Impact of Climate Change on Eye Diseases and Associated Economical Costs
Abstract
:1. Introduction
2. Methods
2.1. Cornea, Sclera and Conjunctive
- (A).
- Increasing inflammation: Allergic Keratoconjunctivitis, Marginal Keratitis, Dry Keratitis, Chronic Episcleritis, Corneal Metaplasia, and Pterygium;
- (B).
- Increasing infections and superinfections: Corneal and Conjunctival Herpes simplex and Herpes Zoster, Viral Keratoconjunctivitis, Accidental Corneal Fungal Injuries, Infectious Corneal Injuries, non-fungal injury by Contact Lens;
- (C).
- Tumor processes in Cornea, Conjunctive and Ocular Annexes: Epidermoid Neoplasia of Ocular Surface, Basal Cell Carcinoma of Eyelid.
2.2. Glaucoma
- (D).
- Acute Glaucoma;
- (E).
- Chronic open-angle Glaucoma.
2.3. Cataracts
- (F).
- Early Cortical and Subcapsular Cataracts;
- (G).
- Pseudo-exfoliation syndrome.
2.4. Tumor Processes in the Choroid, Iris and Ciliary Body
2.5. Uveitis (Intraocular Inflammatory Processes)
- (I).
- Infectious uveitis: Toxoplasmosis, Tuberculosis, Campylobacter, Chlamydia, Ocular Herpes simplex (VHS) and Zoster (VHZ), West Nile Fever, Borreliosis and Rickettsiosis, Shigellosis, Salmonellosis; the World Health Organization offers information about several pathogens and their infectivity [62,63], including those which are engaged in ocular diseases; a higher frequency of infectious uveitis has been highlighted during the summer [64], plus a change in patterns, incidence and prevalence [65].
- (J).
- Non-infectious uveitis associated with systemic diseases: Rheumatoid arthritis (RA), Ankylosing spondylitis (AS), Sarcoidosis, Multiple Sclerosis (MS), Inflammatory bowel diseases (IBD—Crohn and ulcer colitis), Behçet’s disease, Giant cells arteritis (Horton’s disease), Necrotizing systemic vasculitis.
- (K).
- Connective tissue diseases: Systemic Lupus Erythematosus (SLE), Dermatomyositis.
- (L).
- Uveitis merely as ocular disease, without associated systemic pathologies: Fuchs’ heterochronic Uveitis, Posner-Schlossmann syndrome, Intermedia uveitis and pars planitis, Birdshot choroidopathy, Vogt-Koyanagi-Harada syndrome (VKH), White Dots Syndromes [66].
2.6. Retina
- (M).
- Tractional Retinal Detachment and Retinal Tears.
- (N).
- Posterior Vitreous Detachment.
- (O).
- Age Macular Degeneration (AMD).
- (P).
- Central Serous Choroidopathy.
3. Results
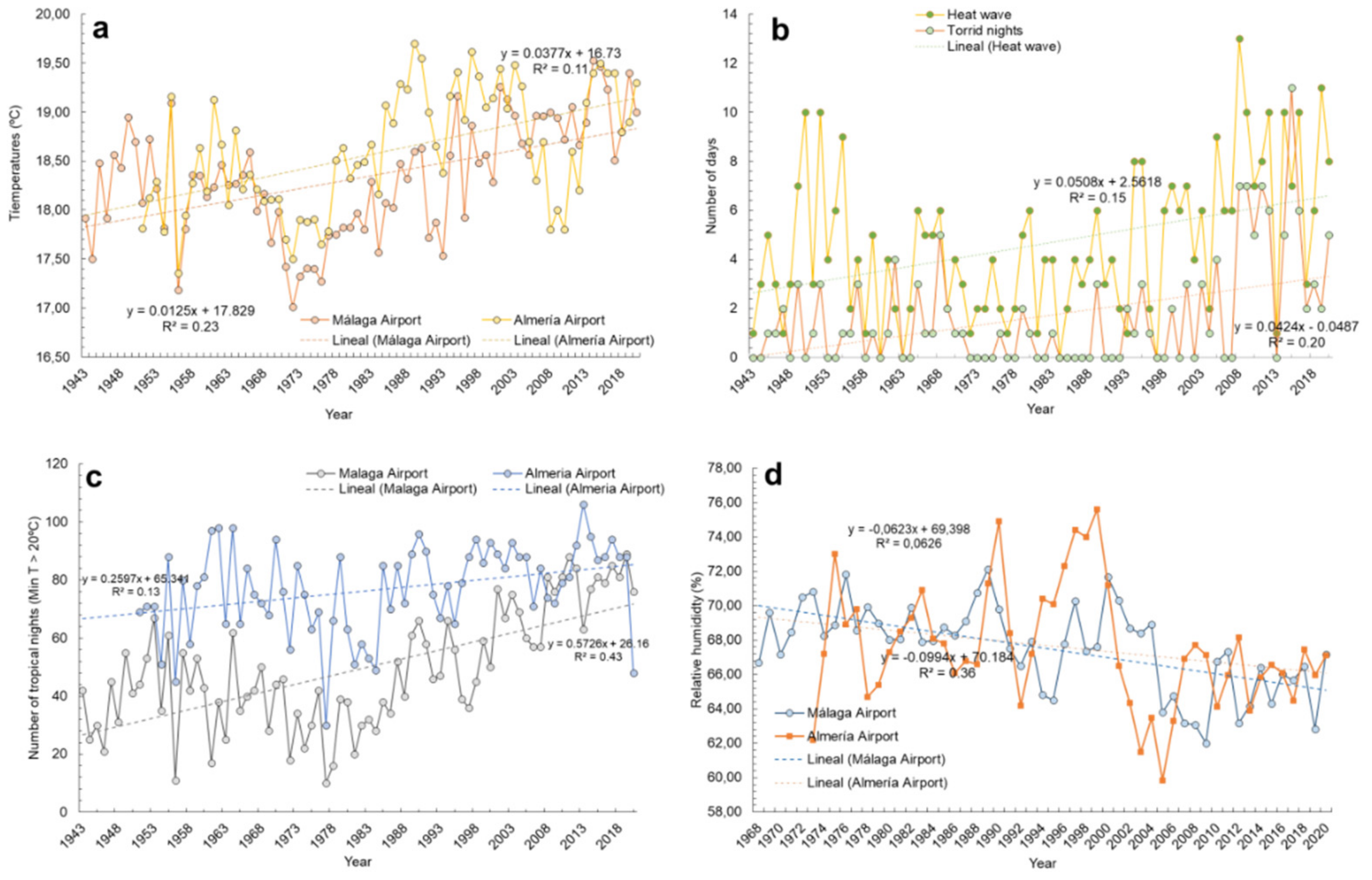
3.1. Background of Climate Conditions in Almería and Málaga
3.2. Ocular Pathologies Related to Climate Variables
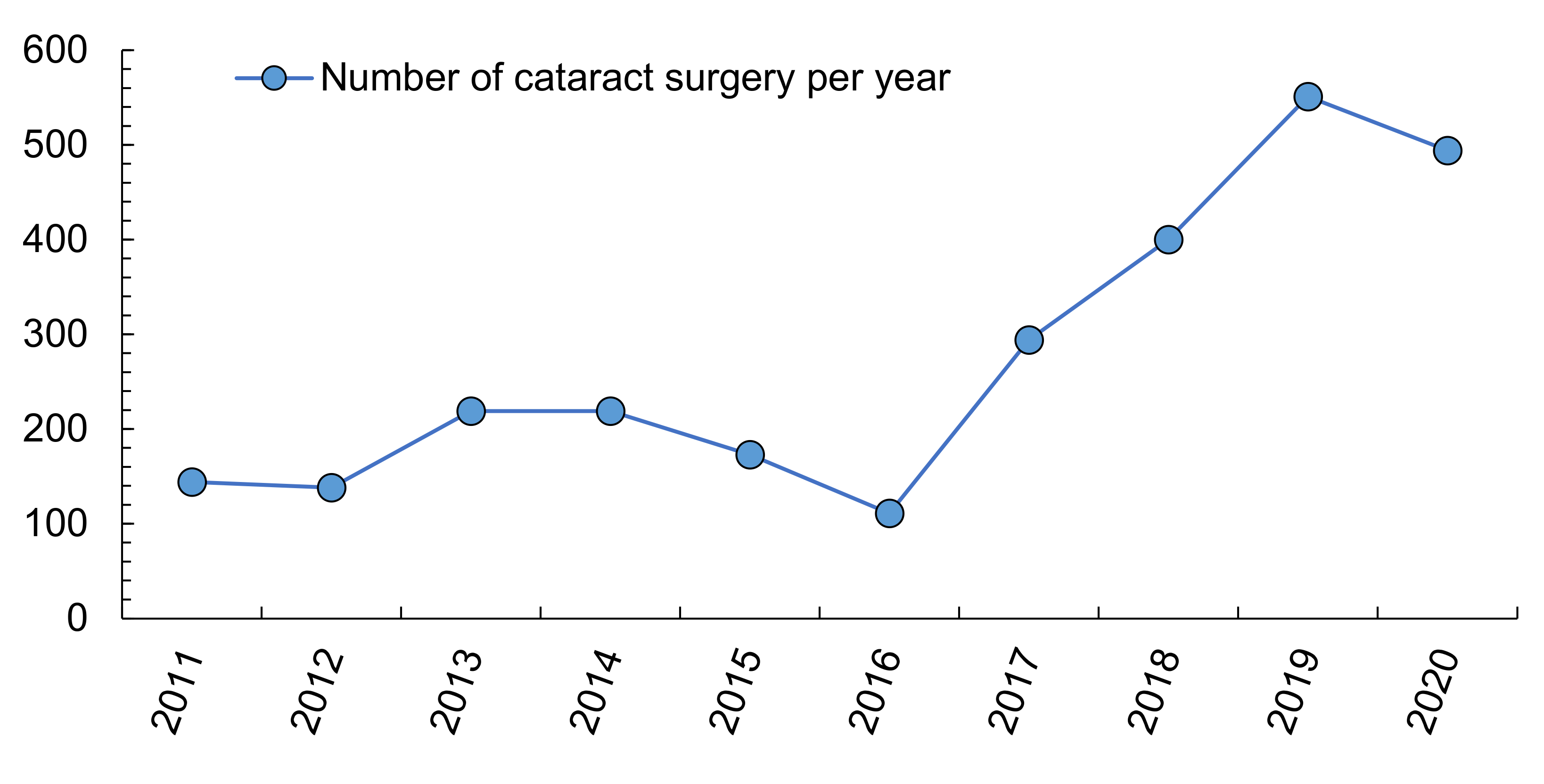
3.3. Estimation of the Health Costs of the Most Frequent Ocular Pathologies Related to Climate Change in Southern Spain
- (a)
- In Rheumatoid Arthritis (RA), genetic factors explain <50% of the risk of developing this disease; however, a 31% increase in risk has been identified in people living within <50 m from the main road (with traffic and, therefore, intense pollution) [107]. In Spain alone, RA accounted for a €1.12 billion/year burden of necessary health care, indirect costs and associated sick leave [108]. Mitigating the pollutant factor could save the Spanish State 350 million (M) euros, as well as undoubtedly reducing the suffering caused by this type of disease.
- (b)
- In Ankylosing Spondylitis (AS), a >60% correlation with pollutant particles (PM2.5) has been found: a prolonged exposure leads to worsening in the control of the inflammatory outbreak of this disease [109]. An average cost to address AS in Spain of 11,462 euros/patient/year has been estimated, including direct costs and productivity losses [110]. In 2017, 1.9% of Spanish people suffered from this disease (900,000 people), implying an annual average cost of €10.3 billion of the Spanish Gross Domestic Product (GDP). Such a cost could be reduced by €6 billion just by controlling environmental factors.
- (c)
- Pollutants determine an association with inflammatory outbreaks of Multiple Sclerosis (MS). Thus, PM10 particles were 8% associated with an increase of relapse during the cold season, whilst ozone was 16% associated during the hot season (therefore, ozone is pathogenically triggered by high temperatures) [111,112]. Around 50,000 people in Spain suffer from this disease, whose average cost is 30,000 euros/patient/year; this implies a global burden of €1.4 billion/year [113], added to the severe disability which it involves. 112 to 224 million euros/year could be saved by controlling pollutants alone, without taking into account the benefit to be gained by controlling high temperatures and, thus, avoiding the exacerbation suffered by MS patients (Uhthoff phenomenon) [112,114].
- (d)
- Inflammatory Bowel Diseases (IBD) include Crohn’s disease (CD), Ulcerative Colitis (UC) and Non-classifiable IBD. Their estimated prevalence is 0.3% of the European population, showing an increasing incidence of 176,000 cases per year. It has been calculated that 2.5–3 million people in Europe suffer from IBD. This implies a very high total annual cost of health care. Based on an average of diagnosed patients in 2010 throughout 28 European medical centers during the first year after diagnosis, a total cost of 5942 euros/patient for CD, 2753 euros/patient for UC, and 2898 euros/patient for non-classifiable IBD was calculated. Furthermore, each day of heatwave increases by 4.6% the outbreak risk for IBD [115,116], and thus the derived visual impairment. For example, Málaga city (Southern Spain) suffered 11 heatwave days only in 2019; therefore, the outbreak risk of IBD was increased by 50.6%, adding to the high health care burden and personal suffering which this implies.
- (e)
- The prevalence of Systemic Lupus Erythematosus (SLE) in Spain is 9/10,000 inhabitants [117]. The patient/year cost ranges between 3604 and 5968 euros, according to the severity [118]. Relating to prevalence and cost per patient, the control of this disease involves a €201 million burden in Spain. Concerning environmental parameters, SLE outbreaks are twice as likely to occur in sun-exposed workers fin a year, and 7.9 times more likely to develop if they suffer sunburn from intense exposure to the sun [119].
- (f)
- Sarcoidosis is a disease with high prevalence in Spain (1/1000 inhabitants) [120]. Although there is no information about the cost of its treatment, data from the USA points out that insurance companies spend 19,714 dollars/patient/year among direct costs of medical attention and secondary costs because of sickness absences [121]. Extrapolating this data to Spain, the disease costs would be equivalent to almost €784 million/year. The relation between Sarcoidosis and environmental factors is essential, given that its pathogenesis is characterized by exposure to dust and both natural and urban pollution (PM10 and PM2.5), together with dryness and high temperatures [122]. Southern Spain records more than 20 events per year with Saharan dust advection from May to September [123]. There are no numerical studies on the evolution of this disease in Spain, but researches from the Midwest of the USA (where dust storms are frequent) reveal that the prevalence doubled between 1995 and 2010, without a relationship to an increase in the population [124]. Extrapolating these data to Spain, a higher frequency of dust storms related to higher dryness and advection from Africa could involve doubling of the health care costs related to Sarcoidosis.
- (1)
- Because of the sustained increase of CO2 concentration in Spain [126]
- (2)
- The trend in Andalucía has been practically stable since 2008, whilst the average of Spain reveals a clear decrease, showing values below the average of Andalucía [127]
- (3)
- In Andalucía during the last decade, the WHO criteria have been permanently broken (>100 µg/m3); there is no aggregated evolutionary data [128]
- (4)
- Considering just the costs of afforestation-reforestation and GG control, and ignoring the benefits of energy-saving
4. Discussion. Challenges and Topics to Be Further Discussed
- (1)
- Increasing the capacity of the carbon sink by means of afforestation and reforestation. From 1990 to 2020, the world lost 420 million (M) hectares (ha) of forest area; 80 M ha of these corresponded to primary woods [137]. Andalucía is one of the three Spanish communities which are developing an emission compensation system using forest projects [138]. The economic cost for one of these projects is 229,351.5 euros for 63.42 ha, and it is expected to capture 16,653 tCO2 in 30 years, that is, to assimilate 262.6 tCO2 per forestry hectare. According to the actual GG emission in Andalucía, where there was an annual production of 51 M tons CO2eq in 2015, it would be necessary to invest €702.27 M to offset through forestry works the GG emissions, and afforest or reforest 194,211.7 ha. Obviously, current efforts are insufficient.
- (2)
- Avoiding ocean acidification. In Andalucía, to avoid the acidification of the sea, and to control the waste of plastics, emissions of nitrogen fertilizers and untreated wastewater, it is essential to control overfishing and highly destructive fisheries to prevent the destruction of carbon sinks [55]. Furthermore, to stabilize emissions at around 450 ppm of CO2eq (recommended by IPCC), it would be necessary to reduce the annualized consumption growth rate by 0.06% per year during the 21st century [56]. While Global GDP (2019) is $87,698 billion [139], the efforts to achieve stabilization would be equivalent to $5.26 billion/year. It has been calculated that Spain would have to reduce its GDP by 1% by 2050 to comply with the limits set by the Kyoto Protocol, i.e., some 12.45 billion euros over 30 years [140].
- (3)
- Promoting the transition of the actual energy generation (electricity) to systems of low carbon emissions. In Spain, the Ministry for Ecological Transition has proposed that 70% of the electric system in 2030 came from renewable energies. Investments in this sector, energy savings and renewable energies (which are cheaper) will allow the GDP to grow by 1.8% by 2030 compared to a scenario without actions: between €16.5 and €25.7 billion [141]. This figure compensates for the reduction in GDP indicated in the previous item.
- (4)
- Increasing building insulation. Global warming makes predictable an increase in energy consumption for cooling buildings and homes. Current energy-efficient systems allow a building with solar protection, efficient ventilation and an insulated façade to save 38% in heating and 52% in cooling [142]. It is expected that the world energy consumption increase will average 57% between 2004 and 2030 [143]. Therefore, energy efficiency and near-zero energy homes will be essential because they have no additional costs [144].
- (5)
- Ensuring that new buildings use more natural air and sunlight. Accordingly, the efficient design of buildings through ventilation with heat recovery, building skin insulation without thermal bridges and dynamic solar control using a blind system (passive house model), would obtain a fairly constant temperature throughout the year, without exceeding 25 °C in summer, i.e., higher energy efficiency [142].
- (6)
- (7)
- Stop playing with Nature [147]. As mentioned above, environmental intervention due to inadequate land management, often because of lack of knowledge, produces irreversible ecological cascades which, increasingly common, affect human beings tragically [148]. One probable origin of Covid19 disease is the uncontrolled alteration of ecosystems (contact with wild animals such as the bat Rhinolophus affinis, or the Malaysian pangolin, Manis javanica) [149] and its triggering due to pollution [150]. Other unfortunate examples of human interventions were the myxomatosis virus [151] and HIV, possibly from apes [152].
5. Conclusions and Implications
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statements
Conflicts of Interest
References
- Smith, K.R.; Woodward, A. Human Health: Impacts, Adaptation, and Co-Benefits—IPCC. 2014. Available online: https://www.ipcc.ch/report/ar5/wg2/human-health-impacts-adaptation-and-co-benefits/ (accessed on 8 April 2020).
- Christidis, N.; Stott, P.A.; Jones, G.S.; Shiogama, H.; Nozawa, T.; Luterbacher, J. Human activity and anomalously warm seasons in Europe. Int. J. Clim. 2010, 32, 225–239. [Google Scholar] [CrossRef]
- Fouillet, A.; Rey, G.; Wagner, V.; Laaidi, K.; Empereur-Bissonnet, P.; Le Tertre, A.; Frayssinet, P.; Bessemoulin, P.; Laurent, F.; De Crouy-Chanel, P.; et al. Has the impact of heat waves on mortality changed in France since the European heat wave of summer 2003? A study of the 2006 heat wave. Int. J. Epidemiol. 2008, 37, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Johnston, F.H.; Henderson, S.B.; Chen, Y.; Randerson, J.T.; Marlier, M.; DeFries, R.S.; Kinney, P.; Bowman, D.M.; Brauer, M. Estimated Global Mortality Attributable to Smoke from Landscape Fires. Environ. Health Perspect. 2012, 120, 695–701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rice, M.B.; Thurston, G.D.; Balmes, J.R.; Pinkerton, K.E. Climate Change. A Global Threat to Cardiopulmonary Health. Am. J. Respir. Crit. Care Med. 2014, 189, 512–519. [Google Scholar] [CrossRef] [Green Version]
- Tsai, M.-J.; Hsu, Y.-L.; Wu, K.-Y.; Yang, R.-C.; Chen, Y.-J.; Yu, H.-S.; Kuo, P.-L. Heat Effect Induces Production of Inflammatory Cytokines Through Heat Shock Protein 90 Pathway in Cornea Cells. Curr. Eye Res. 2013, 38, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Ludema, C.; Cole, S.R.; Poole, C.; Smith, J.S.; Schoenbach, V.J.; Wilhelmus, K.R. Association between unprotected ultraviolet radiation exposure and recurrence of ocular herpes simplex virus. Am. J. Epidemiol. 2013, 179, 208–215. [Google Scholar] [CrossRef] [Green Version]
- Kawai, K.; VoPham, T.; Drucker, A.; Curhan, S.G.; Curhan, G.C. Ultraviolet Radiation Exposure and the Risk of Herpes Zoster in Three Prospective Cohort Studies. Mayo Clin. Proc. 2020, 95, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Van der Leun, J.C.; Piacentini, R.D.; de Gruijl, F.R. Climate change and human skin cancer. Photochem. Photobiol. Sci. 2008, 7, 730–733. [Google Scholar] [CrossRef]
- Aragonés Cruz, B.; Alemañy Martorell, J. Relación de la radiación ultravioleta y el pterigión primario. Rev. Cuba. Oftalmol. 2009, 22. [Google Scholar]
- Caspi, R.R. A look at autoimmunity and inflammation in the eye. J. Clin. Investig. 2010, 120, 3073–3083. [Google Scholar] [CrossRef] [Green Version]
- Cullen, A.P. Ozone Depletion and Solar Ultraviolet Radiation: Ocular Effects, a United Nations Environment Programme Perspective. Eye Contact Lens Sci. Clin. Pract. 2011, 37, 185–190. [Google Scholar] [CrossRef]
- Consejería de Salud. Junta de Andalucia. Informe Inicial Sobre Adaptación al Cambio Climático en el Ámbito de Salud. 2012. Available online: http://www.juntadeandalucia.es/medioambiente/portal_web/web/temas_ambientales/clima/actuaciones_cambio_climatico/adaptacion/vulnerabilidad_impactos_medidas/isis/isi_salud.pdf (accessed on 24 May 2021).
- Yam, J.C.S.; Kwok, A.K.H. Ultraviolet light and ocular diseases. Int. Ophthalmol. 2014, 34, 383–400. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Carcinogenic Risk to Humans. Solar and Ultraviolet Radiation. Available online: https://www.ncbi.nlm.nih.gov/books/NBK304366/ (accessed on 13 April 2020).
- Auger, N.; Rhéaume, M.-A.; Bilodeau-Bertrand, M.; Tang, T.; Kosatsky, T. Climate and the eye: Case-crossover analysis of retinal detachment after exposure to ambient heat. Environ. Res. 2017, 157, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Barcena-Martin, E.; Molina, J.; Ruiz-Sinoga, J.D. Issues and challenges in defining a heat wave: A Mediterranean case study. Int. J. Clim. 2019, 39, 331–342. [Google Scholar] [CrossRef] [Green Version]
- Medina Martín, F. Impactos, Vulnerabilidad y Adaptación al Cambio Climático en el Sector Agrario. Aproximación al Conocimiento y Prácticas de Gestión en España. 2016. Available online: https://www.miteco.gob.es/es/cambio-climatico/temas/impactos-vulnerabilidad-y-adaptacion/impactos_vulnerabilidad_adaptacion_cambio_climatico_sector_agrario__tcm30-178448.pdf (accessed on 24 May 2021).
- Bárcena-Martín, E.; Molina, J.; Hueso, P.; Ruiz-Sinoga, J.D. A Class of Indices and a Graphical Tool to Monitor Temperature Anomalies. Air Soil Water Res. 2020, 13, 1–11. [Google Scholar] [CrossRef]
- Sinoga, J.D.R.; Marin, R.G.; Murillo, J.F.M.; Galeote, M.A.G. Precipitation dynamics in southern Spain: Trends and cycles. Int. J. Clim. 2010, 31, 2281–2289. [Google Scholar] [CrossRef]
- Senciales-González, J.M.; Ruiz Sinoga, J. Análisis espacio-temporal de las lluvias torrenciales en la ciudad de Málaga. Bol. Asoc. Geógrafos Españoles 2013, 61, 7–24. [Google Scholar] [CrossRef]
- Polgreen, P.M.; Polgreen, E.L. Infectious Diseases, Weather, and Climate. Clin. Infect. Dis. 2017, 66, 815–817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burton, M.J. Trachoma: An overview. Br. Med. Bull. 2007, 84, 99–116. [Google Scholar] [CrossRef] [Green Version]
- Last, A.; Versteeg, B.; Abdurahman, O.S.; Robinson, A.; Dumessa, G.; Aga, M.A.; Bejiga, G.S.; Negussu, N.; Greenland, K.; Czerniewska, A.; et al. Detecting extra-ocular Chlamydia trachomatis in a trachoma-endemic community in Ethiopia: Identifying potential routes of transmission. PLoS Negl. Trop. Dis. 2020, 14, e0008120. [Google Scholar] [CrossRef]
- Angelini, P.; Macini, P.; Finarelli, A.C.; Pol, C.; Venturelli, C.; Bellini, R.; Dottori, M. Chikungunya epidemic outbreak in Emilia-Romagna (Italy) during summer 2007. Parasitologia 2008, 50, 97–98. [Google Scholar]
- Bai, L.; Morton, L.; Liu, Q. Climate change and mosquito-borne diseases in China: A review. Glob. Health 2013, 9, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caminade, C.; Ndione, J.-A.; Kebe, C.M.F.; Jones, A.E.; Danuor, S.K.; Tay, S.; Tourre, Y.M.; Lacaux, J.-P.; Vignolles, C.; Duchemin, J.B.; et al. Mapping Rift Valley fever and malaria risk over West Africa using climatic indicators. Atmos. Sci. Lett. 2010, 12, 96–103. [Google Scholar] [CrossRef]
- Anyamba, A.; Linthicum, K.J.; Small, J.L.; Collins, K.M.; Tucker, C.J.; Pak, E.W.; Britch, S.C.; Eastman, J.R.; Pinzon, J.E.; Russell, K.L. Climate Teleconnections and Recent Patterns of Human and Animal Disease Outbreaks. PLoS Negl. Trop. Dis. 2012, 6, e1465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben Yahia, S.; Khairallah, M. Ocular Manifestations of West Nile Virus Infection. Int. J. Med. Sci. 2009, 6, 114–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ECDC. Weekly Updates: 2020 West Nile Virus Transmission Season. 2020. Available online: https://www.ecdc.europa.eu/en/west-nile-fever/surveillance-and-disease-data/disease-data-ecdc (accessed on 2 October 2020).
- Madsen, L.M. How Global Warming May Affect the Prevalence of Lyme Disease. 2019. Available online: https://www.vetfolio.com/learn/article/how-global-warming-may-affect-the-prevalence-of-lyme-disease (accessed on 13 April 2020).
- Yip, V.C.-H.; Sanjay, S.; Koh, Y.T. Ophthalmic Complications of Dengue Fever: A Systematic Review. Ophthalmol. Ther. 2012, 1, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Mahendradas, P.; Avadhani, K.; Shetty, R. Chikungunya and the eye: A review. J. Ophthalmic Inflamm. Infect. 2013, 3, 35. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, D.; Basu, A. Japanese Encephalitis—A Pathological and Clinical Perspective. PLoS Negl. Trop. Dis. 2009, 3, e437. [Google Scholar] [CrossRef] [Green Version]
- Al-Hazmi, A.; Al-Rajhi, A.A.; Abboud, E.B.; Ayoola, E.A.; Al-Hazmi, M.; Saadi, R.; Ahmed, N. Ocular complications of Rift Valley fever outbreak in Saudi Arabia. Ophthalmology 2005, 112, 313–318. [Google Scholar] [CrossRef]
- Bell, M.L.; Peng, R.D.; Dominici, F. The Exposure–Response Curve for Ozone and Risk of Mortality and the Adequacy of Current Ozone Regulations. Environ. Health Perspect. 2006, 114, 532–536. [Google Scholar] [CrossRef] [Green Version]
- Dear, K.; Ranmuthugala, G.; Kjellström, T.; Skinner, C.; Hanigan, I. Effects of Temperature and Ozone on Daily Mortality During the August 2003 Heat Wave in France. Arch. Environ. Occup. Health 2005, 60, 205–212. [Google Scholar] [CrossRef]
- Ren, C.; Williams, G.; Morawska, L.; Mengersen, K.; Tong, S. Ozone modifies associations between temperature and cardiovascular mortality: Analysis of the NMMAPS data. Occup. Environ. Med. 2008, 65, 255–260. [Google Scholar] [CrossRef] [Green Version]
- Rodrigo-Comino, J.; Senciales-González, J.M. A Regional Geography Approach to Understanding the Environmental Changes as a Consequence of the COVID-19 Lockdown in Highly Populated Spanish Cities. Appl. Sci. 2021, 11, 2912. [Google Scholar] [CrossRef]
- Beggs, P.J. Adaptation to Impacts of Climate Change on Aeroallergens and Allergic Respiratory Diseases. Int. J. Environ. Res. Public Health 2010, 7, 3006–3021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saxena, R.; Srivastava, S.; Trivedi, D.; Anand, E.; Joshi, S.; Gupta, S.K. Impact of environmental pollution on the eye. Acta Ophthalmol. Scand. 2003, 81, 491–494. [Google Scholar] [CrossRef]
- Shubhrica, D. Effect of Environment on Eyes: A Review. Indian J. Clin. Pract. 2013, 24, 381–384. [Google Scholar]
- Gupta, P.; Muthukumar, A. Minor to Chronic Eye Disorders Due to Environmental Pollution: A review. J. Ocul. Infect. Inflamm. 2018, 2, 2. [Google Scholar]
- Knox, J.; Hess, T.; Daccache, A.; Wheeler, T. Climate change impacts on crop productivity in Africa and South Asia. Environ. Res. Lett. 2012, 7. [Google Scholar] [CrossRef]
- Brevik, E.C.; Oliver, M.; Zhao, F.-J. Special section on soil and human health—An editorial. Eur. J. Soil Sci. 2019, 70, 859–861. [Google Scholar] [CrossRef] [Green Version]
- Müller, O.; Krawinkel, M. Malnutrition and health in developing countries. Can. Med. Assoc. J. 2005, 173, 279–286. [Google Scholar] [CrossRef] [Green Version]
- Kraemer, K.; Gilbert, C. Do vitamin A deficiency and undernutrition still matter? Community Eye Health 2013, 26, 61–63. [Google Scholar] [PubMed]
- Chandran, R.; Consultant, N.; Gedam, D.S. Ocular manifestations of childhood malnutrition- an overview. Int. J. Med. Res. Rev. 2017, 5, 925–926. [Google Scholar] [CrossRef]
- Dantas, A.P.; Brandt, C.T.; Leal, D.N.B. Manifestações oculares em pacientes que tiveram desnutrição nos primeiros seis meses de vida. Arq. Bras. Oftalmol. 2005, 68, 753–756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-Araque, A.; Aranda, A.G.; Pardo, C.L.; Aragüés, A.R. Los antioxidantes en el proceso de patologías oculares. Nutr. Hosp. 2017, 34, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Sahu, S.; Sett, M.; Kjellstrom, T. Heat Exposure, Cardiovascular Stress and Work Productivity in Rice Harvesters in India: Implications for a Climate Change Future. Ind. Health 2013, 51, 424–431. [Google Scholar] [CrossRef] [Green Version]
- Jovanovic, N.; Peek-Asa, C.; Swanton, A.; Young, T.; Alajbegovic-Halimic, J.; Cavaljuga, S.; Nisic, F. Prevalence and risk factors associated with work-related eye injuries in Bosnia and Herzegovina. Int. J. Occup. Environ. Health 2016, 22, 325–332. [Google Scholar] [CrossRef] [Green Version]
- INE. Available online: https://www.ine.es/jaxiT3/Datos.htm?t=2911 (accessed on 24 May 2021).
- Oppenheimer, M.; Campos, M.; Warren, R.; Birkmann, J.; Luber, G.; O’Neill, B.; Takahashi, K. Emergent risks and key vulnerabilities. In Climate Change 2014: Impacts, Adaptation, and Vulnerability. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Field, C.B., Barros, V.R., Dokken, D.J., Mach, K.J., Mastrandrea, M.D., Bilir, T.E., Chatterjee, M., Ebi, K.L., Estrada, Y.O., Genova, R.C., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014; pp. 1039–1099. [Google Scholar]
- PCC. AR5 Climate Change 2013: The Physical Science Basis—IPCC. 2013. Available online: https://www.ipcc.ch/report/ar5/wg1/ (accessed on 16 April 2020).
- Pachauri, R.K.; Mayer, L. (Eds.) Climate Change 2014: Synthesis Report; Intergovernmental Panel on Climate Change: Geneva, Switzerland, 2015. [Google Scholar]
- Bowling, B. Oftalmología Clínica. Un Enfoque Sistemático; Elsevier Masson: Paris, France, 2016; Available online: https://www.iberlibro.com/KANSKI-OFTALMOLOG%C3%8DA-CL%C3%8DNICA-B-BOWLING-ELSEVIER/20642079458/bd (accessed on 29 April 2020).
- Gómez-Ulla de Irazazábal, F.; Ondategui Parra, S. Informe Sobre la Ceguera en España. 2009. Available online: https://www.seeof.es/archivos/articulos/adjunto_20_1.pdf (accessed on 23 January 2021).
- Díaz-Llopis, M.; Calonge, M.; Sáinz de la Maza, M.T.; Benítez del Castillo, J.M.; Gallego Pinazo, R.; Arévalo, F. Uveítis y escleritis. Diagnóstico y tratamiento. Available online: https://www.oftalmoseo.com/libros_seo/ponencia_seo/uveitis-y-escleritis-diagnostico-y-tratamiento/ (accessed on 18 May 2021).
- Gichuhi, S.; Macharia, E.; Kabiru, J.; Zindamoyen, A.M.; Rono, H.; Ollando, E.; Wachira, J.; Munene, R.; Maina, J.; Onyuma, T.; et al. Topical fluorouracil after surgery for ocular surface squamous neoplasia in Kenya: A randomised, double-blind, placebo-controlled trial. Lancet Glob. Health 2016, 4, e378–e385. [Google Scholar] [CrossRef] [Green Version]
- Estelle, I. Cambio Climático—Escenario Actual, Salud e Implicancias en la Población Chilena. 2020. Available online: https://www.academia.edu/42152825/CAMBIO_CLIM%C3%81TICO_-ESCENARIO_ACTUAL_SALUD_E_IMPLICANCIAS_EN_LA_POBLACI%C3%93N_CHILENA (accessed on 13 April 2020).
- WHO. Infectious Diseases. 2020. Available online: https://www.who.int/topics/infectious_diseases/en/ (accessed on 6 November 2020).
- WHO. Water Sanitation and Health. 2020. Available online: http://www.who.int/water_sanitation_health/en/ (accessed on 8 May 2020).
- Cassel, G.H.; Burrows, A.; Jeffers, J.B.; Fischer, D.H. Anterior nongranulomatous uveitis: A seasonal variation. Ann. Ophthalmol. 1984, 16, 1066–1068. [Google Scholar]
- Luca, C.; Raffaella, A.; Sylvia, M.; Valentina, M.; Fabiana, V.; Marco, C.; Annamaria, S.; Luisa, S.; Alessandro, D.F.; Lucia, B.; et al. Changes in patterns of uveitis at a tertiary referral center in Northern Italy: Analysis of 990 consecutive cases. Int. Ophthalmol. 2017, 38, 133–142. [Google Scholar] [CrossRef] [Green Version]
- Rea, W.J.; Patel, K.D. Reversibility of Chronic Disease and Hypersensitivity: The Effects of Environmental Pollutants on the Organ System; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- NASA. Global Climate Change. Available online: https://climate.nasa.gov/solutions/adaptation-mitigation/ (accessed on 3 June 2021).
- Zhao, C.; Yan, Y.; Wang, C.; Tang, M.; Wu, G.; Ding, D.; Song, Y. Adaptation and mitigation for combating climate change—From single to joint. Ecosyst. Health Sustain. 2018, 4, 85–94. [Google Scholar] [CrossRef] [Green Version]
- Ginbo, T.; Di Corato, L.; Hoffmann, R. Investing in climate change adaptation and mitigation: A methodological review of real-options studies. Ambio 2021, 50, 229–241. [Google Scholar] [CrossRef] [PubMed]
- European Commission. EU Reference Scenario Energy, Transport and GHG Emissions Trends to 2050. 2016. Available online: https://ec.europa.eu/energy/sites/ener/files/documents/20160713%20draft_publication_REF2016_v13.pdf (accessed on 24 May 2021).
- Sillero-Medina, J.; Pérez-González, M.; Martínez-Murillo, J.; Ruiz-Sinoga, J. Factors affecting eco-geomorphological dynamics in two contrasting Mediterranean environments. Geomorphology 2020, 352, 106996. [Google Scholar] [CrossRef]
- Rodrigo-Comino, J.; Senciales, J.M.; Sillero-Medina, J.A.; Gyasi-Agyei, Y.; Ruiz-Sinoga, J.D.; Ries, J.B. Analysis of Weather-Type-Induced Soil Erosion in Cultivated and Poorly Managed Abandoned Sloping Vineyards in the Axarquía Region (Málaga, Spain). Air Soil Water Res. 2019, 12, 1178622119839403. [Google Scholar] [CrossRef]
- Diputación de Málaga. Estudio Sobre la Cuenca del Río Guadalhorce y Cuatro Tramos Representativos. Montes y Caminos. Ingenieros Consultores. 2013. Available online: http://static.malaga.es/malaga/subidas/descargas/archivos/3/2/206223/estudio-cuenca-del-rio-guadalhorce.pdf (accessed on 16 January 2021).
- REDMAR (Red de Medidas del nivel del mar y agitación de Puertos del Estado). Puerto de Málaga. Resumen de Parámetros Relacionados con el Nivel del Mar y la Marea que Afectan a Las Condiciones de Diseño y Explotación Portuaria. 2019. Available online: http://www.puertos.es/es-es/oceanografia/Paginas/portus.aspx (accessed on 6 February 2021).
- Alonso, B.L.; Rosel, A.B.; Villaverde-Royo, M.; Alonso, I.L. Malaria por Plasmodium falciparum en residente en España sin antecedente de viaje reciente a zonas endémicas. Semergen 2016, 42, e71–e72. [Google Scholar] [CrossRef] [PubMed]
- Junta de Andalucía. Informe del Inventario de Emisiones a la Atmósfera de la Comunidad Autónoma de Andalucía; Serie 2003–2016. Edición 2019. 2019. Available online: http://www.juntadeandalucia.es/medioambiente/site/portalweb/menuitem.7e1cf46ddf59bb227a9ebe205510e1ca/?vgnextoid=139f3d9292df3610VgnVCM100000341de50aRCRD&vgnextchannel=a35b445a0b5f4310VgnVCM2000000624e50aRCRD&lr=lang_es (accessed on 21 April 2020).
- Sanz, M.J.; Galán, E. Impactos y Riesgos Derivados del Cambio Climático en España; Ministerio para la Transición Ecológica y el Reto Demográfico (MITECO): Madrid, Spain, 2020.
- Giorgi, F.; Lionello, P. Climate change projections for the Mediterranean region. Glob. Planet. Chang. 2008, 63, 90–104. [Google Scholar] [CrossRef]
- Chang, J.; Smiddy, W.E. Cost-Effectiveness of Retinal Detachment Repair. Ophthalmology 2014, 121, 946–951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Araiz Iribarren, J.; Piñero Bustamante, A. Manejo de las Degeneraciones Periféricas de la Retina; SERV, Sociedad Española de Retina y Vítreo: Madrid, Spain, 2017. [Google Scholar]
- AEMET. Julio de 2020 Finaliza con Récords de Temperatura y Riesgo Extremo de Incendios. 2020. Available online: http://www.aemet.es/es/noticias/2020/07/Olacalor_incendios_jul2020 (accessed on 7 November 2020).
- AEMET. Olas de Calor en España desde Área de Climatología y Aplicaciones Operativas. 2020. Available online: http://www.aemet.es/documentos/es/conocermas/recursos_en_linea/publicaciones_y_estudios/estudios/Olas_calor/Olas_Calor_ActualizacionMarzo2020.pdf (accessed on 24 May 2021).
- Tomany, S.C.; Cruickshanks, K.J.; Klein, R.; Klein, B.E.K.; Knudtson, M.D. Sunlight and the 10-Year Incidence of Age-Related Maculopathy. Arch. Ophthalmol. 2004, 122, 750–757. [Google Scholar] [CrossRef] [Green Version]
- Junta de Andalucía. Ozono y Radiación Ultravioleta en Andalucía. 2014. Available online: http://www.juntadeandalucia.es/medioambiente/site/ima/menuitem.5893969315ab596f7bbe6c6f5510e1ca/?vgnextoid=7be208a2c7001510VgnVCM2000000624e50aRCRD&vgnextchannel=8ac91193c89e0510VgnVCM1000001325e50aRCRD&lr=lang_es) (accessed on 24 May 2021).
- Junta de Andalucía. Relación de Indicadores Ambientales de Andalucía 2019. Índice Ultravioleta. Available online: https://www.juntadeandalucia.es/medioambiente/portal/landing-page-indicador/-/asset_publisher/OGeXai6LtflU/content/espesor-de-la-capa-de-ozono/20151?categoryVal (accessed on 8 May 2021).
- Agencia Estatal de Meteorología—AEMET; Gobierno de España. Radiación y Ozono—Radiación Ultravioleta (UVI) y Ozono. 2020. Available online: http://www.aemet.es/es/serviciosclimaticos/vigilancia_clima/radiacion_ozono?w=1 (accessed on 8 November 2020).
- Xia, N.; Chen, L.; Chen, H.; Luo, X.; Deng, T. Influence of atmospheric relative humidity on ultraviolet flux and aerosol direct radiative forcing: Observation and simulation. Asia Pac. J. Atmos. Sci. 2016, 52, 341–352. [Google Scholar] [CrossRef]
- WHO. Climate Change and Health. 2018. Available online: https://www.who.int/news-room/fact-sheets/detail/climate-change-and-health (accessed on 7 November 2020).
- INE. Índices Nacionales de Subclases. Índices de Precios al Consumo. 2020. Available online: https://www.ine.es/jaxiT3/Datos.htm?t=22347#!tabs-grafico (accessed on 8 November 2020).
- EFEverde + Redacción. Desastres Naturales: Treinta y Cinco Muertos en España en EFEverde. 2020. Available online: https://www.efeverde.com/noticias/desastres-naturales-treinta-y-cinco-muertos-en-espana-en-2012/ (accessed on 8 November 2020).
- Glaucoma Research Fundation. Datos y Estadísticas Sobre el Glaucoma. 2020. Available online: https://www.glaucoma.org/es/datos-y-estadisticas-sobre-el-glaucoma.php (accessed on 8 November 2020).
- Statista. Glaucoma: Número de Casos 2011–2016. Statista. Available online: https://es.statista.com/estadisticas/991862/numero-de-casos-de-glaucoma-en-espana/ (accessed on 17 November 2020).
- Wang, W.; He, M.; Li, Z.; Huang, W. Epidemiological variations and trends in health burden of glaucoma worldwide. Acta Ophthalmol. 2019, 97, e349–e355. [Google Scholar] [CrossRef] [Green Version]
- Osborne, N.N.; Lascaratos, G.; Bron, A.J.; Chidlow, G.; Wood, J.P.M. A hypothesis to suggest that light is a risk factor in glaucoma and the mitochondrial optic neuropathies. Br. J. Ophthalmol. 2006, 90, 237–241. [Google Scholar] [CrossRef] [Green Version]
- Ivanov, I.V.; Mappes, T.; Schaupp, P.; Lappe, C.; Wahl, S. Ultraviolet radiation oxidative stress affects eye health. J. Biophotonics 2018, 11, e201700377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Europa Press. La cirugía de Catarata es Uno de los Procedimientos Quirúrgicos Más Comunes. 2015. Available online: https://www.infosalus.com/asistencia/noticia-cirugia-catarata-procedimientos-quirurgicos-mas-comunes-20150518140228.html (accessed on 9 November 2015).
- Sasaki, K.; Hockwin, O. Progress in Lens and Cataract Research: In Honour of Professor Kazuyuki Sasaki; Karger Medical and Scientific Publishers: Basel, Switzerland, 2002. [Google Scholar]
- Ministerio de Sanidad, Servicios Sociales e Igualdad. Informe Anual del Sistema Nacional de Salud 2015. 2016. Available online: https://www.mscbs.gob.es/estadEstudios/estadisticas/sisInfSanSNS/tablasEstadisticas/Inf_Anual_SNS_2015.1.pdf (accessed on 15 September 2020).
- Vidal, M.J.; Labeaga, J.M.; Casado, P.; Madrigal, A.; López, J.; Montero, A.; Meil, G. Informe 2016: Las Personas Mayores en España. Datos estadísticos estatales y por Comunidades Autónomas; Ministerio de Sanidad, Servicios Sociales e Igualdad, IMSERSO: Madrid, Spain, 2017; p. 540.
- Servicio Andaluz de Salud. Tiempos de Respuesta Asistencial. Listas de Espera. 2020. Available online: https://www.sspa.juntadeandalucia.es/servicioandaluzdesalud/ciudadania/derechos-y-garantias/tiempos-de-respuesta-asistencial-listas-de-espera (accessed on 9 November 2020).
- Zak-Prelich, M.; Borkowski, J.L.; Alexander, F.; Norval, M. The role of solar ultraviolet irradiation in zoster. Epidemiol. Infect. 2002, 129, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Salvador Velázquez, P. Estimación del Coste Económico de la Asistencia a los Pacientes Diagnosticados de Herpes-Zoster; Trabajo de Investigación; Departamento de Farmacología: Terapéutica y Toxicología, UAB (Universidad Autónoma de Barcelona), 2012; p. 25. Available online: https://ddd.uab.cat/pub/trerecpro/2012/hdl_2072_203218/TR-SalvadorVelazquez.pdf (accessed on 22 May 2021).
- Ojeda Ruiz, E.; Vila Cordero, B.; López-Perea, N.; Carmona Alférez, R. Informe Epidemiológico Sobre la Situación de Herpes Zóster en España, 1998–2018. 2020. Available online: https://www.isciii.es/QueHacemos/Servicios/VigilanciaSaludPublicaRENAVE/EnfermedadesTransmisibles/Documents/archivos%20A-Z/HERPES%20ZOSTER/Informe_HZ_Espa%C3%B1a_1998-2018.pdf (accessed on 24 May 2021).
- Centro Nacional de Epidemiologia. Infección Genital por el Virus Herpes Simple. Sistema de Información Microbiológica. España. Años 2000–2008. Boletín epidemiológico Semin. 2011, 19, 1–5. [Google Scholar]
- Villanueva Blandón, R. Estudio Retrospectivo para la Evaluación de la Eficacia del Aciclovir Oral en la Prevención de Recurrencias en Pacientes con Queratitis Herpética Recidivante. 2016. Available online: https://idus.us.es/bitstream/handle/11441/39293/Tesis%20Rafael%20Villanueva.pdf?sequence=1&isAllowed=y (accessed on 24 May 2021).
- Statista. Herpes Simple: Número de casos 2011–2017. 2018. Available online: https://es.statista.com/estadisticas/1038556/numero-de-casos-de-herpes-simple-en-espana/ (accessed on 17 November 2020).
- Hart, J.E.; Laden, F.; Puett, R.C.; Costenbader, K.H.; Karlson, E.W. Exposure to Traffic Pollution and Increased Risk of Rheumatoid Arthritis. Environ. Health Perspect. 2009, 117, 1065–1069. [Google Scholar] [CrossRef] [Green Version]
- Sociedad Española de Reumatología. El Retraso en el Diagnóstico de Pacientes con Artritis Reumatoide se ha Reducido a Menos de 8 Meses. 2018. Available online: https://www.ser.es/retraso-diagnostico-pacientes-artritis-reumatoide-se-ha-reducido-menos-8-meses/ (accessed on 9 November 2020).
- Soleimanifar, N.; Nicknam, M.H.; Bidad, K.; Jamshidi, A.R.; Mahmoudi, M.; Mostafaei, S.; Hosseini-Khah, Z.; Nikbin, B. Effect of food intake and ambient air pollution exposure on ankylosing spondylitis disease activity. Adv. Rheumatol. 2019, 59, 9. [Google Scholar] [CrossRef]
- Plazuelo Ramos, P. Atlas de Espondiloartritis Axial en España 2017: Radiografía de la Enfermedad; Instituto Max Weber: Madrid, Spain, 2017. [Google Scholar]
- Jeanjean, M.; Bind, M.-A.; Roux, J.; Ongagna, J.-C.; de Sèze, J.; Bard, D.; Leray, E. Ozone, NO2 and PM10 are associated with the occurrence of multiple sclerosis relapses. Evidence from seasonal multi-pollutant analyses. Environ. Res. 2018, 163, 43–52. [Google Scholar] [CrossRef] [Green Version]
- Gallego-Gallego, M.; Anillo-Lombana, V.E.; Gómez-Mayordomo, V.; García-Miguel, F.J. Uhthoff’s phenomenon in a patient with multiple sclerosis during the perioperative period for hip surgery. Case report. Colomb. J. Anesthesiol. 2018, 46, 345–348. [Google Scholar] [CrossRef]
- Gaceta Médica. Cada Año se Diagnostican Unos 2000 Nuevos Casos de Esclerosis Múltiple en España. 2020. Available online: https://gacetamedica.com/investigacion/cada-ano-se-diagnostican-unos-2-000-nuevos-casos-de-esclerosis-multiple-en-espana/ (accessed on 9 November 2020).
- Panginikkod, S.; Rayi, A.; Rocha Cabrero, F.; Rukmangadachar, L.A. Uhthoff Phenomenon; StatPearls Publishing: Petersburg, FL, USA, 2020. Available online: http://www.ncbi.nlm.nih.gov/books/NBK470244/ (accessed on 9 November 2020).
- Manser, C.N.; Paul, M.; Rogler, G.; Held, L.; Frei, T. Heat Waves, Incidence of Infectious Gastroenteritis, and Relapse Rates of Inflammatory Bowel Disease: A Retrospective Controlled Observational Study. Am. J. Gastroenterol. 2013, 108, 1480–1485. [Google Scholar] [CrossRef] [PubMed]
- Sambuelli, M.; Negreira, S.; Gil, A.; Goncalves, S.; Chavero, P.; Tirado, P.; Bellicoso, M.; Huernos, S. Manejo de la Enfermedad Inflamatoria Intestinal. Revisión y Algoritmos de Tratamiento. 2019. Available online: http://actagastro.org/manejo-de-la-enfermedad-inflamatoria-intestinal-revision-y-algoritmos-de-tratamientos/ (accessed on 9 November 2020).
- Rúa-Figueroa, I.; López-Longo, F.J.; Calvo-Alén, J.; Galindo, M.; Loza, E.; de Yebenes, M.J.G.; Pego-Reigosa, J.M. Registro nacional de pacientes con lupus eritematoso sistémico de la Sociedad Española de Reumatología: Objetivos y metodología. Reumatol. Clínica 2014, 10, 17–24. [Google Scholar] [CrossRef]
- Cervera, R.; Rúa-Figueroa, I.; Gil-Aguado, A.; Sabio, J.; Pallarés, L.; Hernández-Pastor, L.; Iglesias, M. Coste económico directo del control y el tratamiento del lupus eritematoso sistémico activo y sus brotes en España: Estudio LUCIE. Rev. Clínica Española 2013, 213, 127–137. [Google Scholar] [CrossRef]
- Cooper, G.S.; Wither, J.; Bernatsky, S.; Claudio, J.O.; Clarke, A.; Rioux, J.D.; Fortin, P.R. CaNIOS GenES Investigators Occupational and environmental exposures and risk of systemic lupus erythematosus: Silica, sunlight, solvents. Rheumatology 2010, 49, 2172–2180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- UNIMID. Asociación de Personas con Enfermedades Crónicas Inflamatorias Inmunomediadas. 2019. Available online: http://www.unimid.es/ (accessed on 9 November 2020).
- Rice, J.B.; White, A.; Lopez, A.; Conway, A.; Wagh, A.; Nelson, W.W.; Philbin, M.; Wan, G.J. Economic burden of sarcoidosis in a commercially-insured population in the United States. J. Med. Econ. 2017, 20, 1048–1055. [Google Scholar] [CrossRef]
- Schweitzer, M.D.; Calzadilla, A.S.; Salamo, O.; Sharifi, A.; Kumar, N.; Holt, G.; Campos, M.; Mirsaeidi, M. Lung health in era of climate change and dust storms. Environ. Res. 2018, 163, 36–42. [Google Scholar] [CrossRef] [PubMed]
- AEMET. Informe Annual, 2017. 2018. Available online: https://www.aemet.es/documentos/es/conocenos/a_que_nos_dedicamos/informes/InformeAnualAEMET_2017_web.pdf (accessed on 24 May 2021).
- Erdal, B.S.; Clymer, B.D.; Yildiz, V.O.; Julian, M.W.; Crouser, E.D. Unexpectedly high prevalence of sarcoidosis in a representative U.S. Metropolitan population. Respir. Med. 2012, 106, 893–899. [Google Scholar] [CrossRef] [Green Version]
- AARDA (American Autoimmune Related Diseases Association). The Cost Burden of Autoimmune Disease: The Latest Front in the War on Healthcare Spending. 2020. Available online: http://www.diabetesed.net/page/_files/autoimmune-diseases.pdf (accessed on 1 May 2021).
- El País. Los SUV y la Gasolina, Responsables del Incremento de las Emisiones en Españ. El Motor. 2020. Available online: https://motor.elpais.com/actualidad/los-suv-y-la-gasolina-responsables-del-incremento-de-las-emisiones-en-espana/ (accessed on 18 November 2020).
- INE. Población Urbana Expuesta a Contaminación Del Aire (Micropartículas PM10, PM2,5). 2020. Available online: https://www.ine.es/ss/Satellite?L=es_ES&c=INESeccion_C&cid=1259944618679&p=1254735110672&pagename=ProductosYServicios%2FPYSLayout¶m1=PYSDetalleFichaIndicador¶m3=1259947308577 (accessed on 18 November 2020).
- Ceballos, M.Á. La Contaminación por Ozono en el Estado Español. 2019. Available online: https://www.ecologistasenaccion.org/wp-content/uploads/2019/10/informe-ozono-2019.pdf (accessed on 24 May 2021).
- El Hamichi, S.; Gold, A.; Murray, T.G.; Graversen, V.K. Pandemics, climate change, and the eye. Graefe’s Arch. Clin. Exp. Ophthalmol. 2020, 258, 2597–2601. [Google Scholar] [CrossRef]
- Segnalini, M.; Bernabucci, U.; Vitali, A.; Nardone, A.; Lacetera, N. Temperature humidity index scenarios in the Mediterranean basin. Int. J. Biometeorol. 2012, 57, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Lozano, R. Envisioning sustainability three-dimensionally. J. Clean. Prod. 2008, 16, 1838–1846. [Google Scholar] [CrossRef]
- WHO. World Report on Vision. 2019. Available online: https://www.who.int/publications/i/item/world-report-on-vision (accessed on 15 September 2020).
- Killmann, W. Instruments related to the United Nations Framework Convention on Climate Change and Their Potential for Sustainable Forest Management in Africa. Forest and Climate Change Working Paper FAO. Available online: http://www.fao.org/3/ac836e/AC836E01.htm#P45_290 (accessed on 24 May 2021).
- Gordois, A.; Pezzullo, L.; Cutler, H. The Global Economic Cost of Visual Impairment. 2010. Available online: http://www.icoph.org/dynamic/attachments/resources/globalcostofvi_finalreport.pdf (accessed on 24 May 2021).
- MITECO. Mitigación del Cambio Climático. 2020. Available online: https://www.miteco.gob.es/es/cambio-climatico/temas/mitigacion-politicas-y-medidas/mitigacion.aspx (accessed on 10 November 2020).
- Fischer, G.; Tubiello, F.N.; van Velthuizen, H.; Wiberg, D. Climate change impacts on irrigation water requirements: Effects of mitigation, 1990–2080. Technol. Forecast. Soc. Chang. 2007, 74, 1083–1107. [Google Scholar] [CrossRef] [Green Version]
- FAO; UNEP. The State of the World’s Forests Forests, Biodiversity and People; FAO: Rome, Italy, 2020. [Google Scholar] [CrossRef]
- Xunta de Galicia. LIFE14 CCM/ES/001271 “LIFE FOREST CO2” “Assessment of Forest-Carbon Sinks and Promotion of Compensation Systems as Tools for Climate Change Mitigation.” 2016. Available online: http://lifeforestco2.eu/wp-content/uploads/2017/02/A.1.01_Estudio_diagnostico_iniciativas_compensacion.pdf (accessed on 24 May 2021).
- The World Bank. GDP (Current US $)—Data. 2020. Available online: https://data.worldbank.org/indicator/NY.GDP.MKTP.CD (accessed on 10 November 2020).
- González-Eguino, M. Los costes de mitigar el cambio climático: Un análisis dinámico de equilibrio general aplicado. Rev. Econ. Apl. 2011, XIX, 89–121. [Google Scholar]
- MITECO. Envío a Cortes del Proyecto de Ley de Cambio Climático y Transición Energética Para Alcanzar la Neutralidad de Emisiones a Más Tardar en 2050. 2020. Available online: https://www.miteco.gob.es/es/prensa/ultimas-noticias/el-gobierno-env%C3%ADa-a-las-cortes-el-primer-proyecto-de-ley-de-cambio-clim%C3%A1tico-y-transici%C3%B3n-energ%C3%A9tica-para-alcanzar-la-neutralidad-de-emisiones-a/tcm:30-509229 (accessed on 10 November 2020).
- Hidalgo-Betanzos, J.M.; Iríbar-Solaberrieta, E.; de Lorenzo Urién, A. Guía Básica para el Control Térmico en Edificación. 2014. Available online: https://www.euskadi.eus/contenidos/informacion/area_termica_public/es_def/adjuntos/GB_control_termico.pdf (accessed on 24 May 2021).
- Energía Eficiente. Situación Energética Mundial. 2009. Available online: https://energiaeficiente.wordpress.com/2009/10/22/situacion-energetica-mundial/ (accessed on 10 November 2020).
- García-Pando, C. Cómo afecta la eficiencia energética al valor de la vivienda? 2014. Available online: https://www.pisos.com/aldia/como-afecta-la-eficiencia-energetica-al-valor-de-la-vivienda/44088/ (accessed on 10 November 2020).
- Rose, J.B.; Daeschner, S.; Easterling, D.R.; Curriero, F.C.; Lele, S.; Patz, J.A. Climate and waterborne disease outbreaks. J. Am. Water Work. Assoc. 2000, 92, 77–87. [Google Scholar] [CrossRef]
- Ecologistas en Acción. Propuestas para Reducir la Demanda Energética. 2011. Available online: https://www.ecologistasenaccion.org/19899/propuestas-para-reducir-la-demanda-energetica/ (accessed on 18 November 2020).
- Mukhopadhyay, R.; Karisiddaiah, S.; Mukhopadhyay, J. Climate Change: Alternate Governance Policy for South Asia; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- El País. El 70% de los Últimos Brotes Epidémicos Han Comenzado con la Deforestación. 2021. Available online: https://elpais.com/ciencia/2021-02-05/el-70-de-los-ultimos-brotes-epidemicos-han-comenzado-con-la-deforestacion.html (accessed on 19 May 2021).
- Andersen, K.G.; Rambaut, A.; Lipkin, W.I.; Holmes, E.C.; Garry, R.F. The proximal origin of SARS-CoV-2. Nat. Med. 2020, 26, 450–452. [Google Scholar] [CrossRef] [Green Version]
- SanJuan-Reyes, S.; Gómez-Oliván, L.M.; Islas-Flores, H. COVID-19 in the environment. Chemosphere 2021, 263, 127973. [Google Scholar] [CrossRef]
- OIE—World Organisation for Animal Health. Mixomatosis. 2020. Available online: https://www.oie.int/es/sanidad-animal-en-el-mundo/enfermedades-de-los-animales/mixomatosis (accessed on 18 November 2020).
- Hemelaar, J. The origin and diversity of the HIV-1 pandemic. Trends Mol. Med. 2012, 18, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Byrne, M.P.; O’Gorman, P.A. Understanding Decreases in Land Relative Humidity with Global Warming: Conceptual Model and GCM Simulations. J. Clim. 2016, 29, 9045–9061. [Google Scholar] [CrossRef]
- MITECO. Cambio Climático. Available online: https://www.miteco.gob.es/es/cambio-climatico/temas/default.aspx (accessed on 14 April 2020).
- Gibson, J.H. UVB Radiation: Definition and Characteristics. 2020. Available online: http://uvb.nrel.colostate.edu/UVB/publications/uvb_primer.pdf (accessed on 24 May 2021).
- Manojkumar, N.; Srimuruganandam, B. Assessment of gaseous emissions and radiative forcing in Indian forest fires. Int. J. Environ. Stud. 2019, 76, 541–557. [Google Scholar] [CrossRef]
- Littell, J.S.; Peterson, D.L.; Riley, K.L.; Liu, Y.; Luce, C. A review of the relationships between drought and forest fire in the United States. Glob. Chang. Biol. 2016, 22, 2353–2369. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, A.S.M.; De Huancayo, U.C.; Arredondo, R.E.; Yuli-Posadas, R.A. Determinación de la presencia de partículas (PM10) en Perú producidas por quema de biomasa con ayuda de modelos numéricos. Rev. Int. Contam. Ambient. 2017, 33, 99–108. [Google Scholar] [CrossRef] [Green Version]
- McMichael, A.J.; World Health Organization (Eds.) Climate Change and Human Health: Risks and Responses; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Girardi, R.; Pinheiro, A.; Garbossa, L.H.P.; Torres, É. Water quality change of rivers during rainy events in a watershed with different land uses in Southern Brazil. Rev. Bras. Recur. Hídr. 2016, 21, 514–524. [Google Scholar] [CrossRef] [Green Version]
- García Aróstegui, J.L.; Cruz Sanjulián, J.J.; Hidalgo Estévez, M.C. Control de la intrusión marina y modelización del acuífero de vélez (Málaga, España). In Tecnología de la Intrusión de Agua de Mar en Acuíferos Costeros: Países Mediterráneos; IGME: Madrid, Spain, 2003; pp. 261–270. [Google Scholar]
- Marín Cots, P.; Sánchez Teba, E.M.; Molina Conde, I. Aproximación al Escenario de Cambio Climático en la Ciudad de Málaga. Composición y Estudio de Series Estadísticas. 2008. Available online: http://static.omau-malaga.com/omau/subidas/archivos/8/5/arc_858.pdf (accessed on 24 May 2021).
- McInnes, K.L.; Erwin, T.A.; Bathols, J.M. Global Climate Model projected changes in 10 m wind speed and direction due to anthropogenic climate change. Atmos. Sci. Lett. 2011, 12, 325–333. [Google Scholar] [CrossRef]
- Hallegatte, S.; Green, C.; Nicholls, R.J.; Corfee-Morlot, J. Future flood losses in major coastal cities. Nat. Clim. Chang. 2013, 3, 802–806. [Google Scholar] [CrossRef]
- Méndez Jiménez, M. Análisis Preliminar de la Vulnerabilidad de la Costa de Andalucía a la Potencial Subida del Nivel del Mar Asociada al Cambio Climático. 2011. Available online: https://www.juntadeandalucia.es/medioambiente/portal_web/web/temas_ambientales/clima/actuaciones_cambio_climatico/adaptacion/vulnerabilidad_impactos_medidas/vulnerabilidad_costas.pdf (accessed on 24 May 2021).
- Jiang, C.; Shaw, K.S.; Upperman, C.R.; Blythe, D.; Mitchell, C.; Murtugudde, R.; Sapkota, A.R.; Sapkota, A. Climate change, extreme events and increased risk of salmonellosis in Maryland, USA: Evidence for coastal vulnerability. Environ. Int. 2015, 83, 58–62. [Google Scholar] [CrossRef] [Green Version]
- Ceballos, G.; Ehrlich, P.R.; Barnosky, A.D.; García, A.; Pringle, R.M.; Palmer, T.M. Accelerated modern human–induced species losses: Entering the sixth mass extinction. Sci. Adv. 2015, 1, e1400253. [Google Scholar] [CrossRef] [Green Version]
- Karp, D.S.; Moeller, H.V.; Frishkoff, L.O. Nonrandom extinction patterns can modulate pest control service decline. Ecol. Appl. 2013, 23, 840–849. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A. Habitat Destruction. National Geographic Kids. 2018. Available online: https://kids.nationalgeographic.com/explore/science/habitat-destruction/ (accessed on 14 April 2020).
- Capdevila-Argüelles, L.; Zilletti, B.; Suárez Álvarez, V.Á. Plataforma Nacional de Adaptación al Cambio Climático—Cambio Climático y Especies Exóticas Invasoras en España: Diagnóstico Preliminar y Bases de Conocimiento Sobre Impactos y Vulnerabilidad (2011). 2011. Available online: https://www.adaptecca.es/recursos/buscador/cambio-climatico-y-especies-exoticas-invasoras-en-espana-diagnostico-preliminar-y (accessed on 14 April 2020).
- El Zowalaty, M.E.; Järhult, J.D. From SARS to COVID-19: A previously unknown SARS- related coronavirus (SARS-CoV-2) of pandemic potential infecting humans—Call for a One Health approach. One Health 2020, 9, 100124. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-J.; Lin, C.Y.; Wu, Y.-T.; Wu, P.-C.; Lung, S.-C.; Su, H.-J. Effects of Extreme Precipitation to the Distribution of Infectious Diseases in Taiwan, 1994–2008. PLoS ONE 2012, 7, e34651. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Hong, K.-H.; Hwandon, J.; Young-Jae, P.; Moojong, P.; Young, S. Effect of Precipitation on Air Pollutant Concentration in Seoul, Korea. Asian J. Atmos. Environ. 2014, 8, 202–211. [Google Scholar] [CrossRef] [Green Version]
- Hong, J.; Zhong, T.; Li, H.; Xu, J.; Ye, X.; Mu, Z.; Lu, Y.; Mashaghi, A.; Zhou, Y.; Tan, M.; et al. Ambient air pollution, weather changes and outpatient visits for allergic conjunctivitis: A retrospective registry study. Sci. Rep. 2016, 6, 23858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stach, A.; García-Mozo, H.; Prieto-Baena, J.C.; Czarnecka-Operacz, M.; Jenerowicz, D.; Silny, W.; Galán, C. Prevalence of Artemisia species pollinosis in western Poland: Impact of climate change on aerobiological trends, 1995–2004. J. Investig. Allergol. Clin. Immunol. 2007, 17, 39–47. [Google Scholar]
- Jung, S.J.; Mehta, J.S.; Tong, L. Effects of environment pollution on the ocular surface. Ocul. Surf. 2018, 16, 198–205. [Google Scholar] [CrossRef]
- Jaggernath, J.; Haslam, D.; Naidoo, K.S. Climate change: Impact of increased ultraviolet radiation and water changes on eye health. Health 2013, 05, 921–930. [Google Scholar] [CrossRef] [Green Version]
- Zhong, J.-Y.; Lee, Y.-C.; Hsieh, C.-J.; Tseng, C.-C.; Yiin, L.-M. Association between Dry Eye Disease, Air Pollution and Weather Changes in Taiwan. Int. J. Environ. Res. Public Health 2018, 15, 2269. [Google Scholar] [CrossRef] [Green Version]
- Copeland, R.; Afshari, N.A. Principles and Practice of Cornea, 1st ed.; The Eye Center and The Eye Foundation for Research in Ophthalmology. Jaypee Brothers Medical Publishers: New Delhi, India, 2013; Available online: https://www.jaypeedigital.com/book/9789350901724/chapter/ch46 (accessed on 29 April 2020).
- Miller, J.K.; Laycock, K.A.; Nash, M.M.; Pepose, J.S. Corneal Langerhans cell dynamics after herpes simplex virus reactivation. Investig. Ophthalmol. Vis. Sci. 1993, 34, 2282–2290. [Google Scholar]
- Wegman, D.H. Epidemic Keratoconjunctivitis. Am. J. Public Health Nations Health 1970, 60, 1230–1237. [Google Scholar] [CrossRef]
- Parra-Rodríguez, D.S.; García-Carmona, K.P.; Vázquez-Maya, L.; Bonifaz, A. Incidencia de úlceras corneales microbianas en el Servicio de Oftalmología del Hospital General de México Dr. Eduardo Liceaga. Rev. Mex. Oftalmol. 2016, 90, 209–214. [Google Scholar] [CrossRef] [Green Version]
- Hussein, A.S.; El Mofty, H.; Hassanien, M. Climate change and predicted trend of fungal keratitis in Egypt. East. Mediterr. Health J. 2011, 17, 468–473. [Google Scholar] [CrossRef]
- Chidambaram, J.D.; Prajna, N.V.; Srikanthi, P.; Lanjewar, S.; Shah, M.; Elakkiya, S.; Lalitha, P.; Burton, M.J. Epidemiology, risk factors, and clinical outcomes in severe microbial keratitis in South India. Ophthalmic Epidemiol. 2018, 25, 297–305. [Google Scholar] [CrossRef] [Green Version]
- Psoter, K.J.; De Roos, A.; Wakefield, J.; Mayer, J.; Rosenfeld, M.; Rolain, J.-M. Season is associated with Pseudomonas aeruginosa acquisition in young children with cystic fibrosis. Clin. Microbiol. Infect. 2013, 19, E483–E489. [Google Scholar] [CrossRef] [Green Version]
- Sanda, J. The influences of climate changes in acute glaucoma. Abstract. In Proceedings of the International Multidisciplinary Scientific GeoConference & EXPO SGEM2014, Albena, Bulgaria, 17–26 June 2014; Available online: https://www.sgem.org/SGEMLIB/spip.php?article5110 (accessed on 10 April 2021). [CrossRef]
- Stein, J.D.; Pasquale, L.R.; Talwar, N.; Kim, D.S.; Reed, D.; Nan, B.; Kang, J.H.; Wiggs, J.L.; Richards, J.E. Geographic and Climatic Factors Associated with Exfoliation Syndrome. Arch. Ophthalmol. 2011, 129, 1053–1060. [Google Scholar] [CrossRef] [Green Version]
- Meerburg, B.G.; Kijlstra, A. Changing climate—Changing pathogens: Toxoplasma gondii in North-Western Europe. Parasitol. Res. 2009, 105, 17–24. [Google Scholar] [CrossRef] [Green Version]
- Rothova, A. Ocular involvement in toxoplasmosis. Br. J. Ophthalmol. 1993, 77, 371–377. [Google Scholar] [CrossRef] [Green Version]
- Jones, B.A.; Grace, D.; Kock, R.; Alonso, S.; Rushton, J.; Said, M.; McKeever, D.; Mutua, F.; Young, J.; McDermott, J.; et al. Zoonosis emergence linked to agricultural intensification and environmental change. Proc. Natl. Acad. Sci. USA 2013, 110, 8399–8404. [Google Scholar] [CrossRef] [Green Version]
- Jones, N.P. The Manchester Uveitis Clinic: The First 3000 Patients—Epidemiology and Casemix. Ocul. Immunol. Inflamm. 2013, 23, 118–126. [Google Scholar] [CrossRef]
- Rathinam, S.R.; Namperumalsamy, P. Global variation and pattern changes in epidemiology of uveitis. Indian J. Ophthalmol. 2007, 55, 173–183. [Google Scholar] [CrossRef]
- Albert, D.M.; Raven, M.L. Ocular Tuberculosis. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef] [Green Version]
- Pope, J.E.; Krizova, A.; Garg, A.X.; Thiessen-Philbrook, H.; Ouimet, J.M. Campylobacter Reactive Arthritis: A Systematic Review. Semin. Arthritis Rheum. 2007, 37, 48–55. [Google Scholar] [CrossRef] [Green Version]
- Ntumba Kayembe, J.-M.; Ntumba Kayembe, H.-C. Pneumonia: A Challenging Health Concern with the Climate Change; IntechOpen: London, UK, 2017. [Google Scholar]
- Chee, S.-P.; Khairallah, M. Emerging Infectious Uveitis; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Pleyer, U.; Chee, S.-P. Current aspects on the management of viral uveitis in immunocompetent individuals. Clin. Ophthalmol. 2015, 9, 1017–1028. [Google Scholar] [CrossRef] [Green Version]
- Gorodner, J.O.; Merino, D.E. Fiebre por virus del Nilo Occidental¿ Otra patología emergente relacionada con el cambio climático? Rev. Asoc. Médica Argent. 2012, 125, 9–12. [Google Scholar]
- Brownstein, J.S.; Holford, T.R.; Fish, D. Effect of Climate Change on Lyme Disease Risk in North America. EcoHealth 2005, 2, 38–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bednar, T.; What Will Climate Change Mean for Lyme Disease? Case Studies. Geology and Human Health. 2012. Available online: https://serc.carleton.edu/NAGTWorkshops/health/case_studies/lyme_disease.html (accessed on 30 April 2020).
- Duvall-Young, J. Emergency, Acute and Rapid Access Ophthalmology: Practical, Clinical and Managerial Aspects; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Martinez-Berriotxoa, A.; Fonollosa, A.; Artaraz, J. Aproximación diagnóstica a las uveítis. Rev. Clínica Española 2012, 212, 442–452. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Rueda, A. Retinitis por Rickettsia conorii, una infección emergente en el sureste de la península ibérica. Arch. Sociedad Española Oftalmol. 2020, 95, 507–511. [Google Scholar] [CrossRef]
- Observatorio de Salud y Cambio Climático. Fiebre Botonosa Mediterránea. 2021. Available online: http://www.oscc.gob.es/es/general/salud_cambio_climatico/fiebre_botonosa_mediterranea_es.htm (accessed on 19 May 2021).
- Red Nacional de Vigilancia Epidemiológica-ISCIII (Instituto de Salud Carlos III). Resultados de la Vigilancia Epidemiológica de las enfermedades transmisibles. Informe Anual. Años 2017–2018. Ministerio de Ciencia e Innovación. 2020. Available online: https://www.isciii.es/QueHacemos/Servicios/VigilanciaSaludPublicaRENAVE/EnfermedadesTransmisibles/Documents/INFORMES/INFORMES%20RENAVE/RENAVE_Informe_anual__2017-2018.pdf (accessed on 27 March 2021).
- Song, Y.-J.; Cheong, H.-K.; Ki, M.; Shin, J.-Y.; Hwang, S.-S.; Park, M.; Ki, M.; Lim, J. The Epidemiological Influence of Climatic Factors on Shigellosis Incidence Rates in Korea. Int. J. Environ. Res. Public Health 2018, 15, 2209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.S.; Hoang, T.T.H.; Pham-Duc, P.; Lee, M.; Grace, D.; Hung, N.-V.; Thuc, V.M.; Nguyen-Viet, H. Seasonal and geographical distribution of bacillary dysentery (shigellosis) and associated climate risk factors in Kon Tum Province in Vietnam from 1999 to 2013. Infect. Dis. Poverty 2017, 6, 113. [Google Scholar] [CrossRef] [PubMed]
- Red Nacional de Vigilancia Epidemiológica-ISCIII (Instituto de Salud Carlos III). Resultados de la Vigilancia Epidemiológica de las enfermedades transmisibles. Informe Anual. Año 2016. Ministerio de Ciencia e Innovación. 2020. Available online: http://gesdoc.isciii.es/gesdoccontroller?action=download&id=25/01/2019-d8ee271b6f (accessed on 18 February 2021).
- Cheeti, A.; Chakraborty, R.K.; Ramphul, K. Reactive Arthritis (Reiter Syndrome); StatPearls Publishing: Petersburg, FL, USA, 2021. Available online: http://www.ncbi.nlm.nih.gov/books/NBK499831/ (accessed on 15 June 2020).
- Akil, L.; Ahmad, H.A.; Reddy, R.S. Effects of Climate Change on Salmonella Infections. Foodborne Pathog. Dis. 2014, 11, 974–980. [Google Scholar] [CrossRef] [Green Version]
- Karlson, E.W.; Deane, K. Environmental and Gene-Environment Interactions and Risk of Rheumatoid Arthritis. Rheum. Dis. Clin. N. Am. 2012, 38, 405–426. [Google Scholar] [CrossRef] [Green Version]
- Zlatanović, G.; Veselinović, D.; Cekic, S.; Zivković, M.; Đorđević-Jocić, J.; Zlatanović, M. Ocular manifestation of rheumatoid arthritis-different forms and frequency. Bosn. J. Basic Med. Sci. 2010, 10, 323–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, N.; Agarwal, A. Management of Uveitis in Spondyloarthropathy: Current Trends. Perm. J. 2018, 22. [Google Scholar] [CrossRef] [Green Version]
- Leli, I.; Salimbene, I.; Varone, F.; Fuso, L.; Valente, S. Husband and wife with sarcoidosis: Possible environmental factors involved. Multidiscip. Respir. Med. 2013, 8, 5. [Google Scholar] [CrossRef] [Green Version]
- Ungprasert, P.; Crowson, C.S.; Matteson, E.L. Seasonal variation in incidence of sarcoidosis: A population-based study, 1976–2013. Thorax 2016, 71, 1164–1166. [Google Scholar] [CrossRef] [Green Version]
- Ortiz-Perez, S.; Martínez-Lapiscina, E.H.; Gabilondo, I.; Fraga-Pumar, E.; Martínez-Heras, E.; Saiz, A.; Sanchez-Dalmau, B.F.; Villoslada, P. Retinal periphlebitis is associated with multiple sclerosis severity. Neurology 2013, 81, 877–881. [Google Scholar] [CrossRef] [Green Version]
- Kubaisi, B.; Kopplin, L.; Agarwal, A.; Rosenbaum, J.T.; Foster, C.S.; Nguyen, Q.D. Crohn’s Disease-Associated Uveitis. Uveitis Atlas 2019, 2020, 147–152. [Google Scholar] [CrossRef]
- Eglin, R. Detection of RNA complementary to herpes-simplex virus in mononuclear cells from patients with Behçet’s Syndrome and Recurrent Oral Ulcers. Lancet 1982, 320, 1356–1361. [Google Scholar] [CrossRef]
- Zouboulis, C.C.; May, T. Pathogenesis of Adamantiades-Behçet’s disease. Med. Microbiol. Immunol. 2003, 192, 149–155. [Google Scholar] [CrossRef]
- Watanabe, H.; Yashiro, M.; Asano, T.; Sato, S.; Takahashi, A.; Katakura, K.; Kobayashi, H.; Ohira, H. A case of Behçet’s disease and systemic sclerosis developing after an earthquake disaster. Fukushima J. Med. Sci. 2015, 61, 86–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ness, T.; Bley, T.A.; Schmidt, W.A.; Lamprecht, P. The Diagnosis and Treatment of Giant Cell Arteritis. Dtsch. Aerzteblatt Online 2013, 110, 376–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nordborg, E. Giant cell arteritis: Epidemiological clues to its pathogenesis and an update on its treatment. Rheumatology 2003, 42, 413–421. [Google Scholar] [CrossRef] [Green Version]
- Lyons, H.S.; Quick, V.; Sinclair, A.J.; Nagaraju, S.; Mollan, S.P. A new era for giant cell arteritis. Eye 2019, 34, 1013–1026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vodopivec, I.; Rizzo, J.F. Ophthalmic manifestations of giant cell arteritis. Rheumatology 2018, 57, ii63–ii72. [Google Scholar] [CrossRef] [Green Version]
- Okazaki, T.; Shinagawa, S.; Mikage, H. Vasculitis syndrome-diagnosis and therapy. J. Gen. Fam. Med. 2017, 18, 72–78. [Google Scholar] [CrossRef]
- Abdgawad, M. History, Classification and Pathophysiology of Small Vessel Vasculitis. Updates Diagn. Treat. Vasc. 2013, 20. [Google Scholar] [CrossRef] [Green Version]
- Pagnoux, C.; Bienvenu, B.; Guillevin, L. The spectrum of granulomatous vasculitides. Futur. Rheumatol. 2006, 1, 729–750. [Google Scholar] [CrossRef]
- Thorne, J.E.; Jabs, D.A. Ocular Manifestations o Vasculitis. Rheum. Dis. Clin. N. Am. 2001, 27, 761–779. [Google Scholar] [CrossRef]
- Krause, I.; Shraga, I.; Molad, Y.; Guedj, D.; Weinberger, A. Seasons of the Year and Activity of SLE and Behçet’s Disease. Scand. J. Rheumatol. 1997, 26, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Cansu, D.U.; Kasifoglu, T.; Korkmaz, C. Is there any relationship between season/weather and oral ulcer in Behçet’s disease? Eur. J. Rheumatol. 2014, 1, 89–91. [Google Scholar] [CrossRef] [PubMed]
- Kamen, D.L. Environmental Influences on Systemic Lupus Erythematosus Expression. Rheum. Dis. Clin. N. Am. 2014, 40, 401–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, J. Ultraviolet B irradiation and cytomegalovirus infection synergize to induce the cell surface expression of 52-kD/Ro antigen. Clin. Exp. Immunol. 1996, 103, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Organización Nacional de Trastornos Raros (NORD). Dermatomyositis. 2018. Available online: https://rarediseases.org/rare-diseases/dermatomyositis/ (accessed on 1 May 2020).
- Orione, M.A.M.; Silva, C.A.; Sallum, A.M.E.; Campos, L.M.A.; Omori, C.H.; Braga, A.; Farhat, S.C.L. Risk Factors for Juvenile Dermatomyositis: Exposure to Tobacco and Air Pollutants During Pregnancy. Arthritis Rheum. 2014, 66, 1571–1575. [Google Scholar] [CrossRef] [PubMed]
- Pachman, L.M.; Khojah, A.M. Advances in Juvenile Dermatomyositis: Myositis Specific Antibodies Aid in Understanding Disease Heterogeneity. J. Pediatr. 2018, 195, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Calder, J.A.M.; Calder, D.N. Chikungunya Fever: A New Concern for the Western Hemisphere. Open Infect. Dis. J. 2015, 9, 13–19. [Google Scholar]
- Teoh, S.; Thean, L.; Koay, E. Cytomegalovirus in aetiology of Posner–Schlossman syndrome: Evidence from quantitative polymerase chain reaction. Eye 2004, 19, 1338–1340. [Google Scholar] [CrossRef] [PubMed]
- Willermain, F.; Van Laethem, Y.; Caspers, L. Global Variations and Changes in Patterns of Infectious Uveitis. Emerg. Infect. Uveitis 2017, 2020, 1–7. [Google Scholar] [CrossRef]
- Dion, M.; Hamelin, C. Enhanced reacivation of ultraviolet-irradiated human cytomegalovirus in normal host cells. Mutat. Res. Mol. Mech. Mutagen. 1987, 179, 49–53. [Google Scholar] [CrossRef]
- Moshirfar, M.; Murri, M.S.; Shah, T.J.; Skanchy, D.F.; Tuckfield, J.Q.; Ronquillo, Y.C.; Birdsong, O.C.; Hofstedt, D.; Hoopes, P.C. A Review of Corneal Endotheliitis and Endotheliopathy: Differential Diagnosis, Evaluation, and Treatment. Ophthalmol. Ther. 2019, 8, 195–213. [Google Scholar] [CrossRef] [Green Version]
- Donaldson, M.; Pulido, J.S.; Herman, D.C.; Diehl, N.; Hodge, D. Pars Planitis: A 20-Year Study of Incidence, Clinical Features, and Outcomes. Am. J. Ophthalmol. 2007, 144, 812–817.e2. [Google Scholar] [CrossRef] [PubMed]
- Ortega Madueño, I. Estudio Serológico del Herpesvirus Humano Tipo 6 en Pacientes con Esclerosis Múltiple. 2018. Available online: https://eprints.ucm.es/50311/1/T40714.pdf (accessed on 24 May 2021).
- Blanco Kelly, F. Esclerosis Múltiple: Estudio de la Asociación de la Genética con los Factores Ambientales, la Respuesta Inmune y el Tratamiento. Ph.D. Thesis, Departamento de Microbiología, Universidad Complutense de Madrid, Madrid, Spain, 2012; 220p. Available online: https://eprints.ucm.es/id/eprint/17158/1/T34047.pdf (accessed on 9 January 2021).
- Tang, P.H.; Chandramohan, A.; Do, D.V. Evolving Understanding and Treatment of Vogt-Koyanagi-Harada Syndrome. Retin. Physician 2019, 16, 40–45. Available online: https://www.retinalphysician.com/issues/2019/jan-feb-2019/evolving-understanding-and-treatment-of-vogt-koyan (accessed on 2 May 2020).
- Ng, J.Y.; Luk, F.O.; Lai, T.Y.; Pang, C.-P. Influence of molecular genetics in Vogt-Koyanagi-Harada disease. J. Ophthalmic Inflamm. Infect. 2014, 4, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- AlGahtani, H.; Alkhotani, A.; Shirah, B. Neurological Manifestations of Acute Posterior Multifocal Placoid Pigment Epitheliopathy. J. Clin. Neurol. 2016, 12, 460–467. [Google Scholar] [CrossRef] [Green Version]
- Goldhardt, R.; Patel, H.; Davis, J.L. Acute Posterior Multifocal Placoid Pigment Epitheliopathy Following Dengue Fever: A New Association for an Old Disease. Ocul. Immunol. Inflamm. 2016, 24, 610–614. [Google Scholar] [CrossRef] [PubMed]
- Rouse, S.; Jampol, L. Chapter 157—Acute posterior multifocal placoid pigment epitheliopathy, serpiginous choroiditis, and relentless placoid chorioretinitis. In Albert & Jakobiec’s Principles & Practice of Ophthalmology, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 2008; Available online: https://doctorlib.info/ophthalmology/principles/36.html (accessed on 2 May 2020).
- Lin, H.-C.; Chen, C.-S.; Keller, J.J.; Ho, J.-D.; Lin, C.-C.; Hu, C.-C. Seasonality of Retinal Detachment Incidence and Its Associations with Climate: An 11-Year Nationwide Population-Based Study. Chronobiol. Int. 2011, 28, 942–948. [Google Scholar] [CrossRef]
- Thelen, U.; Gerding, H.; Clemens, S. Rhegmatogene Netzhautablösungen. Ophthalmologe 1997, 94, 638–641. [Google Scholar] [CrossRef]
- Bertelmann, T.; Cronauer, M.; Stoffelns, B.M.; Sekundo, W. Saisonale Variation des Auftretens rhegmatogener Netzhautablösungen zu Beginn des Jahrhunderts. Ophthalmologe 2011, 108, 1155–1163. [Google Scholar] [CrossRef]
- Prabhu, P.B.; Raju, K.V. Seasonal Variation in the Occurrence of Rhegmatogenous Retinal Detachment. Asia Pac. J. Ophthalmol. 2016, 5, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Rahman, R.; Ikram, K.; Rosen, P.H.; Cortina-Borja, M.; Taylor, M.E. Do climatic variables influence the development of posterior vitreous detachment? Br. J. Ophthalmol. 2002, 86, 829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, B.; Imrie, F.; Armbrecht, A.-M.; Dhillon, B. Age-related macular degeneration and recent developments: New hope for old eyes? Postgrad. Med. J. 2007, 83, 301–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mauget-Faÿsse, M.; Kodjikian, L.; Quaranta, M.; Ben Ezra, D.; Trepsat, C.; Mion, F.; Mégraud, F. Helicobacter pylori in central serous chorioretinopathy and diffuse retinal epitheliopathy. Results of the first prospective pilot study. J. Français Ophtalmol. 2002, 25, 1021–1025. [Google Scholar]
- Roshani, M.; Davoodi, N.A.; Seyyedmajidi, M.; Zojaji, H.; Sherafat, S.J.; Hashemi, M.; Zali, M. Association of Helicobacter pylori with central serous chorioretinopathy in Iranian patients. Gastroenterol. Hepatol. Bed Bench 2014, 7, 63–67. [Google Scholar] [PubMed]
- Ahmed, N.; Tenguria, S.; Nandanwar, N. Helicobacter pylori—A seasoned pathogen by any other name. Gut Pathog. 2009, 1, 24. [Google Scholar] [CrossRef] [Green Version]
- Aziz, R.K.; Khalifa, M.M.; Sharaf, R.R. Contaminated water as a source of Helicobacter pylori infection: A review. J. Adv. Res. 2015, 6, 539–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Environmental Variable | Cornea, Sclera and Conjunctive | Glaucoma | Cataracts | Tumours, Iris, Choroid and Ciliary Body | Uv. Inf. | Uv. No inf. | Retina | AMD and Central Serous Choroidopathy |
|---|---|---|---|---|---|---|---|---|
| Rainfall | X | X | X | X | X | |||
| Temperatures | X | X | X | X | X | X | ||
| Humidity | X | X | X | X | X | X | ||
| Wind | X | X | ||||||
| Insolation/UV Radiation | X | X | X | X | X | X | X | X |
| Air Pressure | X | |||||||
| Sea level | X | X | ||||||
| Albedo | X | X | X | X | ||||
| Ozone | X | X | ||||||
| GG | X | X | X | |||||
| PM10 and PM2.5 | X | X | X | |||||
| Other pollutants | X | X | X | |||||
| Other indirectly related factors (malnutrition, malnourishment, water consumption) | X | X | X | X |
| Pathology | National Annual Costs (€M or €B) | Attribution to Climate Change | Attributable Increase to Extreme Events (%) | Estimated Increase | Investment for Mitigation (€B) |
|---|---|---|---|---|---|
| Retinal Detachment | €16.47–66.9 M | Heatwaves | 147 | Doubling in 30 years | |
| AMD | €5.81 B | UVR Summer sun | 114 | 2% × Δ °C−1 | |
| Glaucoma | €1.99 B | UVR PM2.5 | 11 Undetermined | Five-year tripling −0.59% year−1 | |
| Cataracts | € 473 M | UVR | 10–150 | Δ4.8% year−1 | |
| Herpes zoster | €18–55 M | UVR | 14 | 2% × Δ °C−1 | |
| Herpes simplex | €235.83–274.46 M | UVR | 33 | 2% × Δ °C−1; Δ4.4%year−1 | |
| Rheumatoid Arthritis | €1.12 B | Pollution | 31 | 0.8% year−1 (1) | |
| Ankylosing Spondylitis | €10.236 B | PM2.5 | 60 (outbreaks) | −0.59% year−1 in Spain; −0.17% year−1 in Andalucía (2) | |
| Multiple Sclerosis | €1.4 B | PM10 + cold Ozone + heat | 8 (outbreaks) 16 (outbreaks) | −1.05% year−1 in Spain −0.57% year−1 in Andalucía 0.47% Year−1 according to WHO criteria (3). | |
| Inflammatory Bowel diseases | €391–844 M | Heatwaves | 4.6 (outbreaks) | Doubling in 78 years | |
| Systemic Lupus Erythematosus | €153.5–254.2 M | Sun exposition | 100 (outbreaks) 790 (sunburns) | 2% × Δ °C−1 | |
| Sarcoidosis | €784 M | PM10 PM2.5 Dryness (Δ1% decade−1) Rising temperatures (0.13–0.4 °C decade−1) | 82 Undetermined Undetermined Undetermined | −1.05% year−1 −0.59% year−1 | |
| Total Amount | €22.63–23.31 B | 36.46 (€8.37 B) | 13.15 (4) | ||
| % burden with respect National GDP (€1194 B) | 1.9–1.95 | 0.75 | 1.1 | ||
| Benefits of changing energy model | 16.5–25.7 | ||||
| % saving of National GDP (€1194 B) | 1.47–2.29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Echevarría-Lucas, L.; Senciales-González, J.M.; Medialdea-Hurtado, M.E.; Rodrigo-Comino, J. Impact of Climate Change on Eye Diseases and Associated Economical Costs. Int. J. Environ. Res. Public Health 2021, 18, 7197. https://doi.org/10.3390/ijerph18137197
Echevarría-Lucas L, Senciales-González JM, Medialdea-Hurtado ME, Rodrigo-Comino J. Impact of Climate Change on Eye Diseases and Associated Economical Costs. International Journal of Environmental Research and Public Health. 2021; 18(13):7197. https://doi.org/10.3390/ijerph18137197
Chicago/Turabian StyleEchevarría-Lucas, Lucía, José Mᵃ Senciales-González, María Eloísa Medialdea-Hurtado, and Jesús Rodrigo-Comino. 2021. "Impact of Climate Change on Eye Diseases and Associated Economical Costs" International Journal of Environmental Research and Public Health 18, no. 13: 7197. https://doi.org/10.3390/ijerph18137197
APA StyleEchevarría-Lucas, L., Senciales-González, J. M., Medialdea-Hurtado, M. E., & Rodrigo-Comino, J. (2021). Impact of Climate Change on Eye Diseases and Associated Economical Costs. International Journal of Environmental Research and Public Health, 18(13), 7197. https://doi.org/10.3390/ijerph18137197








