Evidence-Based Effects of High-Intensity Interval Training on Exercise Capacity and Health: A Review with Historical Perspective
Abstract
:1. Introduction
2. Interval Training
2.1. High-Intensity Interval Training
2.2. Sprint Interval Training
2.3. Repeated-Sprint Training
3. Effects of HIIT on Exercise Capacity and Health Based on Selected Studies
3.1. Most Early Attempts at Applying Interval Training Model
3.2. Studies on Exercise Capacity and Health in Healthy Populations
3.3. Studies on Exercise Capacity and Health in Clinical Populations
3.4. Studies on the Effects of HIIT on Glucose Tolerance and Insulin Sensitivity
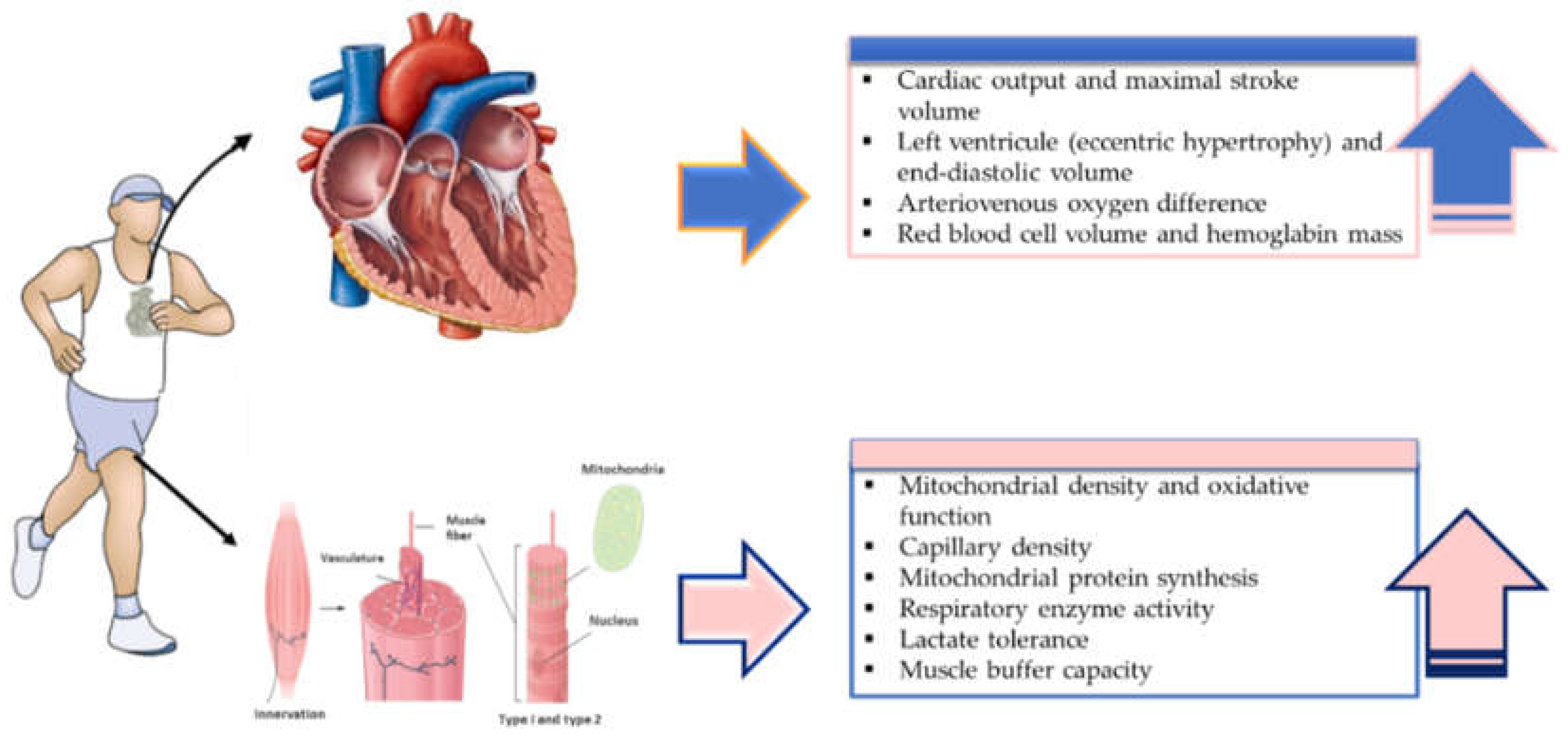
4. Physiological Mechanisms Associated with HIIT-Induced Adaptations
4.1. Adaptations in O2max and Endurance Capacity
4.1.1. Effects on O2max
4.1.2. Effects on Endurance Capacity
4.2. Skeletal Muscle Adaptations to HIIT
4.3. Adaptations to Once- or Twice-Daily HIIT
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Booth, F.W.; Roberts, C.K.; Laye, M.J. Lack of exercise is a major cause of chronic diseases. Compr. Physiol. 2012, 2, 1143–1211. [Google Scholar] [CrossRef] [Green Version]
- Warburton, D.E.; Nicol, C.W.; Bredin, S.S. Health benefits of physical activity: The evidence. CMAJ 2006, 174, 801–809. [Google Scholar] [CrossRef] [Green Version]
- Atakan, M.M.; Kosar, S.N.; Guzel, Y.; Tin, H.T.; Yan, X. The Role of Exercise, Diet, and Cytokines in Preventing Obesity and Improving Adipose Tissue. Nutrients 2021, 13, 1459. [Google Scholar] [CrossRef]
- Sylow, L.; Richter, E.A. Current advances in our understanding of exercise as medicine in metabolic disease. Curr. Opin. Physiol. 2019, 12, 12–19. [Google Scholar] [CrossRef]
- Febbraio, M.A. Exercise metabolism in 2016: Health benefits of exercise - more than meets the eye! Nat. Rev. Endocrinol. 2017, 13, 72–74. [Google Scholar] [CrossRef] [PubMed]
- Murphy, R.M.; Watt, M.J.; Febbraio, M.A. Metabolic communication during exercise. Nat. Metab. 2020, 2, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Azadpour, N.; Tartibian, B.; Kosar, S.N. Effects of aerobic exercise training on ACE and ADRB2 gene expression, plasma angiotensin II level, and flow-mediated dilation: A study on obese postmenopausal women with prehypertension. Menopause 2017, 24, 269–277. [Google Scholar] [CrossRef]
- Pedisic, Z.; Shrestha, N.; Kovalchik, S.; Stamatakis, E.; Liangruenrom, N.; Grgic, J.; Titze, S.; Biddle, S.J.; Bauman, A.E.; Oja, P. Is running associated with a lower risk of all-cause, cardiovascular and cancer mortality, and is the more the better? A systematic review and meta-analysis. Br. J. Sports Med. 2020, 54, 898–905. [Google Scholar] [CrossRef]
- Colberg, S.R.; Sigal, R.J.; Fernhall, B.; Regensteiner, J.G.; Blissmer, B.J.; Rubin, R.R.; Chasan-Taber, L.; Albright, A.L.; Braun, B.; American College of Sports, M.; et al. Exercise and type 2 diabetes: The American College of Sports Medicine and the American Diabetes Association: Joint position statement. Diabetes Care 2010, 33, 147–167. [Google Scholar] [CrossRef] [Green Version]
- Assi, M.; Dufresne, S.; Rébillard, A. Exercise shapes redox signaling in cancer. Redox Biol. 2020, 35, 101439. [Google Scholar] [CrossRef]
- Lee, S.W.; Jo, H.H.; Kim, M.R.; You, Y.O.; Kim, J.H. Association between obesity, metabolic risks and serum osteocalcin level in postmenopausal women. Gynecol. Endocrinol. 2012, 28, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Hallal, P.C.; Andersen, L.B.; Bull, F.C.; Guthold, R.; Haskell, W.; Ekelund, U. Global physical activity levels: Surveillance progress, pitfalls, and prospects. Lancet 2012, 380, 247–257. [Google Scholar] [CrossRef]
- WHO. Guidelines on Physical Activity and Sedentary Behaviour; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.-P.; Chastin, S.; Chou, R.; et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, S.; Thoma, C.; Houghton, D.; Trenell, M.I. High-intensity interval training: A review of its impact on glucose control and cardiometabolic health. Diabetologia 2017, 60, 7–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buchheit, M.; Laursen, P.B. High-intensity interval training, solutions to the programming puzzle: Part I: Cardiopulmonary emphasis. Sports Med. 2013, 43, 313–338. [Google Scholar] [CrossRef]
- Bishop, D.J.; Botella, J.; Genders, A.J.; Lee, M.J.; Saner, N.J.; Kuang, J.; Yan, X.; Granata, C. High-Intensity Exercise and Mitochondrial Biogenesis: Current Controversies and Future Research Directions. Physiology 2019, 34, 56–70. [Google Scholar] [CrossRef]
- MacInnis, M.J.; Gibala, M.J. Physiological adaptations to interval training and the role of exercise intensity. J. Physiol. 2016. [Google Scholar] [CrossRef] [Green Version]
- Gibala, M.J.; Little, J.P.; Macdonald, M.J.; Hawley, J.A. Physiological adaptations to low-volume, high-intensity interval training in health and disease. J. Physiol. 2012, 590, 1077–1084. [Google Scholar] [CrossRef]
- Pattyn, N.; Beulque, R.; Cornelissen, V. Aerobic Interval vs. Continuous Training in Patients with Coronary Artery Disease or Heart Failure: An Updated Systematic Review and Meta-Analysis with a Focus on Secondary Outcomes. Sports Med. 2018, 48, 1189–1205. [Google Scholar] [CrossRef] [PubMed]
- Pescatello, L.S. ACSM’s Guidelines for Exercise Testing and Prescription, 9th ed.; Wolters Kluwer/Lippincott Williams & Wilkins Health: Philadelphia, PA, USA, 2014; pp. 19–38. [Google Scholar]
- Astorino, T.A.; Schubert, M.M. Changes in fat oxidation in response to various regimes of high intensity interval training (HIIT). Eur. J. Appl. Physiol. 2018, 118, 51–63. [Google Scholar] [CrossRef]
- Buchheit, M.; Laursen, P.B. High-intensity interval training, solutions to the programming puzzle. Part II: Anaerobic energy, neuromuscular load and practical applications. Sports Med. 2013, 43, 927–954. [Google Scholar] [CrossRef]
- Gibala, M.J.; Bostad, W.; McCarthy, D.G. Physiological adaptations to interval training to promote endurance. Curr. Opin. Physiol. 2019, 10, 180–184. [Google Scholar] [CrossRef]
- Weston, K.S.; Wisloff, U.; Coombes, J.S. High-intensity interval training in patients with lifestyle-induced cardiometabolic disease: A systematic review and meta-analysis. Br. J. Sports Med. 2014, 48, 1227–1234. [Google Scholar] [CrossRef] [PubMed]
- Girard, O.; Mendez-Villanueva, A.; Bishop, D. Repeated-sprint ability—Part I: Factors contributing to fatigue. Sports Med. 2011, 41, 673–694. [Google Scholar] [CrossRef]
- Gray, S.R.; Ferguson, C.; Birch, K.; Forrest, L.J.; Gill, J.M. High-intensity interval training: Key data needed to bridge the gap from laboratory to public health policy. Br. J. Sports Med. 2016, 50, 1231–1232. [Google Scholar] [CrossRef] [PubMed]
- Gibala, M.J.; Jones, A.M. Physiological and performance adaptations to high-intensity interval training. Nestle Nutr. Inst. Workshop Ser. 2013, 76, 51–60. [Google Scholar] [CrossRef] [Green Version]
- Daussin, F.N.; Zoll, J.; Dufour, S.P.; Ponsot, E.; Lonsdorfer-Wolf, E.; Doutreleau, S.; Mettauer, B.; Piquard, F.; Geny, B.; Richard, R. Effect of interval versus continuous training on cardiorespiratory and mitochondrial functions: Relationship to aerobic performance improvements in sedentary subjects. Am. J. Physiol Regul Integr Comp. Physiol 2008, 295, 264–272. [Google Scholar] [CrossRef] [Green Version]
- Gorostiaga, E.M.; Walter, C.B.; Foster, C.; Hickson, R.C. Uniqueness of interval and continuous training at the same maintained exercise intensity. Eur. J. Appl. Physiol. Occup. Physiol. 1991, 63, 101–107. [Google Scholar] [CrossRef]
- Langan, S.P.; Grosicki, G.J. Exercise Is Medicine…and the Dose Matters. Front. Physiol. 2021, 12, 1–5. [Google Scholar] [CrossRef]
- Thum, J.S.; Parsons, G.; Whittle, T.; Astorino, T.A. High-Intensity Interval Training Elicits Higher Enjoyment than Moderate Intensity Continuous Exercise. PLoS ONE 2017, 12, e0166299. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, B.R.R.; Santos, T.M.; Kilpatrick, M.; Pires, F.O.; Deslandes, A.C. Affective and enjoyment responses in high intensity interval training and continuous training: A systematic review and meta-analysis. PLoS ONE 2018, 13, e0197124. [Google Scholar] [CrossRef] [Green Version]
- Reljic, D.; Lampe, D.; Wolf, F.; Zopf, Y.; Herrmann, H.J.; Fischer, J. Prevalence and predictors of dropout from high-intensity interval training in sedentary individuals: A meta-analysis. Scand. J. Med. Sci. Sports. 2019, 29, 1288–1304. [Google Scholar] [CrossRef]
- Thompson, W.R. Worldwide Survey of Fitness Trends For 2018. ACSM’s Health Fit. J. 2017, 21, 10–19. [Google Scholar] [CrossRef]
- Thompson, W.R. Worldwide Survey of Fitness Trends For 2019. ACSM’s Health Fit. J. 2018, 22, 10–17. [Google Scholar] [CrossRef]
- Batacan, R.B., Jr.; Duncan, M.J.; Dalbo, V.J.; Tucker, P.S.; Fenning, A.S. Effects of high-intensity interval training on cardiometabolic health: A systematic review and meta-analysis of intervention studies. Br. J. Sports Med. 2017, 51, 494–503. [Google Scholar] [CrossRef]
- Atakan, M.M.; Güzel, Y.; Bulut, S.; Koşar, N.; McConell, G.K.; Turnagöl, H.H. Six high-intensity interval training sessions over 5 days increases maximal oxygen uptake, endurance capacity, and sub-maximal exercise fat oxidation as much as 6 high-intensity interval training sessions over 2 weeks. J. Sport Health Sci. 2020. [Google Scholar] [CrossRef]
- Rosenblat, M.A.; Perrotta, A.S.; Thomas, S.G. Effect of High-Intensity Interval Training Versus Sprint Interval Training on Time-Trial Performance: A Systematic Review and Meta-analysis. Sports Med. 2020, 50, 1145–1161. [Google Scholar] [CrossRef]
- Schubert, M.M.; Clarke, H.E.; Seay, R.F.; Spain, K.K. Impact of 4 weeks of interval training on resting metabolic rate, fitness, and health-related outcomes. Appl. Physiol. Nutr. Metab. 2017, 42, 1073–1081. [Google Scholar] [CrossRef]
- Whyte, L.J.; Gill, J.M.; Cathcart, A.J. Effect of 2 weeks of sprint interval training on health-related outcomes in sedentary overweight/obese men. Metabolism. 2010, 59, 1421–1428. [Google Scholar] [CrossRef] [PubMed]
- Talanian, J.L.; Galloway, S.D.; Heigenhauser, G.J.; Bonen, A.; Spriet, L.L. Two weeks of high-intensity aerobic interval training increases the capacity for fat oxidation during exercise in women. J. Appl. Physiol. (1985) 2007, 102, 1439–1447. [Google Scholar] [CrossRef] [PubMed]
- Sultana, R.N.; Sabag, A.; Keating, S.E.; Johnson, N.A. The Effect of Low-Volume High-Intensity Interval Training on Body Composition and Cardiorespiratory Fitness: A Systematic Review and Meta-Analysis. Sports Med. 2019, 49, 1687–1721. [Google Scholar] [CrossRef]
- Jelleyman, C.; Yates, T.; O’Donovan, G.; Gray, L.J.; King, J.A.; Khunti, K.; Davies, M.J. The effects of high-intensity interval training on glucose regulation and insulin resistance: A meta-analysis. Obes. Rev. 2015, 16, 942–961. [Google Scholar] [CrossRef] [Green Version]
- Babraj, J.A.; Vollaard, N.B.; Keast, C.; Guppy, F.M.; Cottrell, G.; Timmons, J.A. Extremely short duration high intensity interval training substantially improves insulin action in young healthy males. BMC Endocr. Disord. 2009, 9, 3. [Google Scholar] [CrossRef] [Green Version]
- Drigny, J.; Gremeaux, V.; Dupuy, O.; Gayda, M.; Bherer, L.; Juneau, M.; Nigam, A. Effect of interval training on cognitive functioning and cerebral oxygenation in obese patients: A pilot study. J. Rehabil. Med. 2014, 46, 1050–1054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsieh, S.S.; Chueh, T.Y.; Huang, C.J.; Kao, S.C.; Hillman, C.H.; Chang, Y.K.; Hung, T.M. Systematic review of the acute and chronic effects of high-intensity interval training on executive function across the lifespan. J. Sports Sci. 2021, 39, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Mekari, S.; Neyedli, H.F.; Fraser, S.; O’Brien, M.W.; Martins, R.; Evans, K.; Earle, M.; Aucoin, R.; Chiekwe, J.; Hollohan, Q.; et al. High-Intensity Interval Training Improves Cognitive Flexibility in Older Adults. Brain Sci. 2020, 10, 796. [Google Scholar] [CrossRef]
- Mijwel, S.; Jervaeus, A.; Bolam, K.A.; Norrbom, J.; Bergh, J.; Rundqvist, H.; Wengström, Y. High-intensity exercise during chemotherapy induces beneficial effects 12 months into breast cancer survivorship. J. Cancer Surviv. 2019, 13, 244–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dun, Y.; Thomas, R.J.; Smith, J.R.; Medina-Inojosa, J.R.; Squires, R.W.; Bonikowske, A.R.; Huang, H.; Liu, S.; Olson, T.P. High-intensity interval training improves metabolic syndrome and body composition in outpatient cardiac rehabilitation patients with myocardial infarction. Cardiovasc. Diabetol. 2019, 18, 104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keogh, J.W.L.; Grigg, J.; Vertullo, C.J. Is Home-Based, High-Intensity Interval Training Cycling Feasible and Safe for Patients With Knee Osteoarthritis?: Study Protocol for a Randomized Pilot Study. Orthop. J. Sports Med. 2017, 5, 2325967117694334. [Google Scholar] [CrossRef]
- Smith-Ryan, A.E.; Blue, M.N.M.; Anderson, K.C.; Hirsch, K.R.; Allen, K.D.; Huebner, J.L.; Muehlbauer, M.J.; Ilkayeva, O.R.; Kraus, V.B.; Kraus, W.E.; et al. Metabolic and physiological effects of high intensity interval training in patients with knee osteoarthritis: A pilot and feasibility study. Osteoarthr. Cartil. Open 2020, 2, 100083. [Google Scholar] [CrossRef]
- Verbrugghe, J.; Agten, A.; Stevens, S.; Hansen, D.; Demoulin, C.; Eijnde, B.O.; Vandenabeele, F.; Timmermans, A. High Intensity Training to Treat Chronic Nonspecific Low Back Pain: Effectiveness of Various Exercise Modes. J. Clin. Med. 2020, 9, 2401. [Google Scholar] [CrossRef]
- Helmhout, P.H.; Harts, C.C.; Staal, J.B.; Candel, M.J.; de Bie, R.A. Comparison of a high-intensity and a low-intensity lumbar extensor training program as minimal intervention treatment in low back pain: A randomized trial. Eur. Spine J. 2004, 13, 537–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da Cruz Fernandes, I.M.; Pinto, R.Z.; Ferreira, P.; Lira, F.S. Low back pain, obesity, and inflammatory markers: Exercise as potential treatment. J. Exerc. Rehabil. 2018, 14, 168–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koes, B.W.; van Tulder, M.W.; Thomas, S. Diagnosis and treatment of low back pain. BMJ 2006, 332, 1430–1434. [Google Scholar] [CrossRef] [Green Version]
- Bartlett, D.B.; Willis, L.H.; Slentz, C.A.; Hoselton, A.; Kelly, L.; Huebner, J.L.; Kraus, V.B.; Moss, J.; Muehlbauer, M.J.; Spielmann, G.; et al. Ten weeks of high-intensity interval walk training is associated with reduced disease activity and improved innate immune function in older adults with rheumatoid arthritis: A pilot study. Arthritis Res. Ther. 2018, 20, 127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norton, K.; Norton, L.; Sadgrove, D. Position statement on physical activity and exercise intensity terminology. J. Sci. Med. Sport 2010, 13, 496–502. [Google Scholar] [CrossRef]
- Kjelkenes, I.; Thorsen, E. Anticipating maximal or submaximal exercise: No differences in cardiopulmonary responses. Clin. Physiol. Funct. Imaging 2010, 30, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, P.; Tschakert, G.; Stark, M.; Schwaberger, G.; Pokan, R.; Wonisch, M.; Smekal, G.; Seibert, F.; Von Duvillard, S. Estimation Error When Using The %HRR Method Compared To The Lactate Turn Point: 1505. Med. Sci. Sports Exerc. 2009, 41, 34–35. [Google Scholar] [CrossRef]
- Wonisch, M.; Hofmann, P.; Fruhwald, F.M.; Kraxner, W.; Hödl, R.; Pokan, R.; Klein, W. Influence of beta-blocker use on percentage of target heart rate exercise prescription. Eur. J. Prev. Cardiol. 2003, 10, 296–301. [Google Scholar] [CrossRef]
- Midgley, A.W.; McNaughton, L.R.; Polman, R.; Marchant, D. Criteria for determination of maximal oxygen uptake: A brief critique and recommendations for future research. Sports Med. 2007, 37, 1019–1028. [Google Scholar] [CrossRef]
- Sabag, A.; Little, J.P.; Johnson, N.A. Low-volume high-intensity interval training for cardiometabolic health. J. Physiol. 2021. [Google Scholar] [CrossRef]
- Metcalfe, R.S.; Babraj, J.A.; Fawkner, S.G.; Vollaard, N.B. Towards the minimal amount of exercise for improving metabolic health: Beneficial effects of reduced-exertion high-intensity interval training. Eur. J. Appl. Physiol. 2012, 112, 2767–2775. [Google Scholar] [CrossRef] [Green Version]
- Tjønna, A.E.; Leinan, I.M.; Bartnes, A.T.; Jenssen, B.M.; Gibala, M.J.; Winett, R.A.; Wisløff, U. Low- and high-volume of intensive endurance training significantly improves maximal oxygen uptake after 10-weeks of training in healthy men. PLoS ONE 2013, 8, e65382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poon, E.T.; Little, J.P.; Sit, C.H.; Wong, S.H. The effect of low-volume high-intensity interval training on cardiometabolic health and psychological responses in overweight/obese middle-aged men. J. Sports Sci. 2020, 38, 1997–2004. [Google Scholar] [CrossRef] [PubMed]
- Kavaliauskas, M.; Steer, T.P.; Babraj, J.A. Cardiorespiratory fitness and aerobic performance adaptations to a 4-week sprint interval training in young healthy untrained females. Sport Sci. Health 2017, 13, 17–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibala, M.J.; Gillen, J.B.; Percival, M.E. Physiological and health-related adaptations to low-volume interval training: Influences of nutrition and sex. Sports Med. 2014, 44 (Suppl. 2), 127–137. [Google Scholar] [CrossRef] [Green Version]
- Gillen, J.B.; Gibala, M.J. Is high-intensity interval training a time-efficient exercise strategy to improve health and fitness? Appl. Physiol. Nutr. Metab. 2014, 39, 409–412. [Google Scholar] [CrossRef]
- Burgomaster, K.A.; Hughes, S.C.; Heigenhauser, G.J.; Bradwell, S.N.; Gibala, M.J. Six sessions of sprint interval training increases muscle oxidative potential and cycle endurance capacity in humans. J. Appl. Physiol. (1985) 2005, 98, 1985–1990. [Google Scholar] [CrossRef]
- Gibala, M.J.; Little, J.P.; van Essen, M.; Wilkin, G.P.; Burgomaster, K.A.; Safdar, A.; Raha, S.; Tarnopolsky, M.A. Short-term sprint interval versus traditional endurance training: Similar initial adaptations in human skeletal muscle and exercise performance. J. Physiol. 2006, 575, 901–911. [Google Scholar] [CrossRef]
- Burgomaster, K.A.; Howarth, K.R.; Phillips, S.M.; Rakobowchuk, M.; Macdonald, M.J.; McGee, S.L.; Gibala, M.J. Similar metabolic adaptations during exercise after low volume sprint interval and traditional endurance training in humans. J. Physiol. 2008, 586, 151–160. [Google Scholar] [CrossRef]
- Richards, J.; Johnson, T.; Kuzma, J.; Lonac, M.; Schweder, M.; Voyles, W.; Bell, C. Short-term sprint interval training increases insulin sensitivity in healthy adults but does not affect the thermogenic response to β-adrenergic stimulation. J. Physiol. 2010, 588, 2961–2972. [Google Scholar] [CrossRef]
- Trapp, E.G.; Chisholm, D.J.; Freund, J.; Boutcher, S.H. The effects of high-intensity intermittent exercise training on fat loss and fasting insulin levels of young women. Int. J. Obes. 2008, 32, 684–691. [Google Scholar] [CrossRef] [Green Version]
- Heydari, M.; Freund, J.; Boutcher, S.H. The effect of high-intensity intermittent exercise on body composition of overweight young males. J. Obes. 2012, 2012, 480467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spencer, M.; Bishop, D.; Dawson, B.; Goodman, C. Physiological and Metabolic Responses of Repeated-Sprint Activities. Sports Med. 2005, 35, 1025–1044. [Google Scholar] [CrossRef] [PubMed]
- Bishop, D.; Girard, O.; Mendez-Villanueva, A. Repeated-Sprint Ability—Part II. Sports Med. 2011, 41, 741–756. [Google Scholar] [CrossRef] [PubMed]
- Ferrari Bravo, D.; Impellizzeri, F.M.; Rampinini, E.; Castagna, C.; Bishop, D.; Wisloff, U. Sprint vs. interval training in football. Int. J. Sports Med. 2008, 29, 668–674. [Google Scholar] [CrossRef] [Green Version]
- Fernandez-Fernandez, J.; Zimek, R.; Wiewelhove, T.; Ferrauti, A. High-intensity interval training vs. repeated-sprint training in tennis. J. Strength Cond. Res. 2012, 26, 53–62. [Google Scholar] [CrossRef]
- Galvin, H.M.; Cooke, K.; Sumners, D.P.; Mileva, K.N.; Bowtell, J.L. Repeated sprint training in normobaric hypoxia. Br. J. Sports Med. 2013, 47 (Suppl. 1), 74–79. [Google Scholar] [CrossRef]
- Taylor, J.; Macpherson, T.; Spears, I.; Weston, M. The effects of repeated-sprint training on field-based fitness measures: A meta-analysis of controlled and non-controlled trials. Sports Med. 2015, 45, 881–891. [Google Scholar] [CrossRef]
- Knuttgen, H.G.; Nordesjö, L.O.; Ollander, B.; Saltin, B. Physical conditioning through interval training with young male adults. Med. Sci. Sports 1973, 5, 220–226. [Google Scholar] [CrossRef]
- Fox, E.L.; Bartels, R.L.; Billings, C.E.; O’Brien, R.; Bason, R.; Mathews, D.K. Frequency and duration of interval training programs and changes in aerobic power. J. Appl. Physiol. 1975, 38, 481–484. [Google Scholar] [CrossRef]
- Henriksson, J.; Reitman, J.S. Quantitative measures of enzyme activities in type I and type II muscle fibres of man after training. Acta Physiol. Scand. 1976, 97, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.D.; Billeter, R.; Howald, H. Anaerobic muscle enzyme changes after interval training. Int. J. Sports Med. 1982, 3, 18–21. [Google Scholar] [CrossRef] [PubMed]
- Sharp, R.L.; Costill, D.L.; Fink, W.J.; King, D.S. Effects of eight weeks of bicycle ergometer sprint training on human muscle buffer capacity. Int. J. Sports Med. 1986, 7, 13–17. [Google Scholar] [CrossRef]
- Tabata, I.; Nishimura, K.; Kouzaki, M.; Hirai, Y.; Ogita, F.; Miyachi, M.; Yamamoto, K. Effects of moderate-intensity endurance and high-intensity intermittent training on anaerobic capacity and VO2max. Med. Sci. Sports Exerc. 1996, 28, 1327–1330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, K.; Lehmann, M.; Sünder, G.; Keul, J.; Weidemann, H. Interval versus continuous exercise training after coronary bypass surgery: A comparison of training-induced acute reactions with respect to the effectiveness of the exercise methods. Clin. Cardiol. 1990, 13, 851–861. [Google Scholar] [CrossRef]
- MacDougall, J.D.; Hicks, A.L.; MacDonald, J.R.; McKelvie, R.S.; Green, H.J.; Smith, K.M. Muscle performance and enzymatic adaptations to sprint interval training. J. Appl. Physiol. 1998, 84, 2138–2142. [Google Scholar] [CrossRef]
- Helgerud, J.; Hoydal, K.; Wang, E.; Karlsen, T.; Berg, P.; Bjerkaas, M.; Simonsen, T.; Helgesen, C.; Hjorth, N.; Bach, R.; et al. Aerobic high-intensity intervals improve VO2max more than moderate training. Med. Sci. Sports Exerc. 2007, 39, 665–671. [Google Scholar] [CrossRef] [Green Version]
- Little, J.P.; Safdar, A.; Wilkin, G.P.; Tarnopolsky, M.A.; Gibala, M.J. A practical model of low-volume high-intensity interval training induces mitochondrial biogenesis in human skeletal muscle: Potential mechanisms. J. Physiol. 2010, 588, 1011–1022. [Google Scholar] [CrossRef]
- Granata, C.; Oliveira, R.S.; Little, J.P.; Renner, K.; Bishop, D.J. Training intensity modulates changes in PGC-1alpha and p53 protein content and mitochondrial respiration, but not markers of mitochondrial content in human skeletal muscle. FASEB J. 2016, 30, 959–970. [Google Scholar] [CrossRef] [Green Version]
- Granata, C.; Oliveira, R.S.; Little, J.P.; Renner, K.; Bishop, D.J. Mitochondrial adaptations to high-volume exercise training are rapidly reversed after a reduction in training volume in human skeletal muscle. FASEB J. 2016, 30, 3413–3423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stensvold, D.; Viken, H.; Steinshamn, S.L.; Dalen, H.; Stoylen, A.; Loennechen, J.P.; Reitlo, L.S.; Zisko, N.; Baekkerud, F.H.; Tari, A.R.; et al. Effect of exercise training for five years on all cause mortality in older adults-the Generation 100 study: Randomised controlled trial. BMJ 2020, 371, m3485. [Google Scholar] [CrossRef] [PubMed]
- Kavanagh, T.; Shephard, R.J. Conditioning of postcoronary patients: Comparison of continuous and interval training. Arch. Phys. Med. Rehabil. 1975, 56, 72–76. [Google Scholar] [PubMed]
- Rognmo, O.; Hetland, E.; Helgerud, J.; Hoff, J.; Slordahl, S.A. High intensity aerobic interval exercise is superior to moderate intensity exercise for increasing aerobic capacity in patients with coronary artery disease. Eur. J. Cardiovasc. Prev. Rehabil. 2004, 11, 216–222. [Google Scholar] [CrossRef]
- Wisloff, U.; Stoylen, A.; Loennechen, J.P.; Bruvold, M.; Rognmo, O.; Haram, P.M.; Tjonna, A.E.; Helgerud, J.; Slordahl, S.A.; Lee, S.J.; et al. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: A randomized study. Circulation 2007, 115, 3086–3094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rognmo, O.; Moholdt, T.; Bakken, H.; Hole, T.; Molstad, P.; Myhr, N.E.; Grimsmo, J.; Wisloff, U. Cardiovascular risk of high- versus moderate-intensity aerobic exercise in coronary heart disease patients. Circulation 2012, 126, 1436–1440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Little, J.P.; Gillen, J.B.; Percival, M.E.; Safdar, A.; Tarnopolsky, M.A.; Punthakee, Z.; Jung, M.E.; Gibala, M.J. Low-volume high-intensity interval training reduces hyperglycemia and increases muscle mitochondrial capacity in patients with type 2 diabetes. J. Appl. Physiol. 2011, 111, 1554–1560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gillen, J.B.; Martin, B.J.; MacInnis, M.J.; Skelly, L.E.; Tarnopolsky, M.A.; Gibala, M.J. Twelve Weeks of Sprint Interval Training Improves Indices of Cardiometabolic Health Similar to Traditional Endurance Training despite a Five-Fold Lower Exercise Volume and Time Commitment. PLoS ONE 2016, 11, e0154075. [Google Scholar] [CrossRef]
- Flockhart, M.; Nilsson, L.C.; Tais, S.; Ekblom, B.; Apro, W.; Larsen, F.J. Excessive exercise training causes mitochondrial functional impairment and decreases glucose tolerance in healthy volunteers. Cell Metab. 2021, 33, 957–970. [Google Scholar] [CrossRef]
- Gibala, M.J.; Hawley, J.A. Sprinting Toward Fitness. Cell Metab. 2017, 25, 988–990. [Google Scholar] [CrossRef]
- Christensen, E.H.; Hedman, R.; Saltin, B. Intermittent and Continuous Running (A further contribution to the physiology of intermittent work.). Acta Physiol. Scand. 1960, 50, 269–286. [Google Scholar] [CrossRef]
- Fox, E.L.; Mathews, D.K. Interval Training; conditioning for sports and general fitness; Saunders: Philadelphia, PA, USA, 1974. [Google Scholar]
- Saleem, A.; Carter, H.N.; Iqbal, S.; Hood, D.A. Role of p53 within the regulatory network controlling muscle mitochondrial biogenesis. Exerc. Sport Sci. Rev. 2011, 39, 199–205. [Google Scholar] [CrossRef]
- Karabiyik, H.; Eser, M.C.; Guler, O.; Yasli, B.C.; Ertetik, G.; Sisman, A.; Koz, M.; Gabrys, T.; Pilis, K.; Karayigit, R. The Effects of 15 or 30 s SIT in Normobaric Hypoxia on Aerobic, Anaerobic Performance and Critical Power. Int. J. Environ. Res. Public Health 2021, 18, 3976. [Google Scholar] [CrossRef]
- Alarcon-Gomez, J.; Calatayud, J.; Chulvi-Medrano, I.; Martin-Rivera, F. Effects of a HIIT Protocol on Cardiovascular Risk Factors in a Type 1 Diabetes Mellitus Population. Int. J. Environ. Res. Public Health 2021, 18, 1262. [Google Scholar] [CrossRef] [PubMed]
- Iellamo, F.; Caminiti, G.; Montano, M.; Manzi, V.; Franchini, A.; Mancuso, A.; Volterrani, M. Prolonged Post-Exercise Hypotension: Effects of Different Exercise Modalities and Training Statuses in Elderly Patients with Hypertension. Int. J. Environ. Res. Public Health 2021, 18, 3229. [Google Scholar] [CrossRef]
- Jakeman, J.; Adamson, S.; Babraj, J. Extremely short duration high-intensity training substantially improves endurance performance in triathletes. Appl. Physiol. Nutr. Metab. 2012, 37, 976–981. [Google Scholar] [CrossRef]
- Liu, H.; Leng, B.; Li, Q.; Liu, Y.; Bao, D.; Cui, Y. The Effect of Eight-Week Sprint Interval Training on Aerobic Performance of Elite Badminton Players. Int. J. Environ. Res. Public Health 2021, 18, 638. [Google Scholar] [CrossRef]
- Alansare, A.; Alford, K.; Lee, S.; Church, T.; Jung, H.C. The Effects of High-Intensity Interval Training vs. Moderate-Intensity Continuous Training on Heart Rate Variability in Physically Inactive Adults. Int. J. Environ. Res. Public Health 2018, 15, 1508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herget, S.; Reichardt, S.; Grimm, A.; Petroff, D.; Kapplinger, J.; Haase, M.; Markert, J.; Bluher, S. High-Intensity Interval Training for Overweight Adolescents: Program Acceptance of a Media Supported Intervention and Changes in Body Composition. Int. J. Environ. Res. Public Health 2016, 13, 1099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuo, T.; Saotome, K.; Seino, S.; Shimojo, N.; Matsushita, A.; Iemitsu, M.; Ohshima, H.; Tanaka, K.; Mukai, C. Effects of a low-volume aerobic-type interval exercise on VO2max and cardiac mass. Med. Sci. Sports Exerc. 2014, 46, 42–50. [Google Scholar] [CrossRef]
- Shepherd, S.O.; Cocks, M.; Tipton, K.D.; Ranasinghe, A.M.; Barker, T.A.; Burniston, J.G.; Wagenmakers, A.J.; Shaw, C.S. Sprint interval and traditional endurance training increase net intramuscular triglyceride breakdown and expression of perilipin 2 and 5. J. Physiol. 2013, 591, 657–675. [Google Scholar] [CrossRef]
- Andrade-Souza, V.A.; Ghiarone, T.; Sansonio, A.; Santos Silva, K.A.; Tomazini, F.; Arcoverde, L.; Fyfe, J.; Perri, E.; Saner, N.; Kuang, J.; et al. Exercise twice-a-day potentiates markers of mitochondrial biogenesis in men. FASEB J. 2020, 34, 1602–1619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, K.; Samek, L.; Schwaibold, M.; Westbrook, S.; Hajric, R.; Lehmann, M.; Essfeld, D.; Roskamm, H. Physical responses to different modes of interval exercise in patients with chronic heart failure--application to exercise training. Eur. Heart J. 1996, 17, 1040–1047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, K.; Foster, C.; Georgakopoulos, N.; Hajric, R.; Westbrook, S.; Ellestad, A.; Tilman, K.; Fitzgerald, D.; Young, H.; Weinstein, H.; et al. Comparison of left ventricular function during interval versus steady-state exercise training in patients with chronic congestive heart failure. Am. J. Cardiol. 1998, 82, 1382–1387. [Google Scholar] [CrossRef]
- Bird, S.R.; Hawley, J.A. Update on the effects of physical activity on insulin sensitivity in humans. BMJ Open Sport Exerc. Med. 2016, 2, e000143. [Google Scholar] [CrossRef] [Green Version]
- Trost, S.G.; Owen, N.; Bauman, A.E.; Sallis, J.F.; Brown, W. Correlates of adults’ participation in physical activity: Review and update. Med. Sci. Sports Exerc. 2002, 34, 1996–2001. [Google Scholar] [CrossRef] [PubMed]
- Francois, M.E.; Little, J.P. Effectiveness and safety of high-intensity interval training in patients with type 2 diabetes. Diabetes Spectr. 2015, 28, 39–44. [Google Scholar] [CrossRef] [Green Version]
- Burgomaster, K.A.; Cermak, N.M.; Phillips, S.M.; Benton, C.R.; Bonen, A.; Gibala, M.J. Divergent response of metabolite transport proteins in human skeletal muscle after sprint interval training and detraining. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, 1970–1976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burgomaster, K.A.; Heigenhauser, G.J.; Gibala, M.J. Effect of short-term sprint interval training on human skeletal muscle carbohydrate metabolism during exercise and time-trial performance. J. Appl. Physiol. 2006, 100, 2041–2047. [Google Scholar] [CrossRef]
- Lora-Pozo, I.; Lucena-Anton, D.; Salazar, A.; Galán-Mercant, A.; Moral-Munoz, J.A. Anthropometric, Cardiopulmonary and Metabolic Benefits of the High-Intensity Interval Training Versus Moderate, Low-Intensity or Control for Type 2 Diabetes: Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2019, 16, 4524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.X.; Zhu, L.; Li, P.J.; Li, N.; Xu, Y.B. Effectiveness of high-intensity interval training on glycemic control and cardiorespiratory fitness in patients with type 2 diabetes: A systematic review and meta-analysis. Aging Clin. Exp. Res. 2019, 31, 575–593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, S.; Cai, X.; Sun, Z.; Zügel, M.; Steinacker, J.M.; Schumann, U. Aerobic Interval Training and Cardiometabolic Health in Patients with Type 2 Diabetes: A Meta-Analysis. Front. Physiol. 2017, 8, 957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saner, N.J.; Lee, M.J.; Kuang, J.; Pitchford, N.W.; Roach, G.D.; Garnham, A.; Genders, A.J.; Stokes, T.; Schroder, E.A.; Huo, Z.; et al. Exercise mitigates sleep-loss-induced changes in glucose tolerance, mitochondrial function, sarcoplasmic protein synthesis, and diurnal rhythms. Mol. Metab. 2021, 43, 101110. [Google Scholar] [CrossRef] [PubMed]
- Hawley, J.A.; Hargreaves, M.; Joyner, M.J.; Zierath, J.R. Integrative biology of exercise. Cell 2014, 159, 738–749. [Google Scholar] [CrossRef] [Green Version]
- Holloszy, J.O.; Coyle, E.F. Adaptations of skeletal muscle to endurance exercise and their metabolic consequences. J. Appl. Physiol. 1984, 56, 831–838. [Google Scholar] [CrossRef]
- Mugele, H.; Freitag, N.; Wilhelmi, J.; Yang, Y.; Cheng, S.; Bloch, W.; Schumann, M. High-intensity interval training in the therapy and aftercare of cancer patients: A systematic review with meta-analysis. J. Cancer Surviv. 2019, 13, 205–223. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Hermoso, A.; Cerrillo-Urbina, A.J.; Herrera-Valenzuela, T.; Cristi-Montero, C.; Saavedra, J.M.; Martinez-Vizcaino, V. Is high-intensity interval training more effective on improving cardiometabolic risk and aerobic capacity than other forms of exercise in overweight and obese youth? A meta-analysis. Obes. Rev. 2016, 17, 531–540. [Google Scholar] [CrossRef]
- Cao, M.; Quan, M.; Zhuang, J. Effect of High-Intensity Interval Training versus Moderate-Intensity Continuous Training on Cardiorespiratory Fitness in Children and Adolescents: A Meta-Analysis. Int. J. Environ. Res. Public Health 2019, 16, 1533. [Google Scholar] [CrossRef] [Green Version]
- Milanovic, Z.; Sporis, G.; Weston, M. Effectiveness of High-Intensity Interval Training (HIT) and Continuous Endurance Training for VO2max Improvements: A Systematic Review and Meta-Analysis of Controlled Trials. Sports Med. 2015, 45, 1469–1481. [Google Scholar] [CrossRef]
- Astorino, T.A.; Edmunds, R.M.; Clark, A.; King, L.; Gallant, R.A.; Namm, S.; Fischer, A.; Wood, K.M. High-Intensity Interval Training Increases Cardiac Output and V˙O2max. Med. Sci. Sports Exerc. 2017, 49, 265–273. [Google Scholar] [CrossRef]
- Raleigh, J.P.; Giles, M.D.; Islam, H.; Nelms, M.; Bentley, R.F.; Jones, J.H.; Neder, J.A.; Boonstra, K.; Quadrilatero, J.; Simpson, C.A.; et al. Contribution of central and peripheral adaptations to changes in maximal oxygen uptake following 4 weeks of sprint interval training. Appl. Physiol. Nutr. Metab. 2018, 43, 1059–1068. [Google Scholar] [CrossRef]
- Lundby, C.; Montero, D.; Joyner, M. Biology of VO2max: Looking under the physiology lamp. Acta Physiol. 2017, 220, 218–228. [Google Scholar] [CrossRef]
- Sloth, M.; Sloth, D.; Overgaard, K.; Dalgas, U. Effects of sprint interval training on VO2max and aerobic exercise performance: A systematic review and meta-analysis. Scand. J. Med. Sci. Sports 2013, 23, 341–352. [Google Scholar] [CrossRef]
- Vollaard, N.B.J.; Metcalfe, R.S.; Williams, S. Effect of Number of Sprints in an SIT Session on Change in V˙O2max: A Meta-analysis. Med. Sci. Sports Exerc. 2017, 49, 1147–1156. [Google Scholar] [CrossRef]
- Bentley, R.F.; Jones, J.H.; Hirai, D.M.; Zelt, J.T.; Giles, M.D.; Raleigh, J.P.; Quadrilatero, J.; Gurd, B.J.; Neder, J.A.; Tschakovsky, M.E. Submaximal exercise cardiac output is increased by 4 weeks of sprint interval training in young healthy males with low initial -O2: Importance of cardiac response phenotype. PLoS ONE 2019, 14, e0195458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horn, T.; Roverud, G.; Sutzko, K.; Browne, M.; Parra, C.; Astorino, T.A. Single session of sprint interval training elicits similar cardiac output but lower oxygen uptake versus ramp exercise to exhaustion in men and women. Int. J. Physiol. Pathophysiol. Pharmacol. 2016, 8, 87–94. [Google Scholar] [PubMed]
- Macpherson, R.E.; Hazell, T.J.; Olver, T.D.; Paterson, D.H.; Lemon, P.W. Run sprint interval training improves aerobic performance but not maximal cardiac output. Med. Sci. Sports Exerc. 2011, 43, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Warburton, D.E.; Haykowsky, M.J.; Quinney, H.A.; Blackmore, D.; Teo, K.K.; Taylor, D.A.; McGavock, J.; Humen, D.P. Blood volume expansion and cardiorespiratory function: Effects of training modality. Med. Sci. Sports Exerc. 2004, 36, 991–1000. [Google Scholar] [CrossRef]
- De Revere, J.L.; Clausen, R.D.; Astorino, T.A. Changes in VO2max and cardiac output in response to short-term high-intensity interval training in Caucasian and Hispanic young women: A pilot study. PLoS ONE 2021, 16, e0244850. [Google Scholar] [CrossRef]
- Astorino, T.A.; Edmunds, R.M.; Clark, A.; King, L.; Gallant, R.M.; Namm, S.; Fischer, A.; Wood, K.A. Increased cardiac output and maximal oxygen uptake in response to ten sessions of high intensity interval training. J. Sports Med. Phys. Fitness 2018, 58, 164–171. [Google Scholar] [CrossRef]
- Krustrup, P.; Hellsten, Y.; Bangsbo, J. Intense interval training enhances human skeletal muscle oxygen uptake in the initial phase of dynamic exercise at high but not at low intensities. J. Physiol. 2004, 559, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Juel, C.; Klarskov, C.; Nielsen, J.J.; Krustrup, P.; Mohr, M.; Bangsbo, J. Effect of high-intensity intermittent training on lactate and H+ release from human skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2004, 286, 245–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortenblad, N.; Lunde, P.K.; Levin, K.; Andersen, J.L.; Pedersen, P.K. Enhanced sarcoplasmic reticulum Ca2+ release following intermittent sprint training. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000, 279, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, B.M.; Zierath, J.R. The Limits of Exercise Physiology: From Performance to Health. Cell Metab. 2017, 25, 1000–1011. [Google Scholar] [CrossRef] [PubMed]
- Egan, B.; Zierath, J.R. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab. 2013, 17, 162–184. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Li, Y.; Atakan, M.M.; Kuang, J.; Hu, Y.; Bishop, D.J.; Yan, X. The Molecular Adaptive Responses of Skeletal Muscle to High-Intensity Exercise/Training and Hypoxia. Antioxidants 2020, 9, 656. [Google Scholar] [CrossRef]
- Egan, B.; Carson, B.P.; Garcia-Roves, P.M.; Chibalin, A.V.; Sarsfield, F.M.; Barron, N.; McCaffrey, N.; Moyna, N.M.; Zierath, J.R.; O’Gorman, D.J. Exercise intensity-dependent regulation of peroxisome proliferator-activated receptor coactivator-1 mRNA abundance is associated with differential activation of upstream signalling kinases in human skeletal muscle. J. Physiol. 2010, 588, 1779–1790. [Google Scholar] [CrossRef]
- Di Donato, D.M.; West, D.W.; Churchward-Venne, T.A.; Breen, L.; Baker, S.K.; Phillips, S.M. Influence of aerobic exercise intensity on myofibrillar and mitochondrial protein synthesis in young men during early and late postexercise recovery. Am. J. Physiol. Endocrinol. Metab. 2014, 306, 1025–1032. [Google Scholar] [CrossRef] [Green Version]
- Bishop, D.J.; Granata, C.; Eynon, N. Can we optimise the exercise training prescription to maximise improvements in mitochondria function and content? Biochim. Biophys. Acta Gen. Subj. 2014, 1840, 1266–1275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Granata, C.; Jamnick, N.A.; Bishop, D.J. Training-Induced Changes in Mitochondrial Content and Respiratory Function in Human Skeletal Muscle. Sports Med. 2018, 48, 1809–1828. [Google Scholar] [CrossRef]
- Philp, A.M.; Saner, N.J.; Lazarou, M.; Ganley, I.G.; Philp, A. The influence of aerobic exercise on mitochondrial quality control in skeletal muscle. J. Physiol. 2020. [Google Scholar] [CrossRef]
- Combes, A.; Dekerle, J.; Webborn, N.; Watt, P.; Bougault, V.; Daussin, F.N. Exercise-induced metabolic fluctuations influence AMPK, p38-MAPK and CaMKII phosphorylation in human skeletal muscle. Physiol. Rep. 2015, 3. [Google Scholar] [CrossRef]
- Ijichi, T.; Hasegawa, Y.; Morishima, T.; Kurihara, T.; Hamaoka, T.; Goto, K. Effect of sprint training: Training once daily versus twice every second day. Eur. J. Sport Sci. 2015, 15, 143–150. [Google Scholar] [CrossRef]
- Tsuchiya, Y.; Ijichi, T.; Goto, K. Effect of sprint training on resting serum irisin concentration - Sprint training once daily vs. twice every other day. Metabolism 2016, 65, 492–495. [Google Scholar] [CrossRef]
- Ghiarone, T.; Andrade-Souza, V.A.; Learsi, S.K.; Tomazini, F.; Ataide-Silva, T.; Sansonio, A.; Fernandes, M.P.; Saraiva, K.L.; Figueiredo, R.; Tourneur, Y.; et al. Twice-a-day training improves mitochondrial efficiency, but not mitochondrial biogenesis, compared with once-daily training. J. Appl. Physiol. 2019. [Google Scholar] [CrossRef]
- Hammond, K.M.; Sale, C.; Fraser, W.; Tang, J.; Shepherd, S.O.; Strauss, J.A.; Close, G.L.; Cocks, M.; Louis, J.; Pugh, J.; et al. Post-exercise carbohydrate and energy availability induce independent effects on skeletal muscle cell signalling and bone turnover: Implications for training adaptation. J. Physiol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Cochran, A.J.; Myslik, F.; MacInnis, M.J.; Percival, M.E.; Bishop, D.; Tarnopolsky, M.A.; Gibala, M.J. Manipulating Carbohydrate Availability Between Twice-Daily Sessions of High-Intensity Interval Training Over 2 Weeks Improves Time-Trial Performance. Int. J. Sport Nutr. Exerc. Metab. 2015, 25, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Yeo, W.K.; Paton, C.D.; Garnham, A.P.; Burke, L.M.; Carey, A.L.; Hawley, J.A. Skeletal muscle adaptation and performance responses to once a day versus twice every second day endurance training regimens. J. Appl. Physiol. 2008, 105, 1462–1470. [Google Scholar] [CrossRef] [Green Version]
- Cochran, A.J.; Little, J.P.; Tarnopolsky, M.A.; Gibala, M.J. Carbohydrate feeding during recovery alters the skeletal muscle metabolic response to repeated sessions of high-intensity interval exercise in humans. J. Appl. Physiol. 2010, 108, 628–636. [Google Scholar] [CrossRef] [Green Version]
- Hulston, C.J.; Venables, M.C.; Mann, C.H.; Martin, C.; Philp, A.; Baar, K.; Jeukendrup, A.E. Training with low muscle glycogen enhances fat metabolism in well-trained cyclists. Med. Sci. Sports Exerc. 2010, 42, 2046–2055. [Google Scholar] [CrossRef] [Green Version]
- Gejl, K.D.; Vissing, K.; Hansen, M.; Thams, L.; Rokkedal-Lausch, T.; Plomgaard, P.; Meinild Lundby, A.K.; Nybo, L.; Jensen, K.; Holmberg, H.C.; et al. Changes in metabolism but not myocellular signaling by training with CHO-restriction in endurance athletes. Physiol. Rep. 2018, 6, e13847. [Google Scholar] [CrossRef]
- Psilander, N.; Frank, P.; Flockhart, M.; Sahlin, K. Exercise with low glycogen increases PGC-1alpha gene expression in human skeletal muscle. Eur. J. Appl. Physiol. 2013, 113, 951–963. [Google Scholar] [CrossRef] [PubMed]
- Hansen, A.K.; Fischer, C.P.; Plomgaard, P.; Andersen, J.L.; Saltin, B.; Pedersen, B.K. Skeletal muscle adaptation: Training twice every second day vs. training once daily. J. Appl. Physiol. 2005, 98, 93–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fletcher, G.F.; Balady, G.J.; Amsterdam, E.A.; Chaitman, B.; Eckel, R.; Fleg, J.; Froelicher, V.F.; Leon, A.S.; Piña, I.L.; Rodney, R.; et al. Exercise standards for testing and training: A statement for healthcare professionals from the American Heart Association. Circulation 2001, 104, 1694–1740. [Google Scholar] [CrossRef] [Green Version]
- Mittleman, M.A.; Maclure, M.; Tofler, G.H.; Sherwood, J.B.; Goldberg, R.J.; Muller, J.E. Triggering of Acute Myocardial Infarction by Heavy Physical Exertion -- Protection against Triggering by Regular Exertion. N. Engl. J. Med. 1993, 329, 1677–1683. [Google Scholar] [CrossRef] [PubMed]
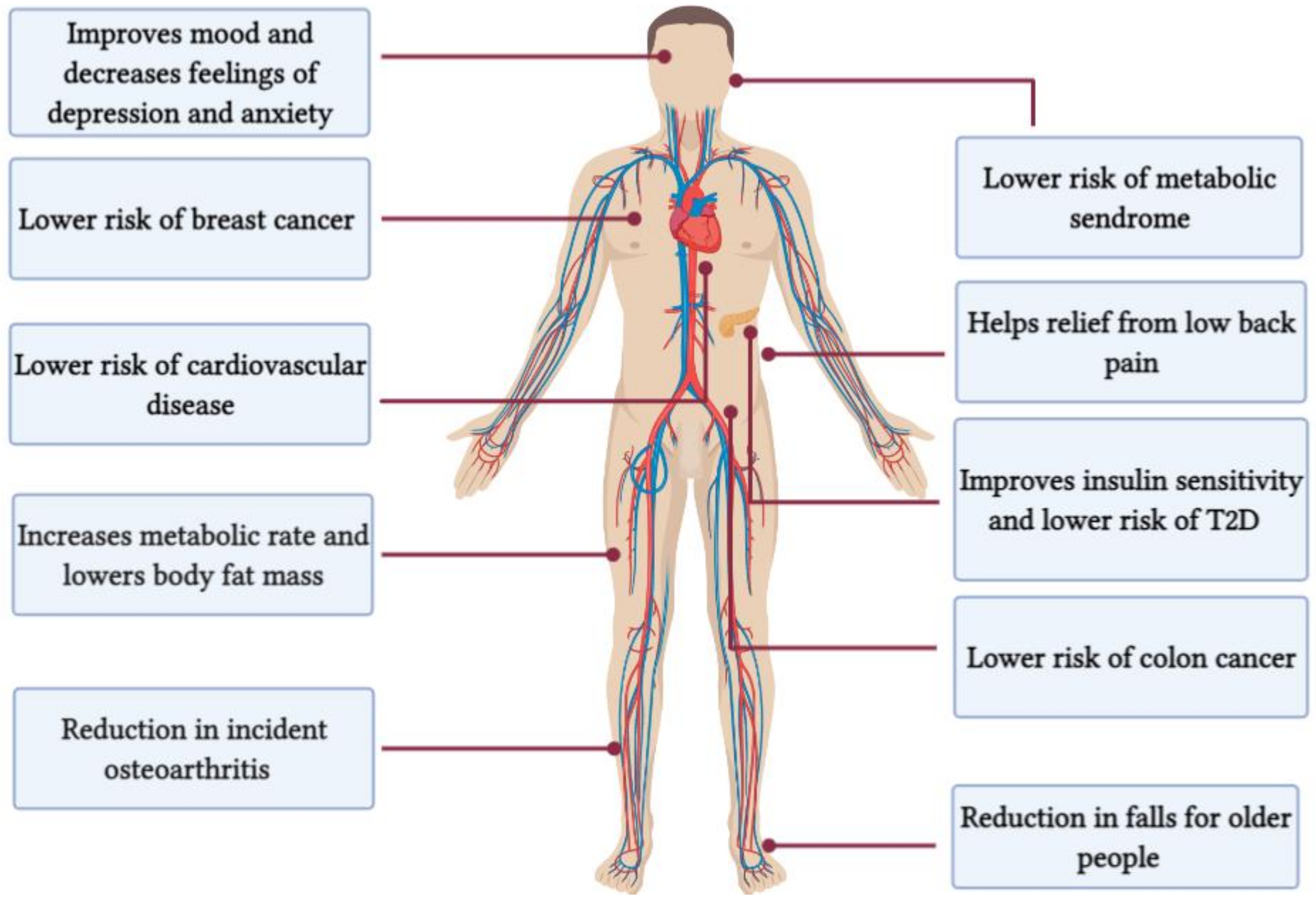

| Author | Year | Participants (O2max)(mL/kg/min) | n (M/F) | Duration; Frequency; Mode | Protocols | Main Findings | |
|---|---|---|---|---|---|---|---|
| 1 | Knuttgen et al. [82] | 1973 | Active male (~45.3 O2max) | (60/0) | 1–2-months; 3–5 days/week; cycling | Group 1: 15 s all-out and 15 s rest 3 days/week for 2 months Group 2: 3 min at O2max and 3 min rest, 3 days/week for 2 months Group 3: 15 min of strenuous exercise/sessions, 5 days/week for 1 month | Increase in O2max, with a concomitant reduction in HR at submaximal exercise. O2max (mL/kg/min) Group 1: 45.8 to 52.6 Group 2: 43.1 to 53.4 Group 3: 46.4 to 57.0 |
| 2 | Fox et al. [83] | 1975 | Young, healthy male (~45.5 O2max) | (69/0) | 7–13-weeks; 2–4 days/week; running | Group 1: 2-day of short- (50–201 m), 1 day of long- (604–1208 m), 1 day of both short- and long-distance running (4 days/week for 7 weeks) Group 2: 1 day of long-, 1 day of both short- and long-distance running (2 days/week for 7 weeks) Group 3: 2-day of short-, 1 day of long-, 1 day of short- and long-distance running (4 days/week for 13 weeks) Group 4: 1 day of long-, 1 day of both short- and long-distance running (2 days/week for 13 weeks) | Increase in O2max, with no difference between the change due to training, training frequency, or training duration. Similar decrease in HRmax in all groups. O2max (mL/kg/min) Group 1: 43.5 to 48.0 Group 2: 44.2 to 48. Group 3: 43.2 to 49.2 Group 4: 41.9 to 47.7 |
| 3 | Henriksson and Reitman [84] | 1976 | Young, healthy male (51.5 O2max) | (9) NS | 7–8-weeks; 3 days/week; cycling | Group 1: 5 × 4 min at 101% O2max, separated by 2 min rest Group 2: 27 min of continuous exercise at 79% of O2max | Increase in maximal activities of SDS in both groups. |
| 4 | Roberts et al. [85] | 1982 | Active male (NR) | (4/0) | 5-weeks; 3–4 days/week; running | 16 sessions of high-intensity interval exercise consisting of eight 200 m run at 90% of the maximal speed (HR ~179 beats/min), separated by 2 min rest periods (HR ~130 beats/min) | Increase in glycolytic enzymes (GAPDH, LDH, MDH, PFK), as well as endurance capacity (~20%). determined by a treadmill test at 16 km/h, 15% grade to exhaustion. |
| 5 | Sharp et el. [86] | 1986 | Young, healthy male (~52.7 O2max) | (15/0) | 8-weeks; 4 days/week; cycling | 8 × 30 s all-out with 4 min of rest | Increase in O2max, buffer capacity, and activity of PFK. |
| 6 | Tabata et al. [87] | 1996 | Young male (50.5 O2max) | (14/0) | 4–6-weeks; 5 days/week; cycling | Group 1: 7–8 × 20 s with 10 s rest (4 days/week)—30 min of cycling at 70% and 4 × 20 s at 170% O2max (1 day/week) Group 2: 60 min of continuous exercise at 70% O2max | Increase in O2max (10–15%) in both groups and concomitant increase in anaerobic capacity only in interval group. |
| 7 | Meyer et al. [88] | 1990 | Patients having undergone coronary bypass surgery (NR) | (18/0) | 3.5-weeks; 7 days/week; cycling | Group 1: 20–25 × 1 at 86% of HRmax, separated by 1 min of recovery at 20 W Group 2: 20–25 min of continuous exercise at 86% of HRmax | Increase physical performance and economization of cardiac function, as well as larger decrease in HR at rest and during exercise, in the interval group. |
| 8 | MacDougall et al. [89] | 1998 | Young, healthy men (47.8 O2max) | (20/0) | 7-weeks; 3 days/week; cycling | 4–10 × 30 s all-out with 2–4 min of recovery. | Increase in O2max, endurance capacity, and glycolytic and oxidative enzyme activity. |
| 9 | Gibala et al. [71] | 2006 | Active male (50.9 O2max) | (16/0) | 2-weeks; 3 days/wk; cycling | Group 1: 4–6 × 30 s at ~250% O2peak with 4 min recovery (Total time commitment: 2.5 h) Group 2: 90–120 min of continuous exercise 70% O2max (Total time commitment: ~10.5 h) | Similar increase in time to trial performance, muscle buffering capacity, and glycogen content in both groups despite markedly less time commitment in group 1. |
| 10 | Helgerud et al. [90] | 2007 | Trained male (57.9 O2max) | (40/0) | 8-weeks; 3 days/wk; running | Group 1: 45 min of running at 70% HRmax Group 2: 25 min of running at 70% HRmax Group 3: 47 × 15 s interval at 90–95% HRmax with 15 s active resting periods Group 4: 4 × 4 min at 90–95% HRmax with 3 min active resting periods at 70% HRmax | Similar increase in O2max and SV only in group 3 and group 4. |
| 11 | Little et al. [91] | 2010 | Young, healthy men (46.0 O2peak) | (7/0) | 2-weeks; 3 days/week; cycling | 8–10 × 1 min at ~100% HRpeak with 75 s recovery | ~10.0%, ~18%, 29%, ~24%, ~56%, ~119 and 17% increase in endurance capacity, CS, COX, PGC-1α, SIRT1, glucose transporter type 4, and resting muscle glycogen, respectively. |
| 12 | Granata et al. [92] | 2016 | Young, healthy men (46.3 O2peak) | (29/0) | 4-weeks; 3 days/week; cycling | Group 1: 4–10 × 30 s all out with a 2 min rest Group 2: 4–7 × 4 min at 90% O2peak with 2 min recovery at 60 W Group 3: 20–36 min at ~90% O2peak | Improved endurance capacity only in group 2 and 3; increase in PGC-1α protein content and mitochondrial respiration only in group 1. |
| 13 | Granata et al. [93] | 2016 | Young, healthy men (45.1 O2peak) | (10/0) | 14-weeks; 3 days/week; cycling | 3 consecutive training programs Program 1: normal volume training, involving 4–7 × 4 min with a 2 min recovery at 60 W (3 times/week for 4 week) Program 2: high volume training, twice a day for 20 consecutive days, involving 5–12 × 4 min intervals or 8–22 × 2 min intervals with a 1 min recovery at 60 W Program 3: 1 and 4 sessions of 4 × 4 min and 1–5 × 2 min, respectively, for 2 weeks | Increase in O2max, endurance performance, mitochondrial content, and mitochondrial respiration following high volume HIIT, and these gains returned to baseline after 2 week of reduced volume training. |
| 14 | Stensvold et al. [94] | 2020 | Older adults (28.7 O2peak) | (777/790) | 12-weeks; 2 days/week; cycling | Group 1: 4 × 4 min at 85–95% HRpeak with 3 min active recovery 60–70% HRpeak Group 2: 50 min of continuous cycling at 70% HRpeak Group 3: National recommendation (30 min of moderate-level physical activity every day without supervision) | Higher increase in O2max and physical component continuous summary score in group 1 than the other groups. No effect on all-cause mortality in group 1 and 2 compared with recommended physical activity levels. |
| 15 | Kavanagh and Shephard [95] | 1975 | Postcoronary patients (NR) | (41/0) | 1 year; 5 days/week; running | Group 1: 24–30 min of continuous training at 60–70% O2max. Group 2: 10–30 × 1 min of jogging or running at 75% of difference resting HR and HRmax, separated by 1 min of recovery at 40% difference resting HR and HRmax. | Substantial increase in aerobic power calculated based on work and oxygen of the Astrand scale in both groups, with higher gains in patients suffering frequent angina, following interval training. |
| 16 | Rognmo et al. [96] | 2004 | Coronary artery disease patients (31.9 O2peak) | (14/3) | 10-weeks; 3 days/week; running | Group 1: 4 × 4 min at 80–90% HRmax with 3 min active resting periods at 60% O2peak Group 2: 41 min of continuous running at 50–60% Opeak | 17.9% and 7.9% increase in O2max in group 1 and group 2, respectively. |
| 17 | Wisløff et al. [97] | 2008 | Postinfarction heart failure patients (13.1 O2peak) | (20/7) | 12-weeks; 3 days/week; running | Group 1: 4 × 4 min at 90–95% HRpeak with 3 min active resting periods at ~60% HRpeak Group 2: 47 min of continuous running at 70–75% HRpeak Group 3: No exercise | 46.0% and 14.0% increase in O2max in group 1 and group 2, respectively, and a 47% increase in PGC-1α only group 1. |
| 18 | Whyte et al. [41] | 2010 | Overweight and obese men (32.8 O2peak) | (10/0) | 2-weeks; 3 days/week; cycling | 4–6 × 30 s all out with 4.5 min recovery at 30 W | 8.4% and 18.2 increase in O2max and resting fat oxidation, respectively, and 24.5% and 4.7 decreases in fasting insulin and systolic blood pressure, respectively. |
| 19 | Rognmo et al. [98] | 2012 | Coronary heart disease patients (NR) | (3393/1453) | - | Group 1: 4 × 4 min at 85–95% HRpeak with 3 min active resting periods at ~60% HRpeak Group 2: 47 min of continuous running at 60–70% HRpeak | 1 nonfatal cardiac arrest during high-intensity interval exercise per 23,182 exercise hours, 1 fatal cardiac arrest during MIT per 129,456 exercise hours. |
| 20 | Babraj et al. [45] | 2009 | Young, healthy men (48.0 O2peak) | (16/0) | 2-weeks; 3 days/week; cycling | Group 1: 4–6 × 30 s all out with 4 min recovery at 30 W Group 2: No exercise | 23% and 6% improvements in insulin sensitivity, endurance capacity, and reduced fasting plasma NEFA concentrations. |
| 21 | Little et al. [99] | 2011 | Patients with type 2 diabetes (NR) | (8) NS | 2-weeks; 3 days/week; cycling | 10 × 1 min at ~90% HRpeak with 60 s rest | Reduced blood glucose concentration and improved glucose transporter type 4 protein content, muscle mitochondrial capacity, and the maximal activity of CS. |
| 22 | Gillen et al. [100] | 2016 | Sedentary men (32.5 O2peak) | (25/0) | 12-weeks; 3 days/week; cycling | Group 1: 3 × 20 s all-out with 3 min recovery at 50 W Group 2: 45 min of continuous cycling at 70% O2peak Group 3: No exercise | Similar increase in O2max, insulin sensitivity, and mitochondrial content and the maximal activity of CS in intervention groups, despite a five-fold lower exercise-volume required in group 1. |
| 25 | Flockhart et al. [101] | 2021 | Young, healthy men and women (48.4 O2max) | (5/6) | 4 weeks; progressively increased work load; cycling | 14 HIIT-sessions in total (~95% of VO2max) 1st week: 2 × 5 × 4-min 2nd week: 2 × 5 × 8-min & 1 × 5 × 4-min 3rd week: 3 × 5 × 8-min & 2 × 5 × 4-min 4th week: 2 × 3 × 8-min & 1 × 3 × 4-min & 1 × 1 × 4-min | At the end of the 1st week and 2nd week: Unaltered glucose AUC and improved PPO. 3rd week: Reduction in mitochondrial intrinsic respiration, glucose tolerance, AUC for plasma insulin, HOMA-β, and higher increase in lipid oxidation compared to 1st and 2nd week. 4th week (recovery): Partly and fully recovered glucose tolerance and HOMA-β, respectively. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atakan, M.M.; Li, Y.; Koşar, Ş.N.; Turnagöl, H.H.; Yan, X. Evidence-Based Effects of High-Intensity Interval Training on Exercise Capacity and Health: A Review with Historical Perspective. Int. J. Environ. Res. Public Health 2021, 18, 7201. https://doi.org/10.3390/ijerph18137201
Atakan MM, Li Y, Koşar ŞN, Turnagöl HH, Yan X. Evidence-Based Effects of High-Intensity Interval Training on Exercise Capacity and Health: A Review with Historical Perspective. International Journal of Environmental Research and Public Health. 2021; 18(13):7201. https://doi.org/10.3390/ijerph18137201
Chicago/Turabian StyleAtakan, Muhammed Mustafa, Yanchun Li, Şükran Nazan Koşar, Hüseyin Hüsrev Turnagöl, and Xu Yan. 2021. "Evidence-Based Effects of High-Intensity Interval Training on Exercise Capacity and Health: A Review with Historical Perspective" International Journal of Environmental Research and Public Health 18, no. 13: 7201. https://doi.org/10.3390/ijerph18137201
APA StyleAtakan, M. M., Li, Y., Koşar, Ş. N., Turnagöl, H. H., & Yan, X. (2021). Evidence-Based Effects of High-Intensity Interval Training on Exercise Capacity and Health: A Review with Historical Perspective. International Journal of Environmental Research and Public Health, 18(13), 7201. https://doi.org/10.3390/ijerph18137201







