What Does CLARITY-BPA Mean for Canadians?
Abstract
:1. Introduction
2. Regulation of BPA in Canada
3. New Data Resolves Discrepancy around BPA Toxicity
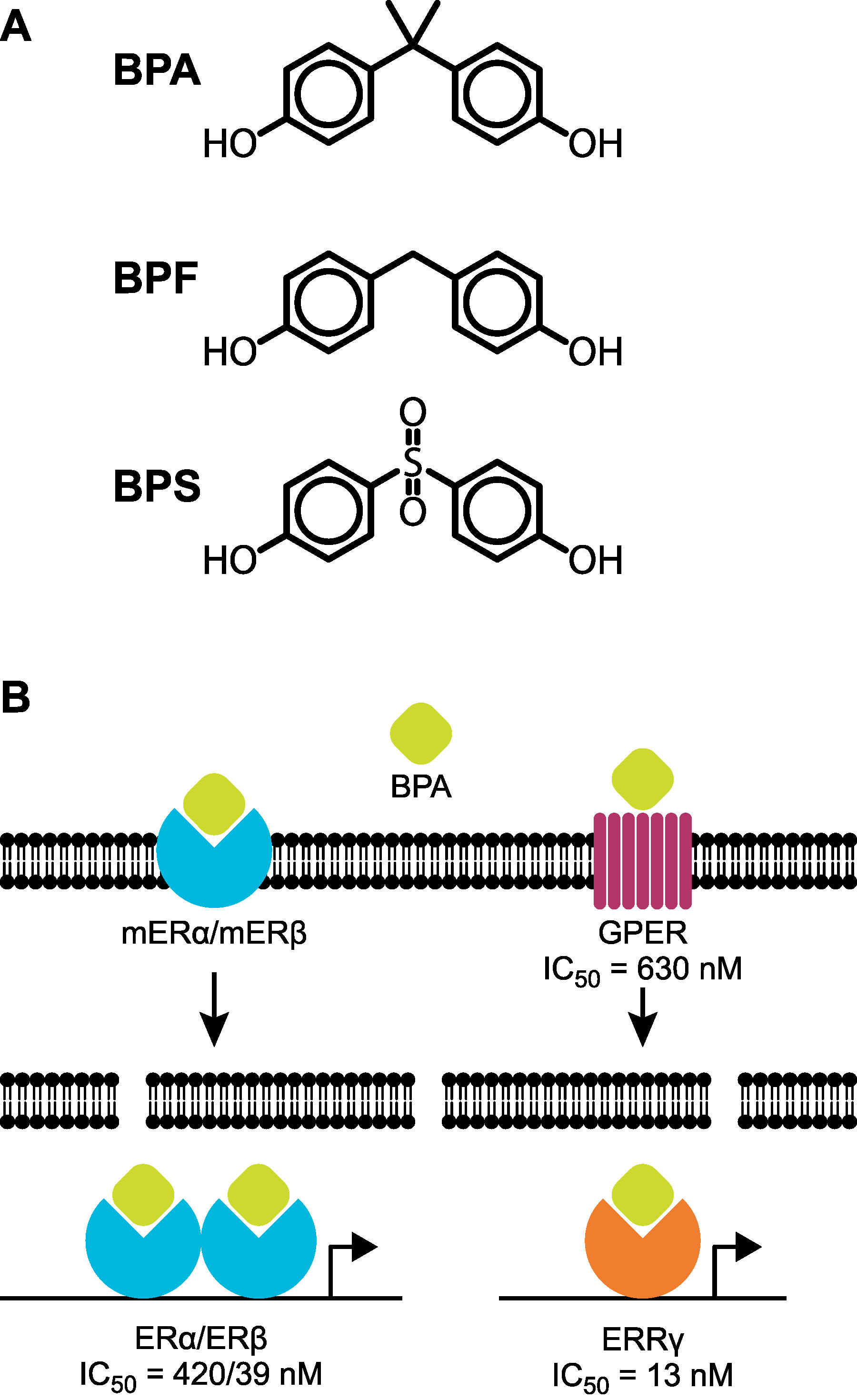
4. Questioning the Safety of BPA Alternatives
5. Regulation of BPA Outside of Canada
6. Canada Needs Revised Assessment around the Toxicity of Bisphenols
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Michałowicz, J. Bisphenol A—Sources, toxicity and biotransformation. Environ. Toxicol. Pharmacol. 2014, 37, 738–758. [Google Scholar] [CrossRef] [PubMed]
- Global Bisphenol A Market Report 2018: Analysis 2013–2017 & Forecasts 2018–2023. Available online: https://www.prnewswire.com/news-releases/global-bisphenol-a-market-report-2018-analysis-2013-2017--forecasts-2018-2023-300757673.html (accessed on 24 November 2020).
- Global Bisphenol-A Market Overview 2016–2022—Market is Projected to Reach US$22.5 Billion by 2022, Up from $15.6 Billion in 2016—Research and Markets. Available online: https://www.prnewswire.com/news-releases/global-bisphenol-a-market-overview-2016-2022---market-is-projected-to-reach-us225-billion-by-2022-up-from-156-billion-in-2016---research-and-markets-300303934.html (accessed on 24 November 2020).
- Bushnik, T.; Haines, D.; Levallois, P.; Levesque, J.; Van Oostdam, J.; Viau, C. Lead and bisphenol A concentrations in the Canadian population. Health Rep. 2010, 21, 7–18. [Google Scholar] [PubMed]
- ARCHIVED—Health Risk Assessment of Bisphenol A from Food Packaging Applications—Canada.ca. Available online: https://www.canada.ca/en/health-canada/services/food-nutrition/food-safety/packaging-materials/bisphenol/health-risk-assessment-bisphenol-food-packaging-applications.html (accessed on 24 November 2020).
- Nagel, S.C.; vom Saal, F.S.; Thayer, K.A.; Dhar, M.G.; Boechler, M.; Welshons, W.V. Relative binding affinity-serum modified access (RBA-SMA) assay predicts the relative in vivo bioactivity of the xenoestrogens bisphenol A and octylphenol. Environ. Health Perspect. 1997, 105, 70–76. [Google Scholar] [CrossRef]
- D’Cruz, S.C.; Jubendradass, R.; Jayakanthan, M.; Rani, S.J.A.; Mathur, P. Bisphenol A impairs insulin signaling and glucose homeostasis and decreases steroidogenesis in rat testis: An in vivo and in silico study. Food Chem. Toxicol. 2012, 50, 1124–1133. [Google Scholar] [CrossRef] [PubMed]
- Villar-Pazos, S.; Martinez-Pinna, J.; Castellano-Muñoz, M.; Magdalena, P.A.; Marroqui, L.; Quesada, I.; Gustafsson, J.-A.; Nadal, A. Molecular mechanisms involved in the non-monotonic effect of bisphenol-a on Ca2+ entry in mouse pancreatic β-cells. Sci. Rep. 2017, 7, 11770. [Google Scholar] [CrossRef]
- Kuiper, G.G.; Carlsson, B.; Grandien, K.; Enmark, E.; Häggblad, J.; Nilsson, S.; Gustafsson, J.A. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology 1997, 138, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Takayanagi, S.; Tokunaga, T.; Liu, X.; Okada, H.; Matsushima, A.; Shimohigashi, Y. Endocrine disruptor bisphenol A strongly binds to human estrogen-related receptor gamma (ERRgamma) with high constitutive activity. Toxicol. Lett. 2006, 167, 95–105. [Google Scholar] [CrossRef]
- Thomas, P.; Dong, J. Binding and activation of the seven-transmembrane estrogen receptor GPR30 by environmental estrogens: A potential novel mechanism of endocrine disruption. J. Steroid Biochem. Mol. Biol. 2006, 102, 175–179. [Google Scholar] [CrossRef]
- Krishnan, A.V.; Stathis, P.; Permuth, S.F.; Tokes, L.; Feldman, D. Bisphenol-A: An estrogenic substance is released from polycarbonate flasks during autoclaving. Endocrinology 1993, 132, 2279–2286. [Google Scholar] [CrossRef]
- Vandenberg, L.N.; Ehrlich, S.; Belcher, S.M.; Ben-Jonathan, N.; Dolinoy, D.C.; Hugo, E.R.; Hunt, P.A.; Newbold, R.R.; Rubin, B.S.; Saili, K.S.; et al. Low dose effects of bisphenol A. Endocr. Disruptors 2013, 1, e26490. [Google Scholar] [CrossRef]
- Peretz, J.; Vrooman, L.; Ricke, W.; Hunt, P.A.; Ehrlich, S.; Hauser, R.; Padmanabhan, V.; Taylor, H.S.; Swan, S.; Vandevoort, C.A.; et al. Bisphenol A and reproductive health: Update of experimental and human evidence, 2007–2013. Environ. Health Perspect. 2014, 122, 775–786. [Google Scholar] [CrossRef]
- Saal, F.S.V.; Vandenberg, L.N. Update on the health effects of bisphenol A: Overwhelming evidence of harm. Endocrinology 2021, 162, 162. [Google Scholar] [CrossRef]
- Perez-Lobato, R.; Mustieles, V.; Calvente, I.; Jimenez-Diaz, I.; Ramos, R.; Caballero-Casero, N.; López-Jiménez, F.; Rubio, S.; Olea, N.; Fernandez, M. Exposure to bisphenol A and behavior in school-age children. NeuroToxicology 2016, 53, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Khalil, N.; Ebert, J.R.; Wang, L.; Belcher, S.; Lee, M.; Czerwinski, S.A.; Kannan, K. Bisphenol A and cardiometabolic risk factors in obese children. Sci. Total. Environ. 2014, 470–471, 726–732. [Google Scholar] [CrossRef]
- Eng, D.S.; Lee, J.M.; Gebremariam, A.; Meeker, J.D.; Peterson, K.; Padmanabhan, V. Bisphenol A and chronic disease risk factors in US children. Pediatrics 2013, 132, e637–e645. [Google Scholar] [CrossRef] [Green Version]
- Muñoz-De-Toro, M.; Markey, C.M.; Wadia, P.R.; Luque, E.H.; Rubin, B.S.; Sonnenschein, C.; Soto, A.M. Perinatal exposure to bisphenol-A alters peripubertal mammary gland development in mice. Endocrinology 2005, 146, 4138–4147. [Google Scholar] [CrossRef]
- Kitamura, S.; Suzuki, T.; Sanoh, S.; Kohta, R.; Jinno, N.; Sugihara, K.; Yoshihara, S.; Fujimoto, N.; Watanabe, H.; Ohta, S. Comparative study of the endocrine-disrupting activity of bisphenol A and 19 related compounds. Toxicol. Sci. 2005, 84, 249–259. [Google Scholar] [CrossRef]
- Alonso-Magdalena, P.; Ropero, A.B.; Soriano, S.; García-Arévalo, M.; Ripoll, C.; Fuentes, E.; Quesada, I.; Nadal, Á. Bisphenol-A acts as a potent estrogen via non-classical estrogen triggered pathways. Mol. Cell. Endocrinol. 2012, 355, 201–207. [Google Scholar] [CrossRef]
- Nadal, A.; Fuentes, E.; Ripoll, C.; Villar-Pazos, S.; Castellano-Muñoz, M.; Soriano, S.; Martinez-Pinna, J.; Quesada, I.; Magdalena, P.A. Extranuclear-initiated estrogenic actions of endocrine disrupting chemicals: Is there toxicology beyond paracelsus? J. Steroid Biochem. Mol. Biol. 2018, 176, 16–22. [Google Scholar] [CrossRef]
- Aris, A. Estimation of bisphenol A (BPA) concentrations in pregnant women, fetuses and nonpregnant women in Eastern Townships of Canada. Reprod. Toxicol. 2014, 45, 8–13. [Google Scholar] [CrossRef]
- Yoshihara, S.; Mizutare, T.; Makishima, M.; Suzuki, N.; Fujimoto, N.; Igarashi, K.; Ohta, S. Potent estrogenic metabolites of bisphenol A and bisphenol B formed by rat liver S9 fraction: Their structures and estrogenic potency. Toxicol. Sci. 2004, 78, 50–59. [Google Scholar] [CrossRef] [Green Version]
- Sohoni, P.; Sumpter, J.P. Several environmental oestrogens are also anti-androgens. J. Endocrinol. 1998, 158, 327–339. [Google Scholar] [CrossRef] [Green Version]
- Moriyama, K.; Tagami, T.; Akamizu, T.; Usui, T.; Saijo, M.; Kanamoto, N.; Hataya, Y.; Shimatsu, A.; Kuzuya, H.; Nakao, K. Thyroid hormone action is disrupted by bisphenol A as an antagonist. J. Clin. Endocrinol. Metab. 2002, 87, 5185–5190. [Google Scholar] [CrossRef]
- Qin, T.; Zhang, X.; Guo, T.; Yang, T.; Gao, Y.; Hao, W.; Xiao, X. Epigenetic alteration shaped by the environmental chemical bisphenol A. Front. Genet. 2021, 11, 618966. [Google Scholar] [CrossRef]
- Farahani, M.; Rezaei-Tavirani, M.; Arjmand, B. A systematic review of microRNA expression studies with exposure to bisphenol A. J. Appl. Toxicol. 2021, 41, 4–19. [Google Scholar] [CrossRef] [PubMed]
- Avissar-Whiting, M.; Veiga, K.R.; Uhl, K.M.; Maccani, M.A.; Gagne, L.A.; Moen, E.L.; Marsit, C.J. Bisphenol A exposure leads to specific microRNA alterations in placental cells. Reprod. Toxicol. 2010, 29, 401–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, G.-Z.; Zhao, Y.; Li, H.-X.; Li, W. Bisphenol A-elicited miR-146a-5p impairs murine testicular steroidogenesis through negative regulation of Mta3 signaling. Biochem. Biophys. Res. Commun. 2018, 501, 478–485. [Google Scholar] [CrossRef]
- Santangeli, S.; Consales, C.; Pacchierotti, F.; Habibi, H.R.; Carnevali, O. Transgenerational effects of BPA on female reproduction. Sci. Total. Environ. 2019, 685, 1294–1305. [Google Scholar] [CrossRef]
- Chioccarelli, T.; Manfrevola, F.; Migliaccio, M.; Altucci, L.; Porreca, V.; Fasano, S.; Cobellis, G. Fetal-Perinatal exposure to bisphenol-A affects quality of spermatozoa in adulthood mouse. Int. J. Endocrinol. 2020, 2020, 2750501. [Google Scholar] [CrossRef]
- Balakrishnan, B.; Henare, K.; Thorstensen, E.B.; Ponnampalam, A.P.; Mitchell, M.D. Transfer of bisphenol A across the human placenta. Am. J. Obstet. Gynecol. 2010, 202, 393.e1–393.e7. [Google Scholar] [CrossRef]
- Matsumoto, J.; Yokota, H.; Yuasa, A. Developmental increases in rat hepatic microsomal UDP-glucuronosyltransferase activities toward xenoestrogens and decreases during pregnancy. Environ. Heal. Perspect. 2002, 110, 193–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bisphenol F Market is Anticipated to Reach at a CAGR of 1.2% Globally with Top Countries Data|Analysis by Trends, Growth and Forecast 2021–2027—The Cowboy Channel. Available online: https://www.thecowboychannel.com/story/43809474/bisphenol-f-market-is-anticipated-to-reach-at-a-cagr-of-12-globally-with-top-countries-data-analysis-by-trends-growth-and-forecast-2021-2027 (accessed on 16 June 2021).
- Global Bisphenol S Market is Forecast to Grow at a CAGR of 11.5% During 2015-2031. Available online: https://www.globenewswire.com/fr/news-release/2021/03/08/2188392/28124/en/Global-Bisphenol-S-Market-is-Forecast-to-Grow-at-a-CAGR-of-11-5-During-2015-2031.html (accessed on 16 June 2021).
- Rochester, J.R.; Bolden, A.L. Bisphenol S and F: A systematic review and comparison of the hormonal activity of bisphenol A substitutes. Environ. Health Perspect. 2015, 123, 643–650. [Google Scholar] [CrossRef]
- Viñas, R.; Watson, C.S. Bisphenol S Disrupts Estradiol-Induced Nongenomic Signaling in a Rat Pituitary Cell Line: Effects on Cell Functions. Environ. Health Perspect. 2013, 121, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Björnsdotter, M.K.; De Boer, J.; Ballesteros-Gómez, A. Bisphenol A and replacements in thermal paper: A review. Chemosphere 2017, 182, 691–706. [Google Scholar] [CrossRef] [PubMed]
- Lehmler, H.-J.; Liu, B.; Gadogbe, M.; Bao, W. Exposure to bisphenol A, bisphenol F, and bisphenol S in U.S. adults and children: The national health and nutrition examination survey 2013–2014. ACS Omega 2018, 3, 6523–6532. [Google Scholar] [CrossRef] [Green Version]
- Serra, H.; Beausoleil, C.; Habert, R.; Minier, C.; Picard-Hagen, N.; Michel, C. Evidence for bisphenol B endocrine properties: Scientific and regulatory perspectives. Environ. Health Perspect. 2019, 127, 106001. [Google Scholar] [CrossRef]
- Cao, L.; Ren, X.-M.; Liang-Hong, G.; Zhang, J.; Qin, W.-P.; Yang, Y.; Wan, B.; Guo, L.-H. Bisphenol AF and bisphenol B exert higher estrogenic effects than bisphenol A via G protein-coupled estrogen receptor pathway. Environ. Sci. Technol. 2017, 51, 11423–11430. [Google Scholar] [CrossRef]
- Moreman, J.; Lee, O.; Trznadel, M.; David, A.; Kudoh, T.; Tyler, C.R. Acute toxicity, teratogenic, and estrogenic effects of bisphenol A and its alternative replacements bisphenol S, bisphenol F, and bisphenol AF in Zebrafish embryo-larvae. Environ. Sci. Technol. 2017, 51, 12796–12805. [Google Scholar] [CrossRef]
- Gély, C.A.; Lacroix, M.Z.; Morin, M.; Vayssière, C.; Gayrard, V.; Picard-Hagen, N. Comparison of the materno-fetal transfer of fifteen structurally related bisphenol analogues using an ex vivo human placental perfusion model. Chemosphere 2021, 276, 130213. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF). Scientific Opinion on the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs. EFSA J. 2015, 13, 3978. [Google Scholar] [CrossRef]
- CLARITY-BPA Program. Available online: https://ntp.niehs.nih.gov/whatwestudy/topics/bpa/index.html (accessed on 15 March 2021).
- National Toxicology Program. NTP Research Report on the CLARITY-BPA Core Study: A Perinatal and Chronic Extended-Dose-Range Study of Bisphenol A in Rats; NTP Research Reports; National Toxicology Program, Research Triangle Park (NC): Triangle Park, NC, USA, 2018. [Google Scholar]
- Schug, T.T.; Heindel, J.J.; Camacho, L.; Delclos, K.B.; Howard, P.; Johnson, A.F.; Aungst, J.; Keefe, D.; Newbold, R.; Walker, N.J.; et al. A new approach to synergize academic and guideline-compliant research: The CLARITY-BPA research program. Reprod. Toxicol. 2013, 40, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Vandenberg, L.N.; Hunt, P.A.; Gore, A.C. Endocrine disruptors and the future of toxicology testing—Lessons from CLARITY–BPA. Nat. Rev. Endocrinol. 2019, 15, 366–374. [Google Scholar] [CrossRef]
- Cao, J.; Rebuli, M.; Rogers, J.; Todd, K.L.; Leyrer, S.M.; Ferguson, S.; Patisaul, H.B. Prenatal bisphenol A exposure alters sex-specific estrogen receptor expression in the neonatal rat hypothalamus and amygdala. Toxicol. Sci. 2013, 133, 157–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vandenberg, L.N.; Colborn, T.; Hayes, T.B.; Heindel, J.J.; Jacobs, D.R., Jr.; Lee, D.H.; Shioda, T.; Soto, A.M.; vom Saal, F.S.; Welshons, W.V.; et al. Hormones and endocrine-disrupting chemicals: Low-Dose effects and nonmonotonic dose responses. Endocr. Rev. 2012, 33, 378–455. [Google Scholar] [CrossRef] [PubMed]
- Katchy, A.; Pinto, C.; Jonsson, P.; Nguyen-Vu, T.; Pandelova, M.; Riu, A.; Schramm, K.-W.; Samarov, D.; Gustafsson, J.-Å.; Bondesson, M.; et al. Coexposure to phytoestrogens and bisphenol a mimics estrogenic effects in an additive manner. Toxicol. Sci. 2013, 138, 21–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aichinger, G.; Pantazi, F.; Marko, D. Combinatory estrogenic effects of bisphenol A in mixtures with alternariol and zearalenone in human endometrial cells. Toxicol. Lett. 2020, 319, 242–249. [Google Scholar] [CrossRef]
- Committee Report No. 8—ENVI (42-1)—House of Commons of Canada. Available online: https://www.ourcommons.ca/DocumentViewer/en/42-1/ENVI/report-8 (accessed on 21 April 2021).
- Technical Consultation: Proposed Subgrouping of Bisphenol A (BPA) Structural Analogues and Functional Alternatives—Canada.ca. Available online: https://www.canada.ca/en/environment-climate-change/services/evaluating-existing-substances/technical-consultation-proposed-subgrouping-bpa-structural-analogues-functional-alternatives.html (accessed on 21 April 2021).

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rogers, L.D. What Does CLARITY-BPA Mean for Canadians? Int. J. Environ. Res. Public Health 2021, 18, 7001. https://doi.org/10.3390/ijerph18137001
Rogers LD. What Does CLARITY-BPA Mean for Canadians? International Journal of Environmental Research and Public Health. 2021; 18(13):7001. https://doi.org/10.3390/ijerph18137001
Chicago/Turabian StyleRogers, Lindsay D. 2021. "What Does CLARITY-BPA Mean for Canadians?" International Journal of Environmental Research and Public Health 18, no. 13: 7001. https://doi.org/10.3390/ijerph18137001
APA StyleRogers, L. D. (2021). What Does CLARITY-BPA Mean for Canadians? International Journal of Environmental Research and Public Health, 18(13), 7001. https://doi.org/10.3390/ijerph18137001




