactor IBR-2, Frank Laboratory of neutron physics, Joint Institute for Nuclear Research, Dubna, Russia. J. Radioanal. Nucl. Chem. 2016, 309, 27–38. [Google Scholar] [CrossRef] [Green Version]
- Bargagli, R.; Brown, D.H.; Nelli, L. Metal biomonitoring with mosses: Procedures for correcting for soil contamination. Environ. Pollut. 1995, 89, 169–175. [Google Scholar] [CrossRef]
- Mingorance, M.D.; Valdés, B.; Olivioa, S.R. Strategies of heavy metal uptake by plants growing under industrial emissions. Environ. Int. 2007, 33, 514–520. [Google Scholar] [CrossRef]
- Kohatsu, M.Y.; Pelegrino, M.T.; Monteiro, L.R.; Freire, B.M.; Pereira, R.M.; Fincheira, P.; Rubilar, O.; Tortella, G.; Batista, B.L.; de Jesus, T.A.; et al. Comparison of foliar spray and soil irrigation of biogenic CuO nanoparticles (NPs) on elemental uptake and accumulation in lettuce. Environ. Sci. Pollut. Res. 2021. [Google Scholar] [CrossRef]
- Dey, A.; Manna, S.; Chattopadhyay, S.; Mondal, D.; Chattopadhyay, D.; Raj, A.; Das, S.; Bag, B.G.; Roy, S. Azadirachta indica leaves mediated green synthesized copper oxide nanoparticles induce apoptosis through activation of TNF-α and caspases signaling pathway against cancer cells. J. Saudi Chem. Soc. 2019, 23, 222–238. [Google Scholar] [CrossRef]
- Ankamwar, B. Biosynthesis of gold nanoparticles (green-gold) using leaf extract of Terminalia catappa. J. Chem. 2010, 7, 1334–1339. [Google Scholar] [CrossRef] [Green Version]
- Kumar, R.; Ghoshal, G.; Jain, A.; Goyal, M. Rapid green synthesis of silver nanoparticles (AgNPs) using (Prunus persica) plants extract: Exploring its antimicrobial and catalytic activities. J. Nanomed. Nanotechnol. 2017, 8, 1000452. [Google Scholar] [CrossRef] [Green Version]
- Rengasamy, K.R.R.; Arumugam, R.; Anantharaman, P. Fourier transform infrared spectroscopy analysis of seagrass polyphenols. Curr. Bioact. Compd. 2011, 7, 118–125. [Google Scholar] [CrossRef]
- Azam, A.; Ahmed, A.S.; Oves, M.; Khan, M.S.; Memic, A. Size-dependent antimicrobial properties of CuO nanoparticles against Gram-positive and -negative bacterial strains. Int. J. Nanomed. 2012, 7, 3527–3535. [Google Scholar] [CrossRef] [Green Version]
- Tahir, K.; Nazir, S.; Li, B.; Khan, A.U.; Khan, Z.U.H.; Gong, P.Y.; Khan, S.U.; Ahmad, A. Nerium oleander leaves extract mediated synthesis of gold nanoparticles and its antioxidant activity. Mater. Lett. 2015, 156, 198–201. [Google Scholar] [CrossRef]
- Khan, M.A.; Ullah, M.; Iqbal, T.; Mahmood, H.; Khan, A.A.; Shafique, M.; Majid, A.; Ahmed, A.; Khan, N.A. Surfactant assisted synthesis of cuprous oxide (Cu2O) nanoparticles via solvothermal process. Nanosci. Nanotechnol. Res. 2015, 3, 16–22. [Google Scholar] [CrossRef]
- Sahai, A.; Goswami, N.; Kaushik, S.D.; Tripathi, S. Cu/Cu2O/CuO nanoparticles: Novel synthesis by exploding wire technique and extensive characterization. Appl. Surf. Sci. 2016, 390, 974–983. [Google Scholar] [CrossRef]
- Armstrong, G.A.; Heast, J.E. Carotenoids 2: Genetics and molecular biology of carotenoid pigment biosynthesis. FASEB J. 1996, 10, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Katsube, T.; Tabata, H.; Ohta, Y.; Yamasaki, Y.; Anuurad, E.; Shiwaku, K.; Yamane, Y. Screening for antioxidant activity in edible plant products: Comparison of low-density lipoprotein oxidation assay, DPPH radical scavenging assay, and Folin-Ciocalteu assay. J. Agric. Food Chem. 2004, 52, 2391–2396. [Google Scholar] [CrossRef]
- Da Costa, M.V.J.; Sharma, P.K. Effect of copper oxide nanoparticles on growth, morphology, photosynthesis, and antioxidant response in Oryza sativa. Photosynthetica 2016, 54, 110–119. [Google Scholar] [CrossRef]
- Shi, J.; Peng, C.; Yang, Y.; Yang, J.; Zhang, H.; Yuan, X.; Chen, Y.; Hu, T. Phytoxicity and accumulation of copper oxide nanoparticles to the Cu tolerant plant Elshotlzia splendens. Nanotoxicology 2014, 8, 179–188. [Google Scholar] [CrossRef]
- Atha, D.H.; Wang, H.; Petersen, E.J.; Cleveland, D.; Holbrook, R.D.; Jaruga, P.; Dizdaroglu, M.; Xing, B.; Nelson, B.C. Copper oxide nanoparticle mediated DNA damage in terrestrial plant models. Environ. Sci. Technol. 2011, 46, 1819–1827. [Google Scholar] [CrossRef]
- Honga, J.; Rico, C.M.; Zhao, L.; Adeleye, A.S.; Keller, A.A.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Toxic effects of copper-based nanoparticles or compounds to lettuce (Lactuca sativa) and alfalfa (Medicago sativa). Environ. Sci. Process Impacts 2015, 17, 177–185. [Google Scholar] [CrossRef] [Green Version]
- Xiong, T.T.; Dumat, C.; Dappe, V.; Vezin, H.; Schreck, E.; Shahid, M.; Pierart, A.; Sobanska, S. Copper oxide nanoparticle foliar uptake. Phytotoxicity, and consequences for sustainable urban agriculture. Environ. Sci. Technol. 2017, 51, 5242–5251. [Google Scholar] [CrossRef]
- Nekrasova, G.F.; Ushakova, O.S.; Ermakov, A.E.; Uimin, M.A.; Byzov, I.V. Effects of copper(II) ions and copper oxide nanoparticles on Elodea densa planch. Russ. J. Ecol. 2011, 42, 458–463. [Google Scholar] [CrossRef]
- Hafeez, A.; Razzaq, A.; Mahmood, T.; Jhanzab, H.M. Potential of copper nanoparticles to increase growth and yield of wheat. J. Nanosci. Adv. Technol. 2015, 1, 6–11. [Google Scholar] [CrossRef]
- Dimkpa, C.O.; McLean, J.E.; Latta, D.E.; Manangon, E.; Britt, D.W.; Johnson, W.P.; Boyanov, M.I.; Anderson, A.J. CuO and ZnO nanoparticles: Phytotoxicity, metal speciation, and induction of oxidative stress in sand-grown wheat. J. Nanopart. Res. 2012, 14, 1125. [Google Scholar] [CrossRef]
- Lee, W.M.; An, Y.J.; Yoon, H.; Kweon, H.S. Toxicity and bioavailability of copper nanoparticles to the terrestrial plants mung bean (Phaseolus radiatus) and wheat (Triticum aestivum): Plant agar test for water-insoluble nanoparticles. Environ. Toxicol. Chem. 2008, 27, 1915–1921. [Google Scholar] [CrossRef]
- Yasmeen, F.; Razzaq, A.; Iqbal, M.N.; Jhanzab, H.M. Effect of silver, copper and iron nanoparticles on wheat germination. Int. J. Biosci. 2015, 6, 112–117. [Google Scholar] [CrossRef]
- Zhang, Z.; Ke, M.; Qu, Q.; Peijnenburg, W.J.G.M.; Lu, T.; Zhang, Q.; Ye, Y.; Xu, P.; Du, B.; Sun, L.; et al. Impact of copper nanoparticles and ionic copper exposure on wheat (Triticum aestivum L.) root morphology and antioxidant response. Environ. Pollut. 2018, 239, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, M.; Nigro, A.; Sappa, G. Soil contamination evaluation by Enrichment Factor (EF) and Geoaccumulation Index (Igeo). Senses Sci. 2015, 2, 94–97. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, C.L. Riverine composition and estuarine geochemistry of particulate metals in China—Weathering features, anthropogenic impact and chemical fluxes. Estuar. Coast. Shelf Sci. 2002, 54, 1051–1070. [Google Scholar] [CrossRef]
- Jaberzadeh, A.; Moaveni, P.; Moghadam, H.R.T.; Zahedi, H. Influence of bulk and nanoparticles titanium foliar application on some agronomic traits, seed gluten and starch contents of wheat subjected to water deficit stress. Not. Bot. Horti Agrobot. Cluj Napoca 2013, 41, 201–207. [Google Scholar] [CrossRef] [Green Version]
- Jacob, D.L.; Borchardt, J.D.; Navaratnam, L.; Otte, M.L.; Bezbaruah, A.N. Uptake and translocation of Ti from nanoparticles in crops and wetland plants. Int. J. Phytoremediat. 2013, 15, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Larue, C.; Veronesi, G.; Flank, A.M.; Surble, S.; Herlin-Boime, N.; Carriere, M. Comparative uptake and impact of TiO2 nanoparticles in wheat and rapeseed. J. Toxicol. Environ. Health A 2012, 75, 722–734. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodzadeh, H.; Aghili, R. Effect on germination and early growth characteristics in wheat plants (Triticum aestivum L.) seeds exposed to TiO2 nanoparticles. J. Chem. Health Risks 2014, 4, 29–36. [Google Scholar] [CrossRef]
- Mishra, S.; Singh, B.R.; Singh, A.; Keswani, C.; Naqvi, A.H.; Singh, H.B. Biofabricated silver nanoparticles act as a strong fungicide against Bipolaris sorokiniana causing spot blotch disease in wheat. PLoS ONE 2014, 9, e97881. [Google Scholar] [CrossRef]
- Riahi-Madvar, A.; Rezaee, F.; Jalali, V. Effects of alumina nanoparticles on morphological properties and antioxidant system of Triticum aestivum. Iran J. Plant Physiol. 2012, 3, 595–603. [Google Scholar]
- Farghaly, F.A.; Nafady, N.A. Green synthesis of silver nanoparticles using leaf extract of Rosmarinus officinalis and its effect on tomato and wheat plants. J. Agric. Sci. 2015, 7, 277. [Google Scholar] [CrossRef]
- Gaiser, B.K.; Fernandes, T.F.; Jepson, M.; Lead, J.R.; Tyler, C.R.; Stone, V. Assessing exposure, uptake and toxicity of silver and cerium dioxide nanoparticles from contaminated environments. Environ. Health 2009, 8, S2. [Google Scholar] [CrossRef] [Green Version]
- Xia, F.; Zuo, X.; Yang, R.; Xiao, Y.; Kang, D.; Vallee-Belisle, A.; Plaxco, K.W. Colorimetric detection of DNA, small molecules, proteins, and ions using unmodified gold nanoparticles and conjugated polyelectrolytes. Proc. Nat. Acad. Sci. USA 2010, 107, 10837–10841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakizadeh, M.; Mirzaei, R.; Ghorbani, H. Accumulation and soil-to-plant transfer factor of lead and manganese in some plant species in Semnan province, central Iran. Iran. J. Toxicol. 2016, 10, 29–33. [Google Scholar] [CrossRef]
- Sakizadeh, M.; Sharafabadi, F.M.; Shayegan, E.; Ghorbani, H. Concentrations and soil-to-plant transfer factor of selenium in soil and plant species from an arid area. IOP Conf. Ser. Earth Environ. Sci. 2016, 44, 052027. [Google Scholar] [CrossRef] [Green Version]
- Mishra, M.; Sahu, R.K.; Sahu, S.K.; Padhy, R.N. Growth, yield and elements content of wheat (Triticum aestivum) grown in composted municipal solid wastes amended soil. Environ. Dev. Sustain. 2009, 11, 115–126. [Google Scholar] [CrossRef]
- Munir, T.; Rizwan, M.; Kashif, M.; Shahzad, A.; Ali, S.; Amin, N.; Zahid, R.; Alam, M.F.E.; Imran, M. Effect of zinc oxide nanoparticles on the growth and Zn uptake in wheat (Triticum aestivum L.) by seed priming method. Dig. J. Nanomater. Biostruct. 2018, 13, 315–323. [Google Scholar]
- Svecnjak, Z.; Jenel, M.; Bujan, M.; Vitali, D.; Dragojevic, I.V. Trace element concentrations in the grain of wheat cultivars as affected by nitrogen fertilization. Agric. Food Sci. 2013, 22, 445–451. [Google Scholar] [CrossRef] [Green Version]
- Suchowilska, E.; Wiwart, M.; Kandler, W.; Krska, R. A comparison of macro- and microelement concentrations in the whole grain of four Triticum species. Plant Soil Environ. 2012, 58, 141–147. [Google Scholar] [CrossRef] [Green Version]
- Chamberlain, A.C. Fallout of lead and uptake by crops. Atmos. Environ. 1983, 17, 693–706. [Google Scholar] [CrossRef]
- Baker, A.J.M. Accumulators and excluders: Strategies in the response of plants to heavy metals. J. Plant Nutr. 1981, 3, 643–654. [Google Scholar] [CrossRef]
- Fageria, N.K.; Nascente, A.S. Management of soil acidity of South American soils for sustainable crop production. Adv. Agron. 2014, 128, 221–275. [Google Scholar] [CrossRef]
- Rayment, G. Total potassium to exchangeable potassium ratios as a guide to sustainable soil potassium supply. Commun. Soil Sci. Plant Anal. 2013, 44, 113–119. [Google Scholar] [CrossRef]
- Kozak, M.; Stêpieñ, M.; Joarder, A. Relationships between available and exchangeable potassium content and other soil properties. Pol. J. Soil Sci. 2005, XXXVIII(2), 179–186. [Google Scholar]
- McLean, E.O. Contrasting concepts in soil test interpretation: Sufficiency levels of available nutrients versus basic cation saturation ratios. In Soil Testing: Correlation and Interpretation Analytical Results; American Society of Agronomy Special Publication No 29; ASA, CSSA, and SSSA: Madison, WI, USA, 1977. [Google Scholar]
- McLean, E.O.; Brown, J.R. Crop responses to lime in the Midwestern United States. In Soil Acidity and Liming, 2nd ed.; Adams, F., Ed.; ASA, CSAA ad SSSA: Madison, WI, USA, 1984. [Google Scholar]
- Bear, F.E.; Prince, A.L.; Malcolm, J.L. Potassium needs of New Jersey soils. N. J. Agric. Exp. Stn. Bull. 1945, 721, 19. [Google Scholar]
- Pavlović, D.; Nikolić, B.; Đurović, S.; Waisi, H.; Anđelković, A.; Marisavljević, D. Chlorophyll as a measure of plant health: Agroecological aspects. Pestic. Phytomed. 2014, 29, 21–34. [Google Scholar] [CrossRef]
- Yruela, I. Copper in plants: Acquisition, transport and interactions. Funct. Plant Biol. 2009, 36, 409–430. [Google Scholar] [CrossRef] [Green Version]
- Lombardi, L.; Sebastiani, L. Copper toxicity in Prunus cerasifera: Growth and antioxidant enzymes responses of in vitro grown plants. Plant Sci. 2005, 168, 797–802. [Google Scholar] [CrossRef]
- Herbik, A.; Bölling, C.; Buckhout, T.J. The involvement of a multicopper oxidase in iron uptake by the green algae Chlamydomonas reinhardtii. Plant Physiol. 2002, 130, 2039–2048. [Google Scholar] [CrossRef] [Green Version]
- Pätsikkä, E.; Kairavuo, M.; Sersen, F.; Aro, E.M.; Tyystjärvi, E. Excess copper predisposes photosystem II to photoinhibition in vivo by outcompeting iron and causing decrease in leaf chlorophyll. Plant Physiol. 2002, 129, 1359–1367. [Google Scholar] [CrossRef] [Green Version]
- Panou-Filotheou, H.; Basabalidis, A.M. Root structural aspects associated with copper toxicity in oregano (Origanum vulgare subsp. hirtum). Plant Sci. 2004, 166, 1497–1504. [Google Scholar] [CrossRef]
- Bernal, M.; Cases, R.; Picorel, R.; Yruela, I. Foliar and root Cu supply affect differently Fe and Zn uptake and photosynthetic activity in soybean plants. Environ. Exp. Bot. 2007, 60, 145–150. [Google Scholar] [CrossRef] [Green Version]
- Hailu, H.; Mamo, T.; Keskinen, R.; Karltun, E.; Gebrekidan, H.; Bekele, T. Soil fertility status and wheat nutrient content in Vertisol cropping systems of central highlands of Ethiopia. Agric. Food Secur. 2015, 4, 19. [Google Scholar] [CrossRef] [Green Version]
- Markert, B. Establishing of “reference plant” for inorganic characterization of different plant species by chemical fingerprint. Water Air Soil Pollut. 1992, 64, 533–538. [Google Scholar] [CrossRef]
- Mousavi, S.R.; Galavi, M.; Rezaei, M. The interaction of zinc with other elements in plants: A review. Int. J. Agric. Crop Sci. 2012, 4, 1881–1884. [Google Scholar]
- Marschner, H.; Marschner, P. Marschner’s Mineral Nutrition of Higher Plants; Academic Press: London, UK, 2012. [Google Scholar]
- Verbruggen, N.; Hermans, C. Physiological and molecular responses to magnesium nutritional imbalance in plants. Plant Soil 2013, 368, 87–99. [Google Scholar] [CrossRef]
- Plank, C.O.; Kissel, D.E. Plant Analysis Handbook for Georgia. Agricultural & Environmental Services Laboratories. 2020. Available online: http://aesl.ces.uga.edu/publications/plant/Nutrient.asp (accessed on 15 March 2021).
- White, P.J.; Broadley, M.R. Calcium in plants. Ann. Bot. 2003, 92, 487–511. [Google Scholar] [CrossRef]



| Parameter | Control Soil | Soil with CuO-NP | Soil with CuO-NP-cel | Soil with CuO-NP-bth | Remarks |
|---|---|---|---|---|---|
| Elements determined in soil | Ca > Mg > Al > Fe > K> Na > Ti > Mn > Cl > Ba > Sr > Zn > Zr > V>Rb> Cr > Ce > Br > La > Ni> Nd > As > Co > Sc > Th > Sb > W>Hf > Sm > Cs > Sn > U > Mo > Yb > Ta > Au > Tb > Tm > Eu | Ca > Mg > Al > Fe > K > Na > Ti > Cl > Mn > Cu > Ba > Sr > Zn > Zr > Rb > V > Cr > Ce > Br > Ni > La > Nd > As > Co > Sc > Sb > Th > W > Hf > Sn > Sm > Cs > U > Mo > Yb > Ta > Tb > Eu > Tm > Au | Ca > Al > K > Mg > Fe > Na > Ti > Cl > Mn > Ba > Cu > Sr > Zn > Zr > Rb > V > Cr > Ce > Nd > Ni > La > Br > As > Co > Sc > Th > Sb > Hf > Sn > Sm > Cs > W > U > Mo > Yb > Tb > Ta > Eu > Tm > Au | Ca > Mg > Al > Fe > K > Na > Cl > Ti > Mn > Ba > Cu > Sr > Zr > Zn > Sb > Rb > V > Cr > Ce > Ni > Nd > Br > La > As > Co > Sc > Th > Hf > Sm > Cs > U > Yb > W > Mo > Ta > Eu > Tb > Tm > Au | Cl always higher Ti and Cs always lower |
| EF/Al soil | EF (Cl, Fe, Eu, Nd) > 1.5 | EF (Cl, Fe, Eu, K, Ni, Rb) > 1.5 | EF (Cl, Fe, Eu, K, Ni, As, Sb, Yb) > 1.5 | EF (Cl, Fe, and Eu) > 1.5 for all amended soils |
| Parameter | Control Soil | Soil with CuO-NP | Soil with CuO-NP-cel | Soil with CuO-NP-bth | Remarks |
|---|---|---|---|---|---|
| Elements determined in plants | Cl > Fe > K>Ca > Mg> Na > Ba > Zr > Al > Cr> Rb > Zn > Ni > Sr > Ce > Mn > La > Nd > Co > Sc > Th > As > Hf > Sm > Cs > U>Br > Sb > Ta > Eu> Tb > Yb > Tm > Mo | K > Cl > Ca > Mg > Br > Na > Al > Zn > Rb > Cu > Mn > Ba > Sr | K > Cl > Ca > Mg > Na > Al > Zn > Rb > Mn > Ba > Sr > Br > Mo > As > Sb > Sm | K > Cl > Ca > Mg > Na > Al > Fe > Zn > Rb > Mn > Ba > Br > Sr > Mo > As > Sb > Sm | |
| EF/Al wheat | K, Br >> 1.5 | K, Br, Mo >> 1.5 | K, Br, Mo >> 1.5 |
| Parameter | Control Soil | Soil with CuO-NP | Soil with CuO-NP-cel | Soil with CuO-NP-bth | Remarks |
|---|---|---|---|---|---|
| Soil to plant mobility ratio (MR) | Excl.: Na, Mg, Al, Ca, Mn, As, Sr, Sb, Ba, Yb, Ti, Br, Mo Accum.: Sc, Cr, Co, Ni, Zr, La, Ce, Nd, Sm, Tb, Tm, Hf, Ta, Th, Cl, Fe, Rb Indiff.: U, K, Cs | Excl.: Na, Mg, Al, Ca, Mn, As, Sr, Sb, Ba Accum.: Cl, K, Br Indiff: Zn | Excl.: Na, Mg, Al, Ca, Mn, As, Sr, Sb, Ba, Rb, Cs, Sm Accum.: Cl, K, Br Indiff.: Ti, Mo, Zn | Excl.: Na, Mg, Al, Ca, Mn, As, Sr, Sb, Ba, Fe, Sm Accum.: Cl, K, Ti, Rb, Zn Indiff.: Br, Mo | MRCl: control >> chem > cel >> bth |
| Parameter | Control Soil | Soil with CuO-NP | Soil with CuO-NP-cel | Soil with CuO-NP-bth | Remarks |
|---|---|---|---|---|---|
| CHL a | <control | <control | <control | chem > bth > cel | |
| CHL b | <control | <control | <control | chem > cel > bth | |
| CARO | <control | <control | <control | chem > bth > cel | |
| TP | >control | <control | >control | chem > bth > cel | |
| DPPH | >control | >control | >control | chem~cel < bth | |
| R2 | R2 (CMo-CHL a) = −0.99; R2 (CMo-CHL b) = −0.96; R2 (CMo-CARO) = −0.90, R2 (CAl,Mg, K, Zn, Ca –CHL a, CHL b, CARO) < 0 R2 (CAl, Mg, K, Zn –DPPH) = 0.93 R2 (CBr –TP) = 0.67; R2 (CAl –TP) = −0.43 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lung, I.; Opriş, O.; Soran, M.-L.; Culicov, O.; Ciorîță, A.; Stegarescu, A.; Zinicovscaia, I.; Yushin, N.; Vergel, K.; Kacso, I.; et al. The Impact Assessment of CuO Nanoparticles on the Composition and Ultrastructure of Triticum aestivum L. Int. J. Environ. Res. Public Health 2021, 18, 6739. https://doi.org/10.3390/ijerph18136739
Lung I, Opriş O, Soran M-L, Culicov O, Ciorîță A, Stegarescu A, Zinicovscaia I, Yushin N, Vergel K, Kacso I, et al. The Impact Assessment of CuO Nanoparticles on the Composition and Ultrastructure of Triticum aestivum L. International Journal of Environmental Research and Public Health. 2021; 18(13):6739. https://doi.org/10.3390/ijerph18136739
Chicago/Turabian StyleLung, Ildiko, Ocsana Opriş, Maria-Loredana Soran, Otilia Culicov, Alexandra Ciorîță, Adina Stegarescu, Inga Zinicovscaia, Nikita Yushin, Konstantin Vergel, Irina Kacso, and et al. 2021. "The Impact Assessment of CuO Nanoparticles on the Composition and Ultrastructure of Triticum aestivum L." International Journal of Environmental Research and Public Health 18, no. 13: 6739. https://doi.org/10.3390/ijerph18136739






