Long-Term Residential Exposure to Particulate Matter and Its Components, Nitrogen Dioxide and Ozone—A Northern Sweden Cohort Study on Mortality
Abstract
1. Introduction
2. Methods and Materials
2.1. Study Participants
2.2. Mortality Outcomes
2.3. Exposure Assessment
2.3.1. Model Evaluation
2.3.2. Individual Exposures
2.4. Confounders
2.5. Statistical Methods
3. Results
3.1. Participant Characteristics
3.2. Particle Concentrations
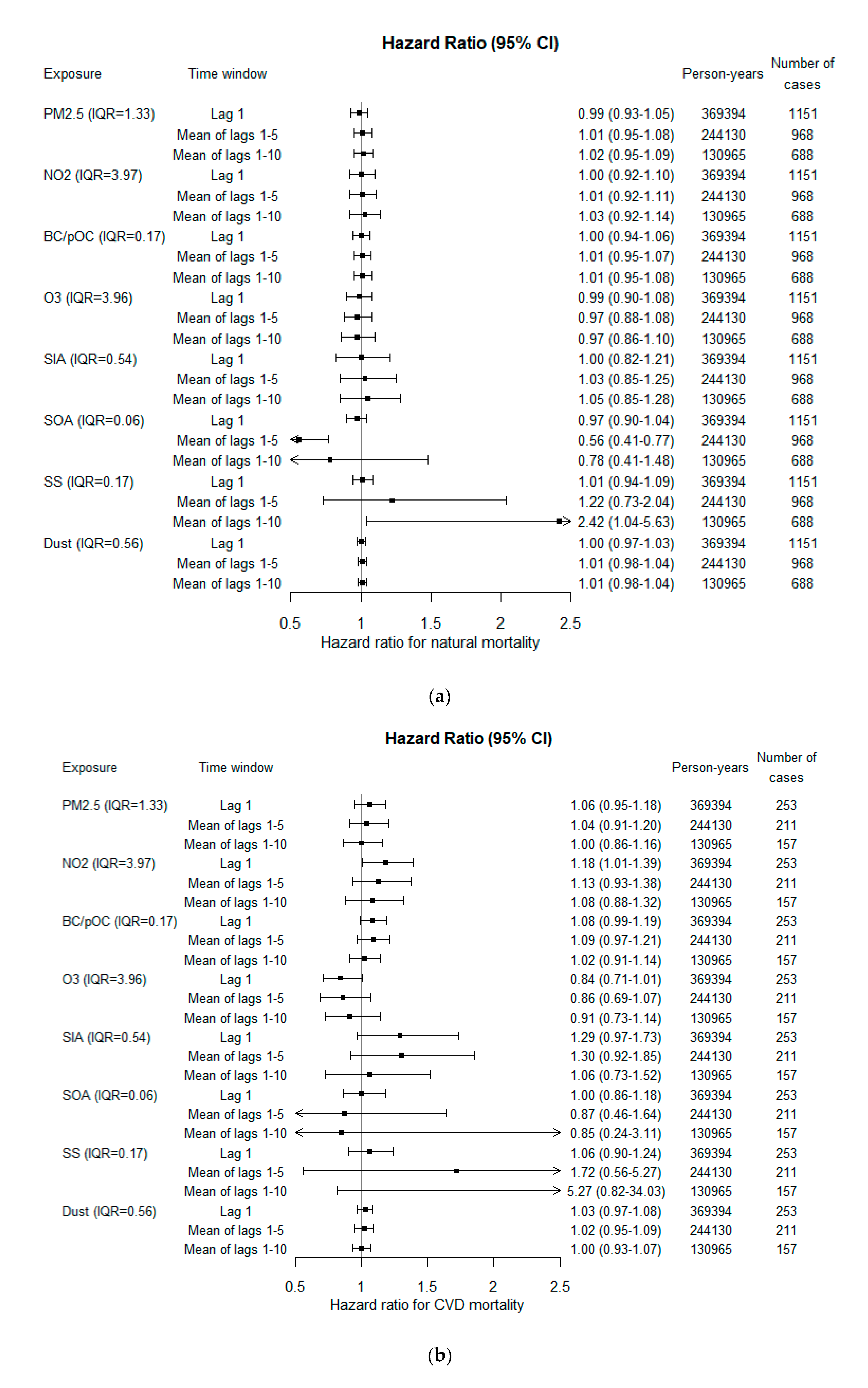
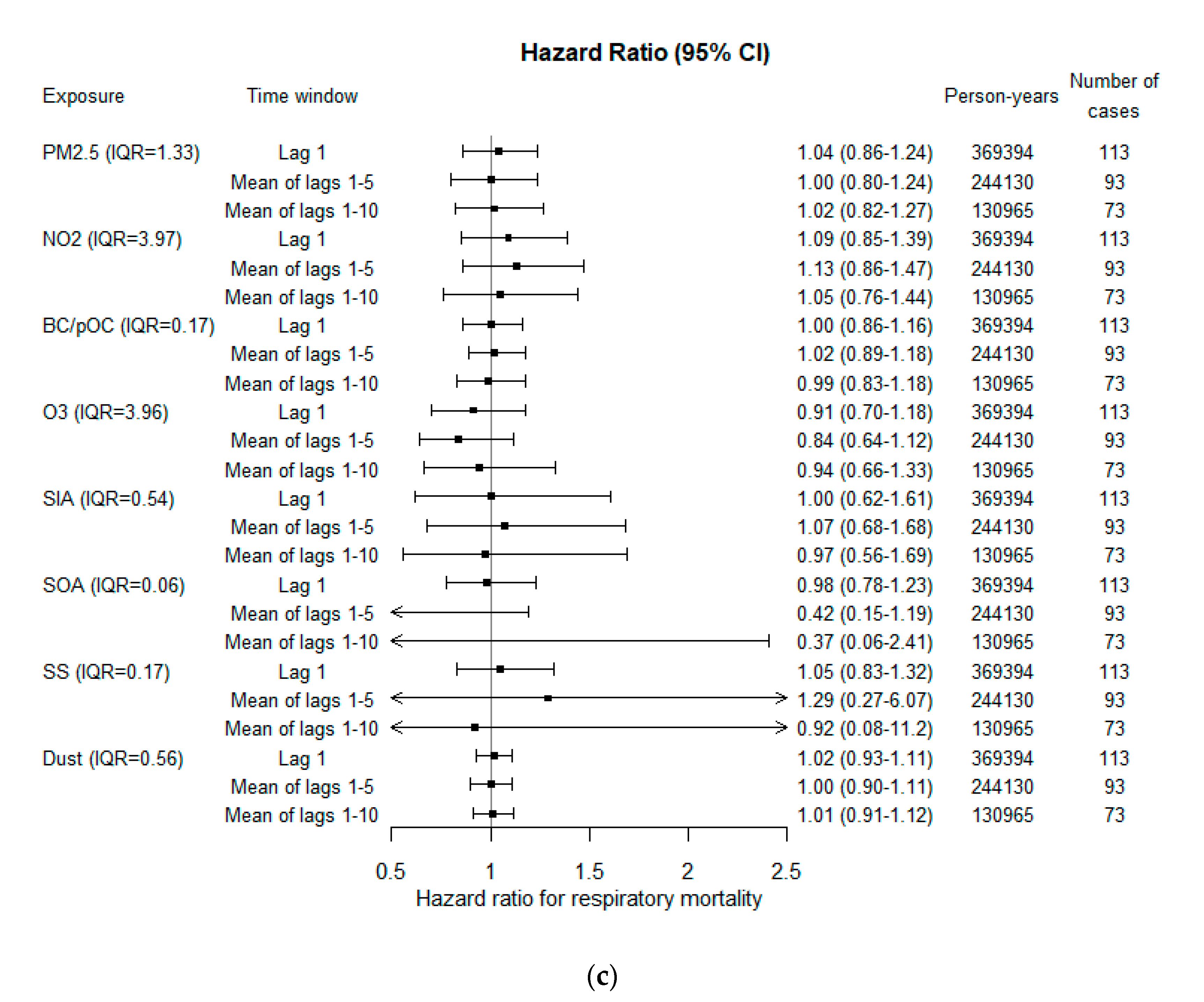
3.3. Associations with Mortality
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vodonos, A.; Abu Awad, Y.; Schwartz, J. The concentration-response between long-term PM2.5 exposure and mortality; A meta-regression approach. Environ. Res. 2018, 166, 677–689. [Google Scholar] [CrossRef]
- Pope, C.A.; Coleman, N.; Pond, Z.A.; Burnett, R.T. Fine particulate air pollution and human mortality: 25+ years of cohort studies. Environ. Res. 2020, 183, 108924. [Google Scholar] [CrossRef] [PubMed]
- Hoek, G.; Krishnan, R.M.; Beelen, R.; Peters, A.; Ostro, B.; Brunekreef, B.; Kaufman, J.D. Long-term air pollution exposure and cardio—respiratory mortality: A review. Environ. Health 2013, 12, 43. [Google Scholar] [CrossRef]
- Beelen, R.; Raaschou-Nielsen, O.; Stafoggia, M.; Andersen, Z.J.; Weinmayr, G.; Hoffmann, B.; Wolf, K.; Samoli, E.; Fischer, P.; Nieuwenhuijsen, M.; et al. Effects of long-term exposure to air pollution on natural-cause mortality: An analysis of 22 European cohorts within the multicentre ESCAPE project. Lancet 2014, 383, 785–795. [Google Scholar] [CrossRef]
- Turner, M.C.; Jerrett, M.; Pope, C.A.; Krewski, D.; Gapstur, S.M.; Diver, W.R.; Beckerman, B.S.; Marshall, J.D.; Su, J.; Crouse, D.; et al. Long-Term Ozone Exposure and Mortality in a Large Prospective Study. Am. J. Respir. Crit. Care Med. 2016, 193, 1134–1142. [Google Scholar] [CrossRef]
- Atkinson, R.W.; Butland, B.; Anderson, H.R.; Maynard, R.L. Long-term Concentrations of Nitrogen Dioxide and Mortality. Epidemiology 2018, 29, 460–472. [Google Scholar] [CrossRef]
- Huangfu, P.; Atkinson, R. Long-term exposure to NO2 and O3 and all-cause and respiratory mortality: A systematic review and meta-analysis. Environ. Int. 2020, 144, 105998. [Google Scholar] [CrossRef] [PubMed]
- Mills, I.C.; Atkinson, R.; Anderson, H.R.; Maynard, R.L.; Strachan, D.P. Distinguishing the associations between daily mortality and hospital admissions and nitrogen dioxide from those of particulate matter: A systematic review and meta-analysis. BMJ Open 2016, 6, e010751. [Google Scholar] [CrossRef]
- Mills, I.C.; Atkinson, R.; Kang, S.; Walton, H.; Anderson, H.R. Quantitative systematic review of the associations between short-term exposure to nitrogen dioxide and mortality and hospital admissions. BMJ Open 2015, 5, e006946. [Google Scholar] [CrossRef] [PubMed]
- WHO. Review of Evidence on Health Aspects of Air Pollution—REVIHAAP Project; WHO Regional Office for Europe: Copenhagen, Denmark, 2013. [Google Scholar]
- Ostro, B.; Lipsett, M.; Reynolds, P.; Goldberg, D.; Hertz, A.; Garcia, C.; Henderson, K.D.; Bernstein, L. Long-Term Exposure to Constituents of Fine Particulate Air Pollution and Mortality: Results from the California Teachers Study. Environ. Health Perspect. 2010, 118, 363–369. [Google Scholar] [CrossRef]
- Thurston, G.D.; Ahn, J.; Cromar, K.; Shao, Y.; Reynolds, H.; Jerrett, M.; Lim, C.C.; Shanley, R.; Park, Y.; Hayes, R. Ambient Particulate Matter Air Pollution Exposure and Mortality in the NIH-AARP Diet and Health Cohort. Environ. Health Perspect. 2016, 124, 484–490. [Google Scholar] [CrossRef]
- Luben, T.J.; Nichols, J.L.; Dutton, S.J.; Kirrane, E.; Owens, E.O.; Datko-Williams, L.; Madden, M.; Sacks, J. A systematic review of cardiovascular emergency department visits, hospital admissions and mortality associated with ambient black carbon. Environ. Int. 2017, 107, 154–162. [Google Scholar] [CrossRef]
- Gan, W.Q.; Koehoorn, M.; Davies, H.W.; Demers, P.A.; Tamburic, L.; Brauer, M. Long-Term Exposure to Traffic-Related Air Pollution and the Risk of Coronary Heart Disease Hospitalization and Mortality. Environ. Health Perspect. 2011, 119, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Ostro, B.; Hu, J.; Goldberg, D.; Reynolds, P.; Hertz, A.; Bernstein, L.; Kleeman, M.J. Associations of Mortality with Long-Term Exposures to Fine and Ultrafine Particles, Species and Sources: Results from the California Teachers Study Cohort. Environ. Health Perspect. 2015, 123, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Dockery, D.W.; Pope, C.A.; Xu, X.; Spengler, J.D.; Ware, J.H.; Fay, M.E.; Ferris, B.G.; Speizer, F.E. An Association between Air Pollution and Mortality in Six, U.S. Cities. N. Engl. J. Med. 1993, 329, 1753–1759. [Google Scholar] [CrossRef] [PubMed]
- Krewski, D.; Burnett, R.; Goldberg, M.; Hoover, B.K.; Siemiatycki, J.; Jerrett, M.; Abrahamowicz, M.; White, W. Overview of the Reanalysis of the Harvard Six Cities Study and American Cancer Society Study of Particulate Air Pollution and Mortality. J. Toxicol. Environ. Health Part A 2003, 66, 1507–1552. [Google Scholar] [CrossRef] [PubMed]
- Pope, C.A., III; Burnett, R.T.; Thun, M.J.; Calle, E.E.; Krewski, D.; Ito, K.; Thurston, G.D. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. J. Am. Med. Assoc. 2002, 287, 1132–1141. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ruan, Z.; Wang, X.; Yang, Y.; Mason, T.G.; Lin, H.; Tian, L. Short-term and long-term exposures to fine particulate matter constituents and health: A systematic review and meta-analysis. Environ. Pollut. 2019, 247, 874–882. [Google Scholar] [CrossRef]
- Chen, J.; Rodopoulou, S.; de Hoogh, K.; Strak, M.; Andersen, Z.J.; Atkinson, R.; Bauwelinck, M.; Bellander, T.; Brandt, J.; Cesaroni, G.; et al. Long-Term Exposure to Fine Particle Elemental Components and Natural and Cause-Specific Mortality—a Pooled Analysis of Eight European Cohorts within the ELAPSE Project. Environ. Health Perspect. 2021, 129, 47009. [Google Scholar] [CrossRef] [PubMed]
- Hvidtfeldt, U.A.; Sorensen, M.; Geels, C.; Ketzel, M.; Khan, J.; Tjonneland, A.; Overvad, K.; Brandt, J.; Raaschou-Nielsen, O. Long-term residential exposure to PM2.5, PM10, black carbon, NO2, and ozone and mortality in a Danish cohort. Environ. Int. 2019, 123, 265–272. [Google Scholar] [CrossRef]
- Hvidtfeldt, U.A.; Geels, C.; Sorensen, M.; Ketzel, M.; Khan, J.; Tjonneland, A.; Christensen, J.H.; Brandt, J.; Raaschou-Nielsen, O. Long-term residential exposure to PM2.5 constituents and mortality in a Danish cohort. Environ. Int. 2019, 133, 105268. [Google Scholar] [CrossRef]
- Chen, J.; Hoek, G. Long-term exposure to PM and all-cause and cause-specific mortality: A systematic review and meta-analysis. Environ. Int. 2020, 143, 105974. [Google Scholar] [CrossRef]
- Lefler, J.S.; Higbee, J.D.; Burnett, R.T.; Ezzati, M.; Coleman, N.C.; Mann, D.D.; Marshall, J.D.; Bechle, M.; Wang, Y.; Robinson, A.L.; et al. Air pollution and mortality in a large, representative U.S. cohort: Multiple-pollutant analyses, and spatial and temporal decompositions. Environ. Health 2019, 18, 101. [Google Scholar] [CrossRef] [PubMed]
- Norberg, M.; Wall, S.; Boman, K.; Weinehall, L. The Västerbotten Intervention Programme: Background, design and implications. Glob. Health Action 2010, 3. [Google Scholar] [CrossRef]
- Brandt, J.; Silver, J.; Frohn, L.; Geels, C.; Gross, A.; Hansen, A.; Hansen, K.M.; Hedegaard, G.; Skjøth, C.A.; Villadsen, H.; et al. An integrated model study for Europe and North America using the Danish Eulerian Hemispheric Model with focus on intercontinental transport of air pollution. Atmos. Environ. 2012, 53, 156–176. [Google Scholar] [CrossRef]
- Mareckova, K.; Wankmüller, R.; Anderl, M.; Muik, B.; Poupa, S.; Wieser, M. Inventory Review 2008: Emission Data Reported under the LRTAP Convention and NEC Directive. Status of Gridded Data. Technical Report, EMEP Centre on Emission Inventories and Projections. Available online: https://www.umweltbundesamt.at/fileadmin/site/publikationen/REP0175.pdf (accessed on 5 August 2021).
- Skamarock, W.C.; Klemp, J.B.; Dudhia, J.; Gill, D.O.; Barker, D.M.; Wang, W.; Powers, J.G. A Description of the Advanced Research WRF Version 3, Technical Report, NCAR, nCAR Tech Notes-468+STR; University Corporation for Atmospheric Research: Boulder, CO, USA, 2008. [Google Scholar]
- Soares, J.; Sofiev, M.; Geels, C.; Christensen, J.H.; Andersson, C.; Tsyro, S.; Langner, J. Impact of climate change on the production and transport of sea salt aerosol on European seas. Atmos. Chem. Phys. Discuss. 2016, 16, 13081–13104. [Google Scholar] [CrossRef]
- Zare, A.; Christensen, J.H.; Gross, A.; Irannejad, P.; Glasius, M.; Brandt, J. Quantifying the contributions of natural emissions to ozone and total fine PM concentrations in the Northern Hemisphere. Atmos. Chem. Phys. Discuss. 2014, 14, 2735–2756. [Google Scholar] [CrossRef]
- Frost, G.J.; Middleton, P.; Tarrason, L.; Granier, C.; Guenther, A.; Cárdenas, B.; Van Der Gon, H.D.; Janssens-Maenhout, G.; Kaiser, J.W.; Keating, T.; et al. New Directions: GEIA’s 2020 vision for better air emissions information. Atmos. Environ. 2013, 81, 710–712. [Google Scholar] [CrossRef][Green Version]
- Brandt, J.; Christensen, J.H.; Frohn, L.; Berkowicz, R. Air pollution forecasting from regional to urban street scale—implementation and validation for two cities in Denmark. Phys. Chem. Earth Parts A/B/C 2003, 28, 335–344. [Google Scholar] [CrossRef]
- Paunu, V.-V.; Karvosenoja, N.; Segersson, D.; López-Aparicio, S.; Nielsen, O.-K.; Schmidt Plejdrup, M.; Thorsteinsson, T.; Niemi, J.V.; Vo, D.T.; Denier van der Gon, H.A.C.; et al. Spatial distribution of residential wood combustion emissions in the Nordic countries: How well national inventories represent local emissions? Atmos. Environ. 2020. submitted. [Google Scholar]
- Ellermann, T.; Nygaard, J.; Christensen, J.H.; Løfstrøm, P.; Geels, C.; Nielsen, I.E.; Poulsen, M.B.; Monies, C.; Gyldenkærne, S.; Brandt, J.; et al. Nitrogen Deposition on Danish Nature. Atmosphere 2018, 9, 447. [Google Scholar] [CrossRef]
- Im, U.; Christensen, J.H.; Geels, C.; Hansen, K.M.; Brandt, J.; Solazzo, E.; Alyuz, U.; Balzarini, A.; Baro, R.; Bellasio, R.; et al. Influence of anthropogenic emissions and boundary conditions on multi-model simulations of major air pollutants over Europe and North America in the framework of AQMEII3. Atmos. Chem. Phys. 2018, 18, 8929–8952. [Google Scholar] [CrossRef] [PubMed]
- Vivanco, M.; Theobald, M.R.; García-Gómez, H.; Garrido, J.L.; Prank, M.; Aas, W.; Adani, M.; Alyuz, U.; Andersson, C.; Bellasio, R.; et al. Modeled deposition of nitrogen and sulfur in Europe estimated by 14 air quality model systems: Evaluation, effects of changes in emissions and implications for habitat protection. Atmos. Chem. Phys. Discuss. 2018, 18, 10199–10218. [Google Scholar] [CrossRef]
- Amini, H.; Dehlendorff, C.; Lim, Y.-H.; Mehta, A.; Jørgensen, J.T.; Mortensen, L.H.; Westendorp, R.; Hoffmann, B.; Loft, S.; Cole-Hunteret, T.; et al. Long-term exposure to air pollution and stroke incidence: A Danish Nurse Cohort study. Environ. Int. 2020, 142, 105891. [Google Scholar] [CrossRef] [PubMed]
- Antonsen, S.; Mok, P.L.H.; Webb, R.T.; Mortensen, P.B.; McGrath, J.J.; Ajerbo, E.; Brandt, J.; Geels, C.; Christensen, J.H.; Pedersen, C.B. Exposure to air pollution during childhood and risk of developing schizophrenia: A national cohort study. Lancet Planet Health 2020, 4, e64–e73. [Google Scholar] [CrossRef]
- Raaschou-Nielsen, O.; Thorsteinson, E.; Antonsen, S.; Holst, G.J.; Sigsgaard, T.; Geels, C.; Frohn, L.M.; Christensen, J.H.; Brandt, J.; Pedersen, C.B.; et al. Long-term exposure to air pollution and mortality in the Danish population a nationwide study. EClinicalMedicine 2020, 28, 100605. [Google Scholar] [CrossRef]
- Thygesen, M.; Holst, G.J.; Hansen, B.; Geels, C.; Kalkbrenner, A.; Schendel, D.; Brandt, J.; Pedersen, C.B.; Dalsgaard, S. Exposure to air pollution in early childhood and the association with Attention-Deficit Hyperactivity Disorder. Environ. Res. 2020, 183, 108930. [Google Scholar] [CrossRef]
- Ellermann, T.; Nygaard, J.; Nøjgaard, J.K.; Nordstrøm, C.; Brandt, J.; Christensen, J.; Ketzel, M.; Massling, A.; Bossi, R.; Jensen, S.S.; et al. The Danish Air Quality Monitoring Programme. Annual Summary for 2017. Scientific Report from DCE—Danish Centre for Environment and Energy No. 281. Aarhus University. 2018. Available online: http://dce2.au.dk/pub/SR281.pdf (accessed on 5 August 2021).
- Im, U.; Christensen, J.H.; Ketzel, M.; Ellermann, T.; Geels, C.; Hansen, K.M.; Hertel, O.; Nielsen, O.-K.; Plejdrup, M.S.; Brandt, J. Air Pollutant Trends over Denmark over the Last 37 Years as Simulated by the Integrated Model System THOR. In Air Pollution Modeling and Its Application XXV. ITM 2016; Mensink, C., Kallos, G., Eds.; Springer: Cham, Switzerland, 2018; pp. 49–54. [Google Scholar] [CrossRef]
- R: A Language and Environment for Statistical Computing, R Foundation for Statistical Computing. 2020. Available online: https://www.R-project.org/ (accessed on 5 August 2021).
- Brook, R.D.; Rajagopalan, S.; Pope, C.A., III; Brook, J.R.; Bhatnagar, A.; Diez-Roux, A.V.; Holguin, F.; Hong, Y.; Luepker, R.V.; Mittleman, M.A.; et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation 2010, 121, 2331–2378. [Google Scholar] [CrossRef] [PubMed]
- Beelen, R.; Stafoggia, M.; Raaschou-Nielsen, O.; Andersen, Z.J.; Xun, W.W.; Katsouyanni, K.; Dimakopoulou, K.; Brunekreef, B.; Weinmayr, G.; Hoffmann, B.; et al. Long-term exposure to air pollution and cardiovascular mortality: An analysis of 22 European cohorts. Epidemiology 2014, 25, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Achilleos, S.; Kioumourtzoglou, M.-A.; Wu, C.-D.; Schwartz, J.D.; Koutrakis, P.; Papatheodorou, S.I. Acute effects of fine particulate matter constituents on mortality: A systematic review and meta-regression analysis. Environ. Int. 2017, 109, 89–100. [Google Scholar] [CrossRef]
- Berger, K.; Malig, B.; Hasheminassab, S.; Pearson, D.L.; Sioutas, C.; Ostro, B.; Basu, R. Associations of Source-apportioned Fine Particles with Cause-specific Mortality in California. Epidemiology 2018, 29, 639–648. [Google Scholar] [CrossRef]
- Mar, T.F.; Ito, K.; Koenig, J.Q.; Larson, T.V.; Eatough, D.J.; Henry, R.C.; Kim, E.; Laden, F.; Lall, R.; Neas, L.; et al. PM source apportionment and health effects. 3. Investigation of inter-method variations in associations between estimated source contributions of PM2.5 and daily mortality in Phoenix, AZ. J. Expo. Sci. Environ. Epidemiol. 2005, 16, 311–320. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, L.; Lee, M.; Liu, P.; Di, Q.; Zanobetti, A.; Schwartz, J.D. Long-term Exposure to PM2.5 and Mortality among Older Adults in the Southeastern US. Epidemiology 2017, 28, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Thurston, G.D.; Burnett, R.T.; Turner, M.C.; Shi, Y.; Krewski, D.; Lall, R.; Ito, K.; Jerrett, M.; Gapstur, S.M.; Diver, W.R.; et al. Ischemic Heart Disease Mortality and Long-Term Exposure to Source-Related Components of U.S. Fine Particle Air Pollution. Environ. Health Perspect. 2016, 124, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.; Dominici, F.; Wang, Y.; Coull, B.A.; Bell, M.L. Associations between Long-Term Exposure to Chemical Constituents of Fine Particulate Matter (PM2.5) and Mortality in Medicare Enrollees in the Eastern United States. Environ. Health Perspect. 2015, 123, 467–474. [Google Scholar] [CrossRef]
- Beelen, R.; Hoek, G.; Raaschou-Nielsen, O.; Stafoggia, M.; Andersen, Z.J.; Weinmayr, G.; Hoffmann, B.; Wolf, K.; Samoli, E.; Fischer, P.H.; et al. Natural-Cause Mortality and Long-Term Exposure to Particle Components: An Analysis of 19 European Cohorts within the Multi-Center ESCAPE Project. Environ. Health Perspect. 2015, 123, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Lippmann, M.; Chen, L.C.; Gordon, T.; Ito, K.; Thurston, G.D. National Particle Component Toxicity (NPACT) Initiative: Integrated epidemiologic and toxicologic studies of the health effects of particulate matter components. Res. Rep. Health Eff. Inst. 2013, 177, 5–13. [Google Scholar]
- Bergström, R.; Denier van der Gon, H.A.C.; Prevot, A.; Yttri, K.E.; Simpson, D. Modelling of organic aerosols over Europe (2002–2007) using a volatility basis set (VBS) framework: Application of different assumptions regarding the formation of secondary organic aerosol. Atmos. Chem. Phys. Discuss. 2012, 12, 8499–8527. [Google Scholar] [CrossRef]
- Di, Q.; Wang, Y.; Zanobetti, A.; Wang, Y.; Koutrakis, P.; Choirat, C.; Dominici, F.; Schwartz, J.D. Air Pollution and Mortality in the Medicare Population. N. Engl. J. Med. 2017, 376, 2513–2522. [Google Scholar] [CrossRef] [PubMed]
- Pope, C.A., III; Lefler, J.S.; Ezzati, M.; Higbee, J.D.; Marshall, J.D.; Kim, S.Y.; Bechle, M.; Gilliat, K.S.; Vernon, S.E.; Robinson, A.L.; et al. Mortality Risk and Fine Particulate Air Pollution in a Large, Representative Cohort of U.S. Adults. Environ. Health Perspect 2019, 127, 77007. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Jørgensen, J.T.; Ljungman, P.; Pershagen, G.; Bellander, T.; Leander, K.; Magnusson, P.K.; Rizzuto, D.; Hvidtfeldt, U.A.; Raaschou-Nielsen, O.; et al. Long-term exposure to low-level air pollution and incidence of chronic obstructive pulmonary disease: The ELAPSE project. Environ. Int. 2021, 146, 106267. [Google Scholar] [CrossRef] [PubMed]

| All | In Tertiles of PM2.5 | ||||
|---|---|---|---|---|---|
| <4.36 µg/m3 | 4.36–4.81 µg/m3 | >4.81 µg/m3 | |||
| Participants (n) | 43,216 | 14,261 | 14,261 | 14,694 | |
| Women | 52% | 50% | 52% | 53% | |
| BMI (kg/m2; mean ± sd) | 25.5 (4.1) | 26.0 (4.4) | 25.4 (4.0) | 25.1 (3.9) | |
| Smoking status | Current smoker | 20% | 15% | 21% | 25% |
| Former smoker | 30% | 28% | 31% | 30% | |
| Never smoker | 49% | 56% | 47% | 44% | |
| Missing data | 1% | 1% | 1% | 2% | |
| Leisure time physical activity | Sedentary | 36% | 33% | 38% | 37% |
| Moderate | 42% | 37% | 42% | 45% | |
| Intermediate and vigorous | 21% | 28% | 19% | 16% | |
| Missing data | 2% | 2% | 1% | 2% | |
| Alcohol consumption | Daily | 1% | 1% | 1% | 1% |
| Weekly | 16% | 17% | 18% | 14% | |
| Seldom | 42% | 48% | 46% | 33% | |
| Never | 2% | 3% | 1% | 0% | |
| Missing data | 39% | 31% | 34% | 52% | |
| Low fruit intake | Yes | 5% | 7% | 4% | 3% |
| Missing | 15% | 3% | 9% | 34% | |
| Low vegetable intake | Yes | 4% | 5% | 4% | 4% |
| Missing | 16% | 4% | 10% | 34% | |
| Married/living with partner | Yes | 76% | 78% | 77% | 75% |
| Missing data | 1% | 1% | 1% | 1% | |
| Education level | Primary school or less | 31% | 21% | 33% | 38% |
| Up to secondary school or equivalent | 30% | 35% | 29% | 25% | |
| University degree and more | 39% | 43% | 38% | 36% | |
| Missing data | 1% | 1% | 1% | 1% | |
| Occupation | Gainfully employed | 85% | 87% | 85% | 83% |
| Unemployed/not gainfully employed | 6% | 6% | 6% | 7% | |
| Retired | 4% | 5% | 5% | 3% | |
| Missing data | 4% | 2% | 4% | 7% | |
| Mean (sd) | Median (1st Quartile–3rd Quartile) | IQR | |
|---|---|---|---|
| PM2.5 | 4.90 (1.92) | 4.55 (3.81–5.14) | 1.33 |
| NO2 | 7.09 (3.49) | 6.63 (4.82–8.79) | 3.97 |
| BC/pOC | 0.66 (0.25) | 0.62 (0.54–0.71) | 0.17 |
| O3 | 50.76 (3.27) | 50.93 (48.96–52.92) | 3.96 |
| SIA | 1.67 (0.43) | 1.73 (1.33–1.87) | 0.54 |
| SOA | 0.23 (0.05) | 0.23 (0.20–0.26) | 0.06 |
| SS | 1.02 (0.12) | 1.00 (0.95–1.12) | 0.17 |
| dust | 1.32 (1.54) | 0.98 (0.73–1.29) | 0.56 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sommar, J.N.; Hvidtfeldt, U.A.; Geels, C.; Frohn, L.M.; Brandt, J.; Christensen, J.H.; Raaschou-Nielsen, O.; Forsberg, B. Long-Term Residential Exposure to Particulate Matter and Its Components, Nitrogen Dioxide and Ozone—A Northern Sweden Cohort Study on Mortality. Int. J. Environ. Res. Public Health 2021, 18, 8476. https://doi.org/10.3390/ijerph18168476
Sommar JN, Hvidtfeldt UA, Geels C, Frohn LM, Brandt J, Christensen JH, Raaschou-Nielsen O, Forsberg B. Long-Term Residential Exposure to Particulate Matter and Its Components, Nitrogen Dioxide and Ozone—A Northern Sweden Cohort Study on Mortality. International Journal of Environmental Research and Public Health. 2021; 18(16):8476. https://doi.org/10.3390/ijerph18168476
Chicago/Turabian StyleSommar, Johan N., Ulla A. Hvidtfeldt, Camilla Geels, Lise M. Frohn, Jørgen Brandt, Jesper H. Christensen, Ole Raaschou-Nielsen, and Bertil Forsberg. 2021. "Long-Term Residential Exposure to Particulate Matter and Its Components, Nitrogen Dioxide and Ozone—A Northern Sweden Cohort Study on Mortality" International Journal of Environmental Research and Public Health 18, no. 16: 8476. https://doi.org/10.3390/ijerph18168476
APA StyleSommar, J. N., Hvidtfeldt, U. A., Geels, C., Frohn, L. M., Brandt, J., Christensen, J. H., Raaschou-Nielsen, O., & Forsberg, B. (2021). Long-Term Residential Exposure to Particulate Matter and Its Components, Nitrogen Dioxide and Ozone—A Northern Sweden Cohort Study on Mortality. International Journal of Environmental Research and Public Health, 18(16), 8476. https://doi.org/10.3390/ijerph18168476






