Correlation between Primary, Secondary Stability, Bone Density, Percentage of Vital Bone Formation and Implant Size
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Radiographic Evaluation
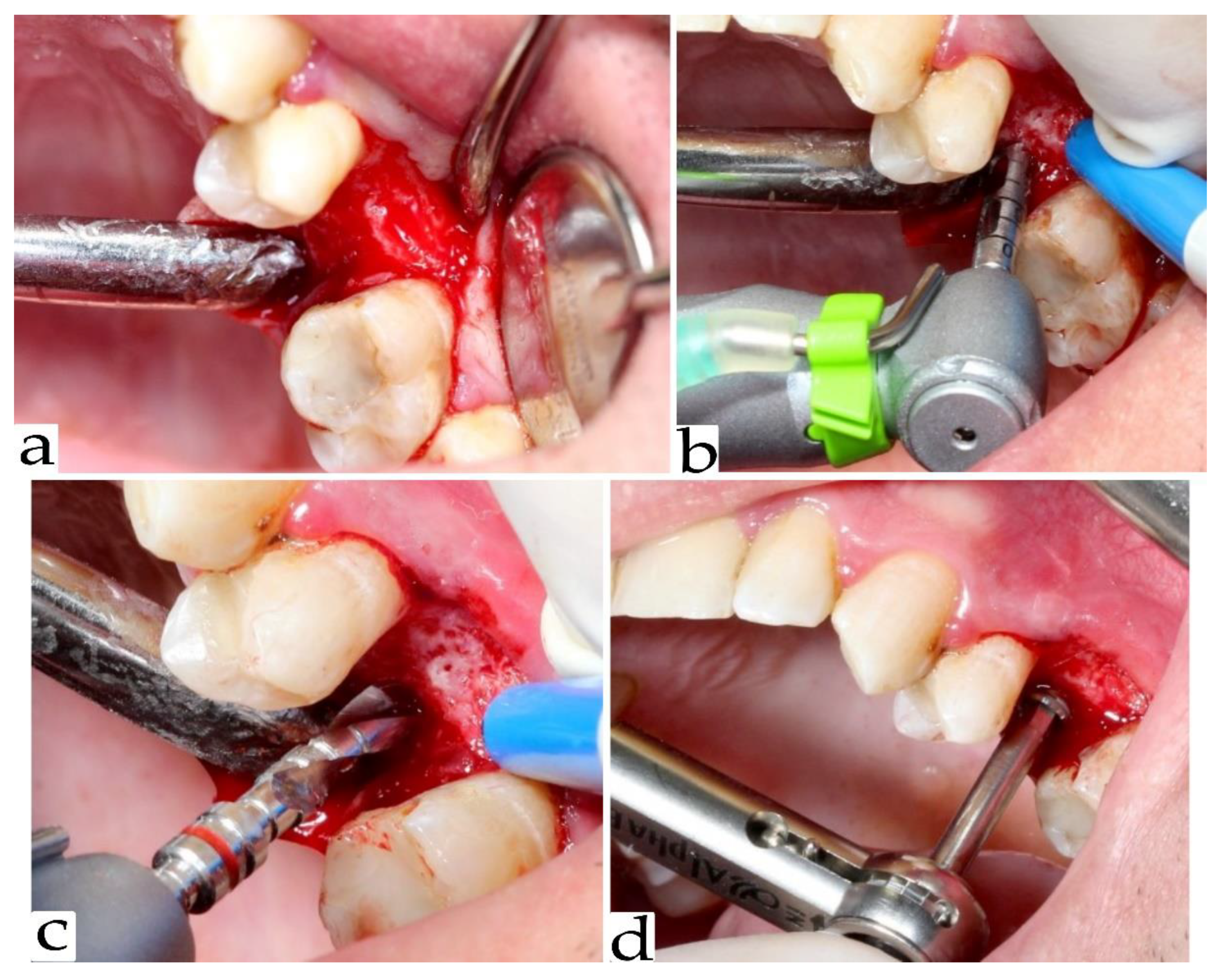
2.3. Re-Entry Surgery and Implant Placement
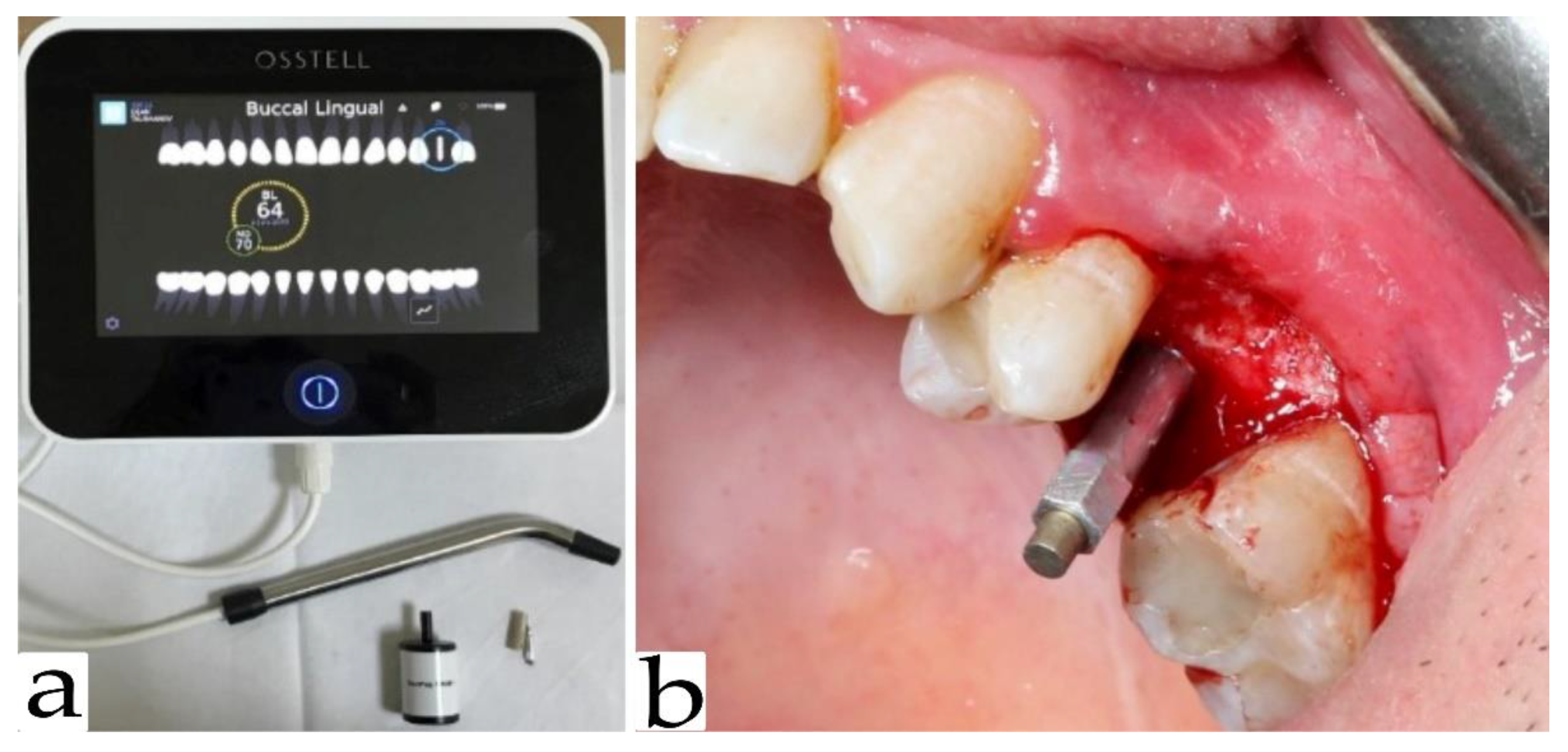
2.4. Implant Stability
2.5. Statistical Methods
3. Results
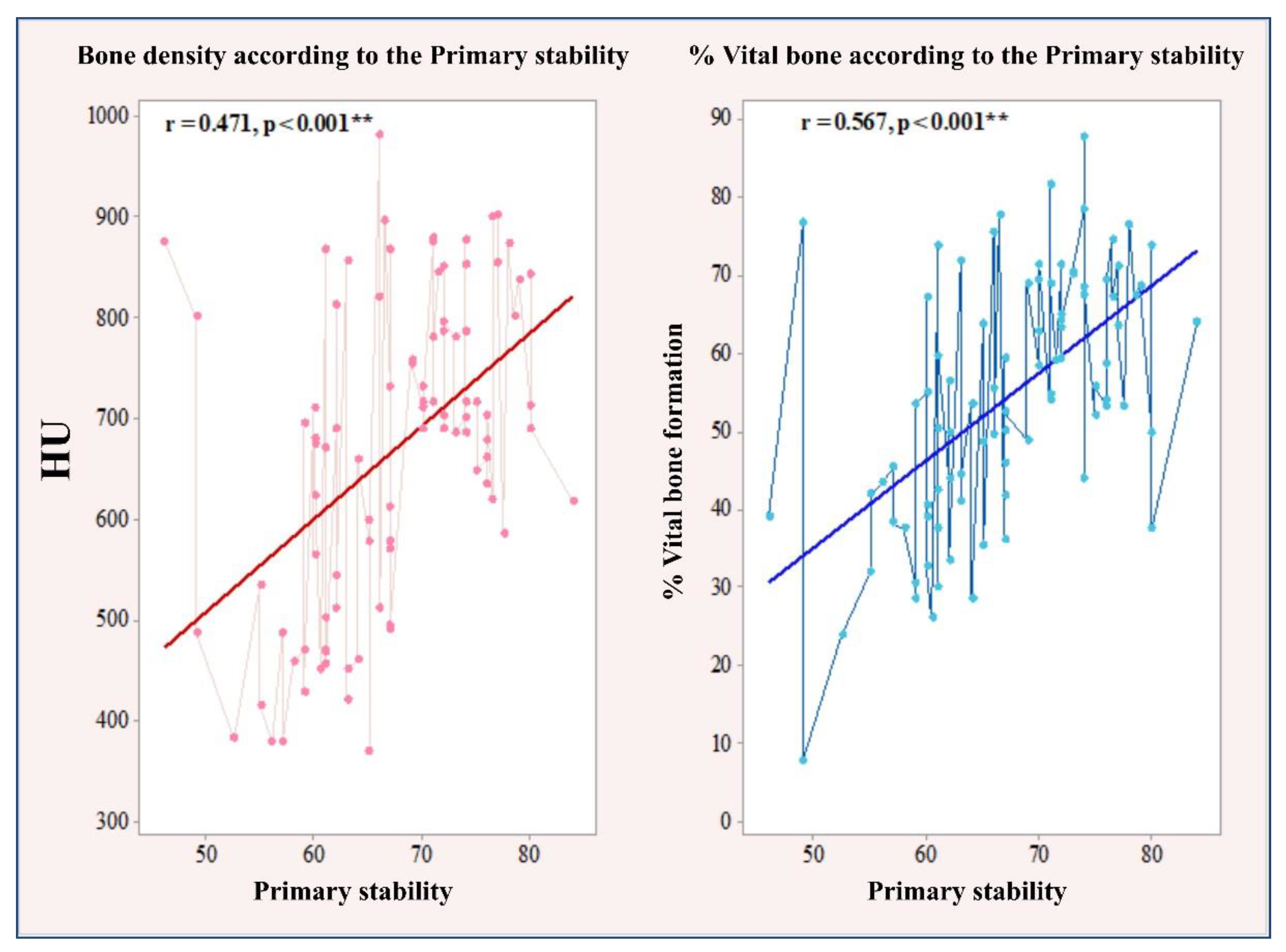
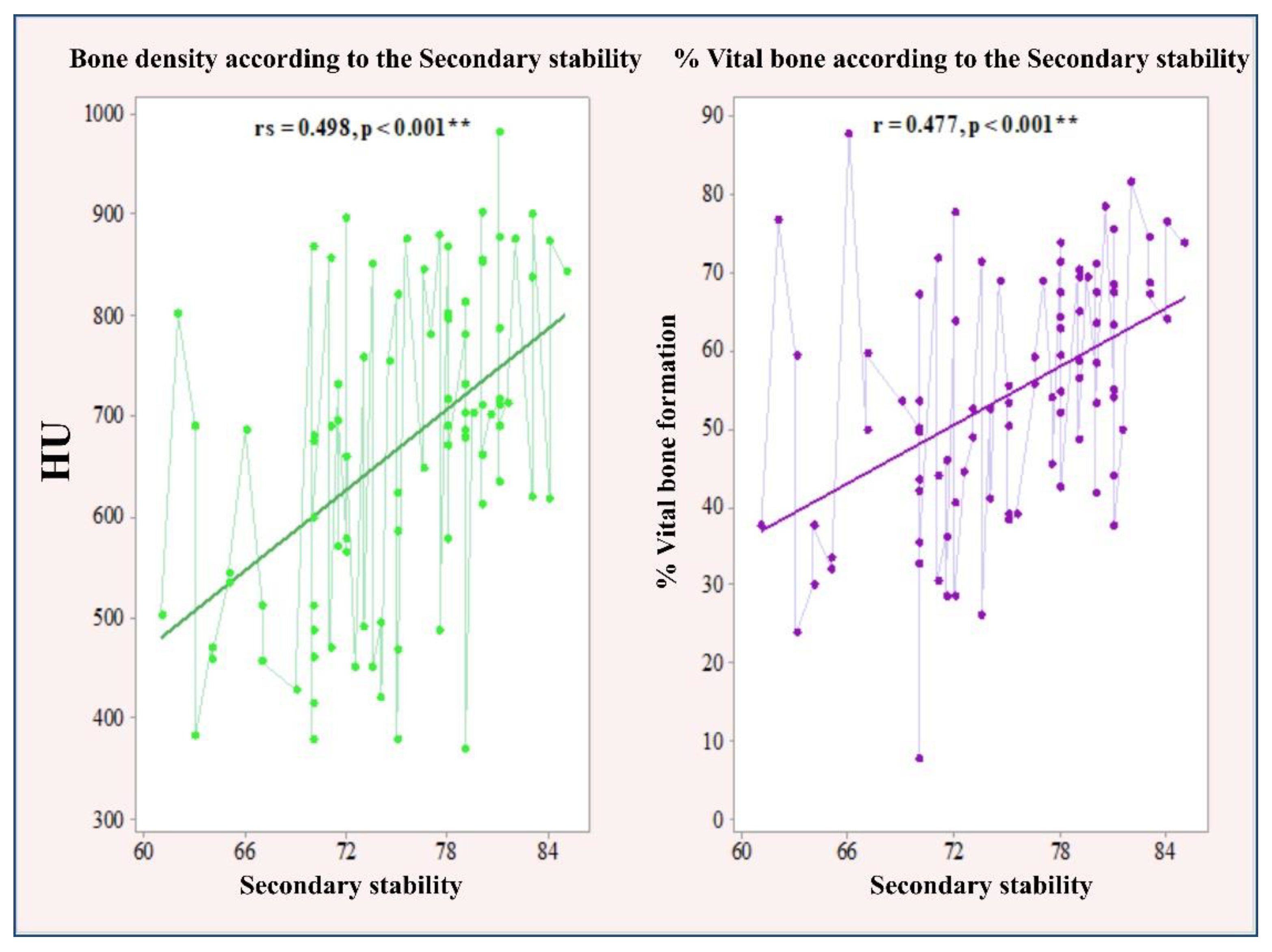
3.1. Correlation between Primary and Secondary Stability with Bone Density and the Amount of Bone Formation in Biopsy Samples
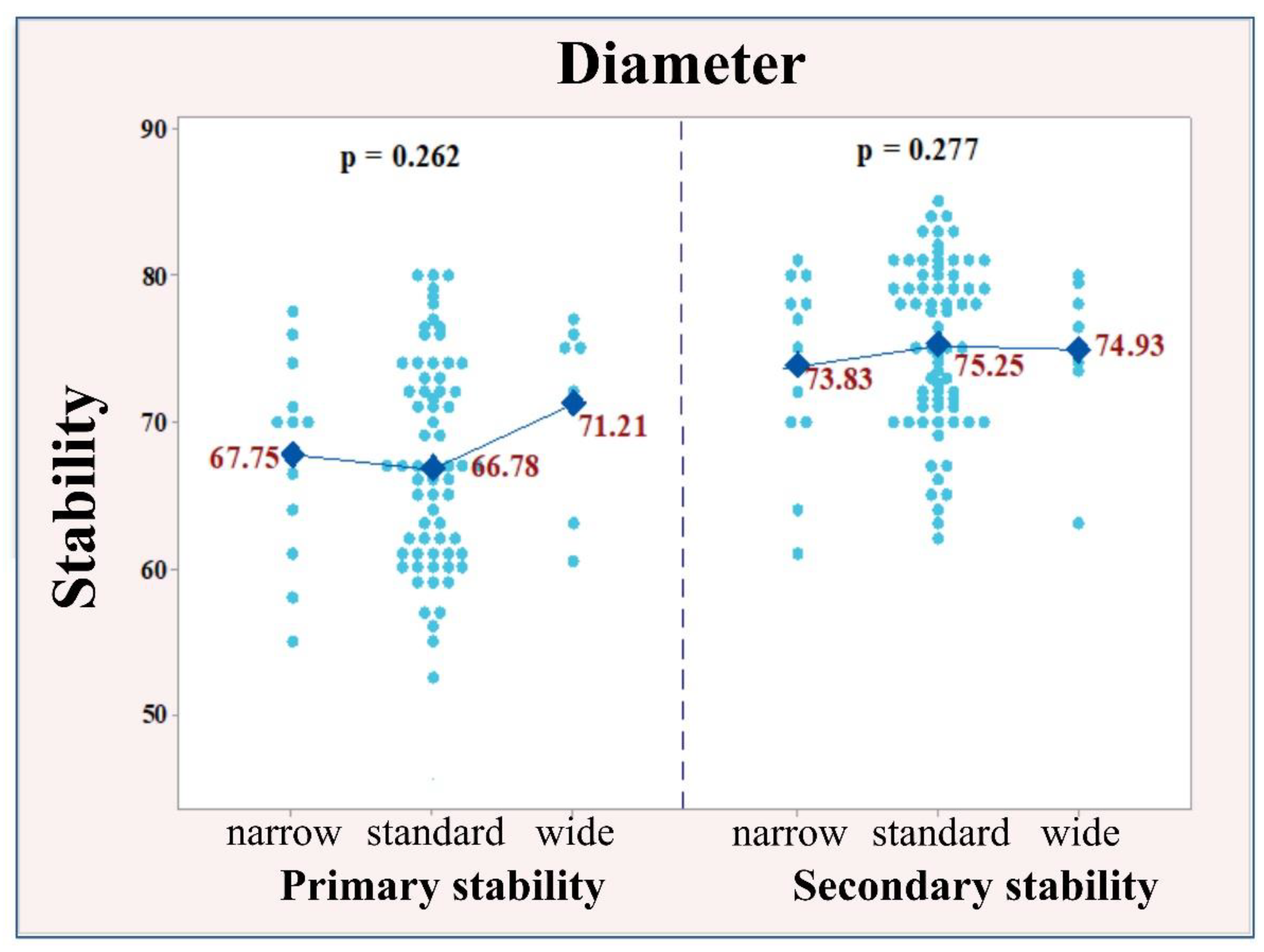
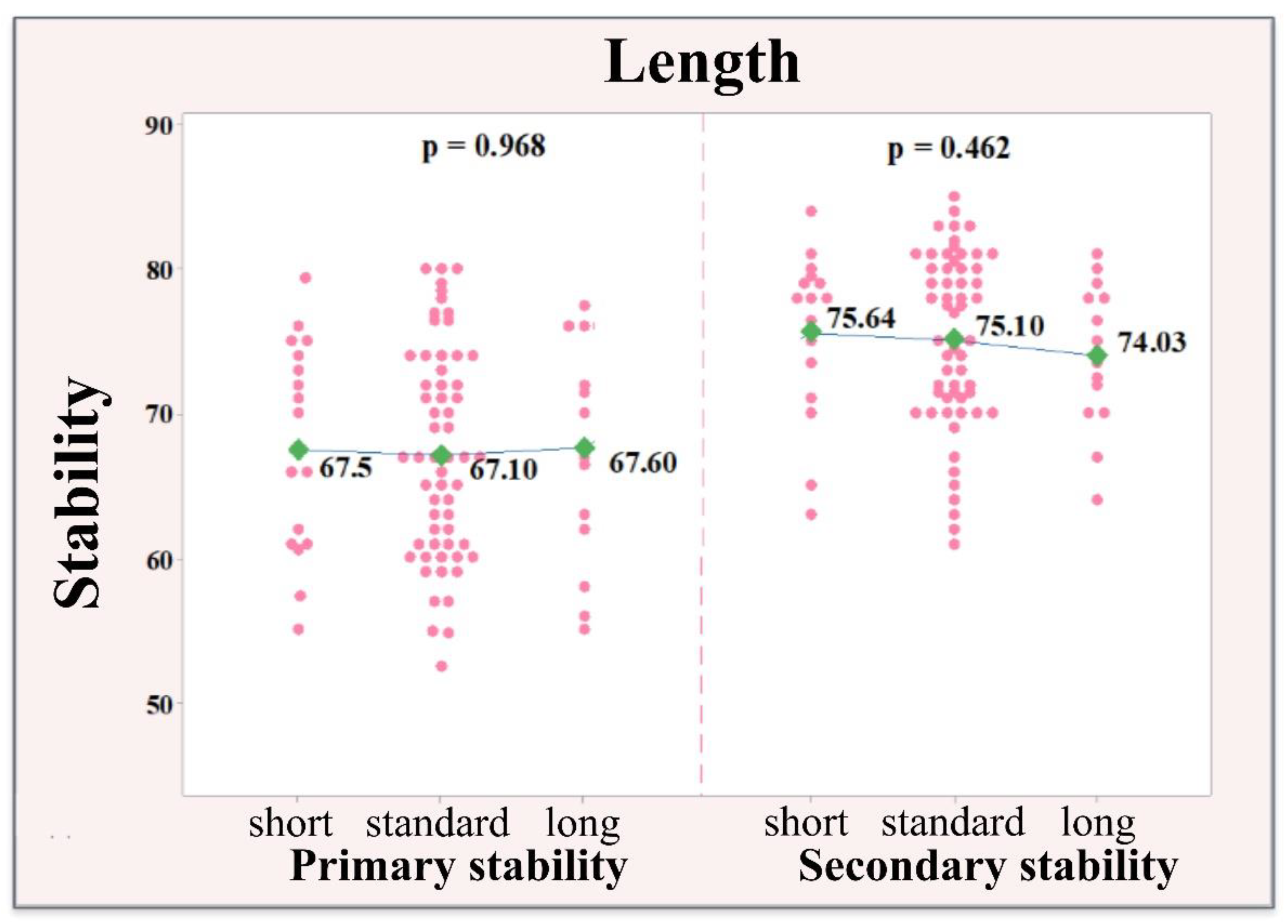
3.2. Correlation between Implant Size and Stability of the Implants
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Franchi, M.; Bacchelli, B.; Giavaresi, G.; De Pasquale, V.; Martini, D.; Fini, M.; Giardino, R.; Ruggeri, A. Influence of different implant surfaces on peri-implant osteogenesis: Histomorphometric analysis in sheep. J. Periodontol. 2007, 78, 879–888. [Google Scholar] [CrossRef]
- Vayron, R.; Nguyen, V.-H.; Lecuelle, B.; Albini Lomami, H.; Meningaud, J.-P.; Bosc, R.; Haiat, G. Comparison of Resonance Frequency Analysis and of Quantitative Ultrasound to Assess Dental Implant Osseointegration. Sensors 2018, 18, 1397. [Google Scholar] [CrossRef] [Green Version]
- Szmukler-Moncler, S.; Salama, H.; Reingewirtz, Y.; Dubruille, J.H. Timing of loading and effect of micromotion on bone-dental implant interface: Review of experimental literature. J. Biomed. Mater. Res. 1998, 43, 192–203. [Google Scholar] [CrossRef]
- Di Stefano, D.A.; Arosio, P.; Perrotti, V.; Iezzi, G.; Scarano, A.; Piattelli, A. Correlation between Implant Geometry, Bone Density, and the Insertion Torque/Depth Integral: A Study on Bovine Ribs. Dent. J. 2019, 7, 25. [Google Scholar] [CrossRef] [Green Version]
- Davies, J.E. Understanding peri-implant endosseous healing. J. Dent. Educ. 2003, 67, 932–949. [Google Scholar] [CrossRef] [PubMed]
- Sakka, S.; Baroudi, K.; Nassani, M.Z. Factors associated with early and late failure of dental implants. J. Investig. Clin. Dent. 2012, 3, 258–261. [Google Scholar] [CrossRef] [PubMed]
- Branemark, P.I. Osseointegration and Its Experimental Background. J. Prosthet. Dent. 1983, 50, 399–410. [Google Scholar] [CrossRef]
- Akoğlan, M.; Tatli, U.; Kurtoğlu, C.; Salimov, F.; Kürkçü, M. Effects of different loading protocols on the secondary stability and peri-implant bone density of the single implants in the posterior maxilla. Clin. Implant. Dent. Relat. Res. 2017, 19, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Cho, I.H.; Lee, Y.I.; Kim, Y.M. A comparative study on the accuracy of the devices for measuring the implant stability. J. Adv. Prosthodont. 2009, 1, 124–128. [Google Scholar] [CrossRef]
- Cehreli, M.C.; Kökat, A.M.; Comert, A.; Akkocaoğlu, M.; Tekdemir, I.; Akça, K. Implant stability and bone density: Assessment of correlation in fresh cadavers using conventional and osteotome implant sockets. Clin. Oral Implant. Res. 2009, 20, 1163–1169. [Google Scholar] [CrossRef] [PubMed]
- Meredith, N. Assessment of implant stability as a prognostic determinant. Int. J. Prosthodont. 1998, 11, 491–501. [Google Scholar]
- Gehrke, S.A.; Neto, U.T.; Rossetti, P.H.; Watinaga, S.E.; Giro, G.; Shibli, J.A. Stability of implants placed in fresh sockets versus healed alveolar sites: Early findings. Clin. Oral Implant. Res. 2016, 27, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Greenstein, G.; Cavallaro, J. Implant Insertion Torque: Its Role in Achieving Primary Stability of Restorable Dental Implants. Compend. Contin. Educ. Dent. 2017, 38, 88–95. [Google Scholar]
- Gehrke, S.A.; da Silva, U.T.; Del Fabbro, M. Does Implant Design Affect Implant Primary Stability? A Resonance Frequency Analysis-Based Randomized Split-Mouth Clinical Trial. J. Oral Implantol. 2015, 41, e281–e286. [Google Scholar] [CrossRef]
- Schwarz, M.S.; Rothman, S.L.; Rhodes, M.L.; Chafetz, N. Computed tomography: Part, I.I. Preoperative assessment of the maxilla for endosseous implant surgery. Int. J. Oral Maxillofac. Implant. 1987, 2, 143–148. [Google Scholar]
- Isoda, K.; Ayukawa, Y.; Tsukiyama, Y.; Sogo, M.; Matsushita, Y.; Koyano, K. Relationship between the bone density estimated by cone-beam computed tomography and the primary stability of dental implants. Clin. Oral Implant. Res. 2012, 23, 832–836. [Google Scholar] [CrossRef]
- Valiyaparambil, J.V.; Yamany, I.; Ortiz, D.; Shafer, D.M.; Pendrys, D.; Freilich, M.; Mallya, S.M. Bone quality evaluation: Comparison of cone beam computed tomography and subjective surgical assessment. Int. J. Oral Maxillofac. Implant. 2012, 27, 1271–1277. [Google Scholar]
- Cassetta, M.; Stefanelli, L.V.; Pacifici, A.; Pacifici, L.; Barbato, E. How accurate is CBCT in measuring bone density? A comparative CBCT-CT in vitro study. Clin. Implant. Dent. Relat. Res. 2014, 16, 471–478. [Google Scholar] [CrossRef]
- Nomura, Y.; Watanabe, H.; Honda, E.; Kurabayashi, T. Reliability of voxel values from cone-beam computed tomography for dental use in evaluating bone mineral density. Clin. Oral Implant. Res. 2010, 21, 558–562. [Google Scholar] [CrossRef] [PubMed]
- Howashi, M.; Tsukiyama, Y.; Ayukawa, Y.; Isoda-Akizuki, K.; Kihara, M.; Imai, Y.; Koyano, K. Relationship between the CT Value and Cortical Bone Thickness at Implant Recipient Sites and Primary Implant Stability with Comparison of Different Implant Types. Clin. Implant Dent. Relat. Res. 2016, 18, 107–116. [Google Scholar] [CrossRef]
- Orlando, F.; Arosio, F.; Arosio, P.; Di Stefano, D.A. Bone Density and Implant Primary Stability. A Study on Equine Bone Blocks. Dent. J. 2019, 7, 73. [Google Scholar] [CrossRef] [Green Version]
- Trisi, P.; Rao, W. Bone classification: Clinical-histomorphometric comparison. Clin. Oral Implant. Res. 1999, 10, 1–7. [Google Scholar]
- Pereira, A.C.; Souza, P.P.; Souza, J.A.; Silva, T.A.; Batista, A.C.; Ribeiro-Rotta, R.F. Histomorphometrical and molecular evaluation of endosseous dental implants sites in humans: Correlation with clinical and radiographic aspects. Clin. Oral Implant. Res. 2013, 24, 414–421. [Google Scholar] [CrossRef]
- Degidi, M.; Piattelli, A.; Iezzi, G.; Carinci, F. Do longer implants improve clinical outcome in immediate loading? Int. J. Oral Maxillofac. Surg. 2007, 36, 1172–1176. [Google Scholar] [CrossRef]
- Ostman, P.O.; Hellman, M.; Wendelhag, I.; Sennerby, L. Resonance Frequency Analysis Measurements of Implants at Placement Surgery. Int. J. Prosthodont. 2006, 19, 77–84. [Google Scholar] [PubMed]
- Barikani, H.; Rashtak, S.; Akbari, S.; Badri, S.; Daneshparvar, N.; Rokn, A. The Effect of Implant Length and Diameter on the Primary Stability in Different Bone Types. J. Dent. 2013, 10, 449–455. [Google Scholar]
- Bergkvist, G.; Koh, K.J.; Sahlholm, S.; Klintström, E.; Lindh, C. Bone Density at Implant Sites and Its Relationship to Assessment of Bone Quality and Treatment Outcome. Int. J. Oral Maxillofac. Implant. 2010, 25, 321–328. [Google Scholar]
- Stocchero, M.; Toia, M.; Cecchinato, D.; Becktor, J.P.; Coelho, P.G.; Jimbo, R. Biomechanical, Biologic, and Clinical Outcomes of Undersized Implant Surgical Preparation: A Systematic Review. Int. J. Oral Maxillofac. Implant. 2016, 31, 1247–1263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Marshood, M.M.; Junker, R.; Al-Rasheed, A.; Al Farraj Aldosari, A.; Jansen, J.A.; Anil, S. Study of the osseointegration of dental implants placed with an adapted surgical technique. Clin. Oral Implant. Res. 2011, 22, 753–759. [Google Scholar] [CrossRef]
- Möhlhenrich, S.C.; Heussen, N.; Elvers, D.; Steiner, T.; Hölzle, F.; Modabber, A. Compensating for poor primary implant stability in different bone densities by varying implant geometry: A laboratory study. Int. J. Oral Maxillofac. Surg. 2015, 44, 1514–1520. [Google Scholar] [CrossRef] [PubMed]
- Sabeva, E.; Peev, S.; Miteva, M.; Georgieva, M. Bone characteristics and implant stability. Scr. Sci. Med. Dent. 2017, 3, 18–22. [Google Scholar] [CrossRef] [Green Version]
- Klinge, B.; Johansson, C.; Albrektsson, T.; Hallström, H.; Engdahl, T. A new method to obtain bone biopsies at implant sites peri-operatively: Technique and bone structure. Clin. Oral Implant. Res. 1995, 6, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Rokn, A.R.; Labibzadeh, A.; Ghohroudi, A.A.R.; Shamshiri, A.R.; Solhjoo, S. Histomorphometric Analysis of Bone Density in Relation to Tactile Sense of the Surgeon During Dental Implant Placement. Open Dent. J. 2018, 12, 46–52. [Google Scholar] [CrossRef] [Green Version]
- Sennerby, L.; Thomsen, P.; Ericson, L.E. A morphometric and biomechanic comparison of titanium implants inserted in rabbit cortical and cancellous bone. Int. J. Oral Maxillofac. Implant. 1992, 7, 62–71. [Google Scholar]
- Chan, H.L.; Lin, G.H.; Fu, J.H.; Wang, H.L. Alterations in bone quality after socket preservation with grafting materials: A systematic review. Int. J. Oral Maxillofac. Implant. 2013, 28, 710–720. [Google Scholar] [CrossRef] [Green Version]
- Horváth, A.; Mardas, N.; Mezzomo, L.A.; Needleman, I.G.; Donos, N. Alveolar ridge preservation. A systematic review. Clin. Oral Investig. 2013, 17, 341–363. [Google Scholar] [CrossRef]
- Lekholm, U.; Zarb, G.A. Patient Selection and Preparation. Tissue Integrated Prostheses: Osseointegration in Clinical Dentistry; Quintessence Publishing Company: Chicago, IL, USA, 1985; pp. 199–209. [Google Scholar]
- Turkyilmaz, I.; Tumer, C.; Ozbek, E.N.; Tözüm, T.F. Relations between the bone density values from computerized tomography, and implant stability parameters: A clinical study of 230 regular platform implants. J. Clin. Periodontol. 2007, 34, 716–722. [Google Scholar] [CrossRef] [PubMed]
- Herekar, M.; Sethi, M.; Ahmad, T.; Fernandes, A.; Patil, V.; Kulkarni, H. A correlation between bone (B), insertion torque (IT), and implant stability (S): BITS score. J. Prosthet Dent. 2014, 112, 805–810. [Google Scholar] [CrossRef] [PubMed]
- Farré-Pagés, N.; Augé-Castro, M.L.; Alaejos-Algarra, F.; Mareque-Bueno, J.; Ferrés-Padró, E.; Hernández-Alfaro, F. Relation between bone density and primary implant stability. Med. Oral Patol Oral Cir. Bucal. 2011, 16, e62–e67. [Google Scholar] [CrossRef] [Green Version]
- Park, I.P.; Kim, S.K.; Lee, S.J.; Lee, J.H. The relationship between initial implant stability quotient values and bone-to-implant contact ratio in the rabbit tibia. J. Adv. Prosthodont. 2011, 3, 76–80. [Google Scholar] [CrossRef]
- Rodrigo, D.; Aracil, L.; Martin, C.; Sanz, M. Diagnosis of implant stability and its impact on implant survival: A prospective case series study. Clin. Oral Implant. Res. 2010, 21, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Pagliani, L.; Sennerby, L.; Petersson, A.; Verrocchi, D.; Volpe, S.; Andersson, P. The relationship between resonance frequency analysis (RFA) and lateral displacement of dental implants: An in vitro study. J. Oral Rehabil. 2013, 40, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Monje, A.; Suarez, F.; Garaicoa, C.A.; Monje, F.; Galindo-Moreno, P.; García-Nogales, A.; Wang, H.L. Effect of location on primary stability and healing of dental implants. Implant. Dent. 2014, 23, 69–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- da Cunha, H.A.; Francischone, C.E.; Filho, H.N.; de Oliveira, R.C. A comparison between cutting torque and resonance frequency in the assessment of primary stability and final torque capacity of standard and TiUnite single-tooth implants under immediate loading. Int. J. Oral Maxillofac. Implant. 2004, 19, 578–585. [Google Scholar]
- Baltayan, S.; Pi-Anfruns, J.; Aghaloo, T.; Moy, P.K. The predictive value of resonance frequency analysis measurements in the surgical placement and loading of endosseous implants. J. Oral Maxillofac. Surg. 2016, 74, 1145–1152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baftijari, D.; Benedetti, A.; Kirkov, A.; Iliev, A.; Stamatoski, A.; Baftijari, F.; Deliverska, E.G.; Gjorgievska, E. Assessment of Primary and Secondary Implant Stability by Resonance Frequency Analysis in Anterior and Posterior Segments of Maxillary Edentulous Ridges. J. IMAB 2018, 24, 2058–2064. [Google Scholar] [CrossRef]
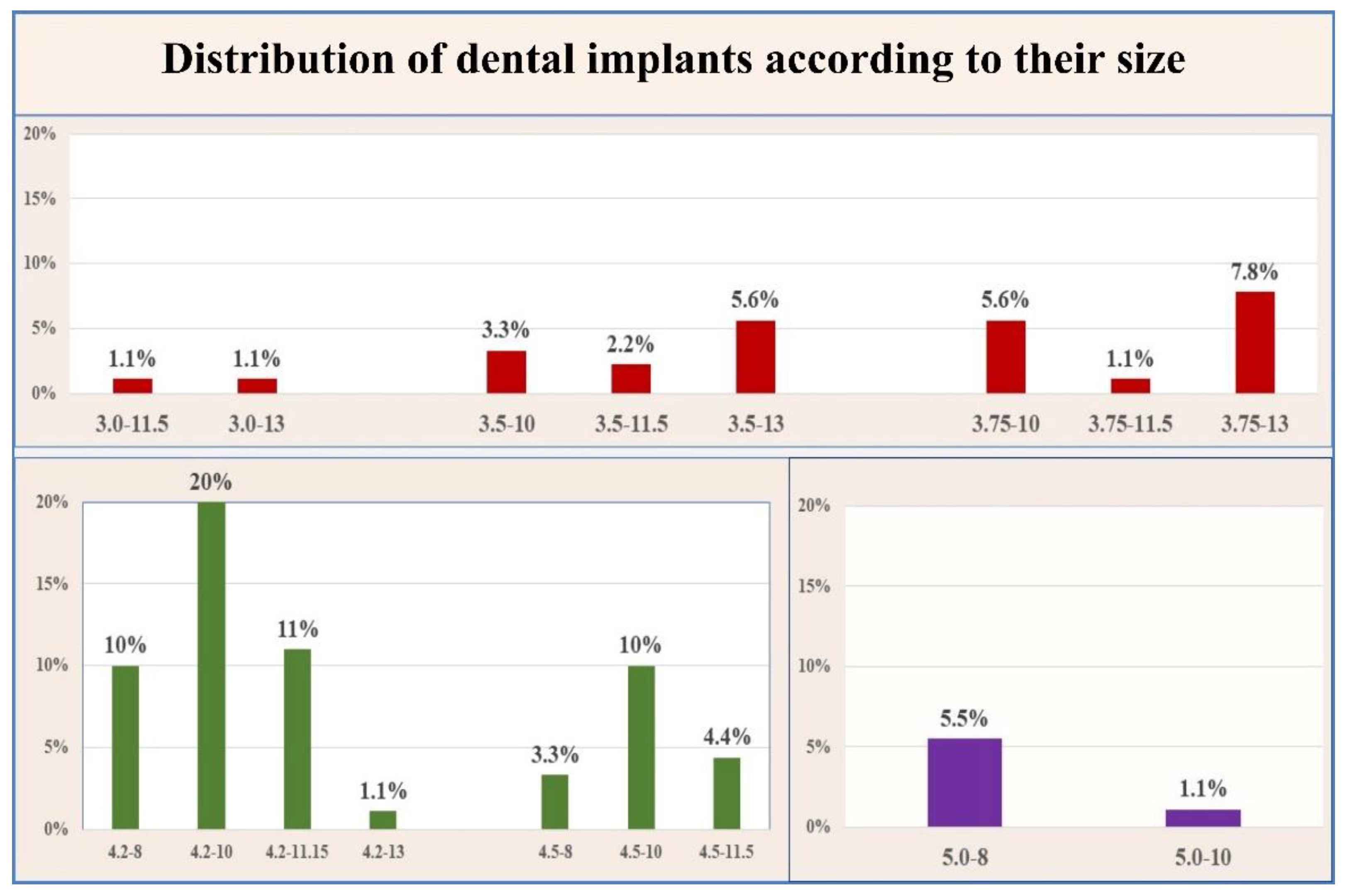
| Implant (I) Diameter | Fisher’s Test | |||
| Term | Narrow | Standard | Wide | p < 0.001 ** |
| Measurements | I < 3.75 mm | 3.75 mm ≤ I ≤ 5 mm | I > 5 | |
| Number (%) | 12 (13%) | 71 (79%) | 7 (8%) | |
| Implant (I) Length | ||||
| Term | Short | Standard | Long | p < 0.001 ** |
| Measurements | I ≤ 6 mm | 6 mm < I < 10 mm | I ≥ 10 mm | |
| Number (%) | 17 (19%) | 59 (65.5%) | 14 (15.50%) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanova, V.; Chenchev, I.; Zlatev, S.; Mijiritsky, E. Correlation between Primary, Secondary Stability, Bone Density, Percentage of Vital Bone Formation and Implant Size. Int. J. Environ. Res. Public Health 2021, 18, 6994. https://doi.org/10.3390/ijerph18136994
Ivanova V, Chenchev I, Zlatev S, Mijiritsky E. Correlation between Primary, Secondary Stability, Bone Density, Percentage of Vital Bone Formation and Implant Size. International Journal of Environmental Research and Public Health. 2021; 18(13):6994. https://doi.org/10.3390/ijerph18136994
Chicago/Turabian StyleIvanova, Vasilena, Ivan Chenchev, Stefan Zlatev, and Eitan Mijiritsky. 2021. "Correlation between Primary, Secondary Stability, Bone Density, Percentage of Vital Bone Formation and Implant Size" International Journal of Environmental Research and Public Health 18, no. 13: 6994. https://doi.org/10.3390/ijerph18136994
APA StyleIvanova, V., Chenchev, I., Zlatev, S., & Mijiritsky, E. (2021). Correlation between Primary, Secondary Stability, Bone Density, Percentage of Vital Bone Formation and Implant Size. International Journal of Environmental Research and Public Health, 18(13), 6994. https://doi.org/10.3390/ijerph18136994







