Abstract
We aimed to investigate whether prior exposure to antiplatelet therapy (anti-PLT) was associated with stroke incidence after the initiation of extracorporeal membrane oxygenation (ECMO) therapy. We conducted a population-based cohort study based on health records obtained from the National Health Insurance Service database in South Korea. Adult patients (aged ≥ 18 years) who underwent ECMO therapy in the intensive care unit during 2009–2018 were enrolled. In total, 17,237 patients who underwent ECMO therapy were included; stroke occurred in 779 (4.5%) of 17,237 patients within 7 days of initiating the ECMO therapy. The number of patients in the anti-PLT and control groups was 3909 (22.7%) and 13,328 (77.3%), respectively. In the multivariable logistic regression analysis, the anti-PLT group showed 33% lower incidence of stroke than the control group (odds ratio (OR): 0.67, 95% confidence interval (CI): 0.55–0.82; p < 0.001). The cardiovascular group showed 35% lower incidence of stroke than the control group (OR: 0.65, 95% CI: 0.52–0.78; p < 0.001), whereas the respiratory group (p = 0.821) and the other group (p = 0.705) did not show any significant association. Prior anti-PLT therapy was associated with a lower incidence of stroke within 7 days of initiating ECMO therapy, which was more evident in the cardiovascular group.
1. Introduction
To treat patients with refractory cardiac and/or respiratory failure, extracorporeal membrane oxygenation (ECMO) has been used as an option of rescue therapy [1,2]. The clinical indications of ECMO support include post-cardiac surgery management, heart failure, intractable arrhythmia, heart inflammation, pulmonary hypertension, severe trauma, respiratory failure, and acute respiratory distress syndrome (ARDS) [3,4,5,6,7]. In South Korea, 21,129 ECMO procedures were conducted in adult patients from 2005 to 2018, and the preference for ECMO therapy continuously increased [8].
Initiation of extracorporeal circulation stimulates inflammation and activates the coagulation cascade, leading to thrombosis of the ECMO circuit and the occurrence of thromboembolic complications after ECMO therapy [9]. A recent study reported a 100% occurrence of venous thromboembolism among 13 patients with coronavirus disease (COVID-19)-related ARDS who underwent venovenous (VV) ECMO therapy [10]. Although the clinical use of ECMO therapy as a life-saving therapy has been expanding [11], the systemic inflammatory response to extracorporeal circulation triggers various complications [12]. One of the most severe complications in critically ill patients undergoing ECMO therapy is stroke [13,14,15]. Moreover, bleeding is a frequent complication associated with ECMO therapy [16], and hemorrhagic stroke has also been frequently reported in patients undergoing ECMO therapy [13].
Thus, risk evaluation and prevention of stroke in patients undergoing ECMO are critical safety issues [17]. Antiplatelet (anti-PLT) drugs (aspirin and clopidogrel) have been used in monotherapy or in combination to prevent acute cardiac or vascular events [18]. They inhibit the coagulation effect of platelets to prevent the formation of blood clots, hence protecting the patients from fatal vascular complications such as stroke [19]. Because patients who undergo ECMO therapy have a higher risk of stroke [17], prior use of anti-PLT drugs might affect the risk of stroke after initiating ECMO therapy. However, to the best of our knowledge, no study has evaluated the impact of prior anti-PLT drug therapy on the incidence of stroke among critically ill patients undergoing ECMO.
Therefore, we aimed to investigate whether prior anti-PLT therapy was associated with the incidence of stroke after initiating ECMO therapy. We hypothesized that prior anti-PLT therapy might decrease the risk of acute ischemic stroke but increase the risk of acute hemorrhagic stroke.
2. Materials and Methods
2.1. Study Design and Ethical Statement
In this population-based cohort study, the guidelines for Reporting of Observational Studies in Epidemiology were followed [20]. The Institutional Review Board of Seoul National University Bundang Hospital (X-2001-586-902) and the Health Insurance Review and Assessment Service approved the study protocol. The need for informed consent was waived because analyses were performed retrospectively with anonymized data, which were derived from the South Korean National Health Insurance Service (NHIS) database.
2.2. NHIS Database and Study Population
In this study, the health records were extracted from the NHIS database. As the sole public health insurance system, information regarding all disease diagnoses and prescriptions corresponding to drugs and/or procedures had to be registered in the NHIS database by physicians for patients to receive financial support for any diagnostic test and treatment charges in South Korea. This study included all adult patients (aged ≥ 18 years old) who received ECMO support in the intensive care unit (ICU; outside the operating room) after hospitalization from 2009 to 2018 (10 years). The cases of Novalung therapy were not considered for ECMO in this study. For patients who received two or more episodes of ECMO therapy, only the first episode of ECMO therapy was considered in this study. Additionally, cases of ECMO therapy for stroke treatment were excluded from the analysis because we focused on the newly developed stroke after initiating ECMO. As the NHIS did not provide distinguishable prescription codes for venoarterial (VA) and VV ECMO, we classified all ECMO patients into three groups using main diagnosis at ECMO therapy: (1) cardiovascular group (cardiovascular disease, shock, or post-cardiac arrest), (2) respiratory group (ARDS or respiratory failure), and (3) other group. The NHIS contains the records of all diagnoses made during hospitalization; the diagnosis registered as the primary morbidity of treatment was then classified as the main diagnosis.
2.3. Exposure (Aspirin and Clopidogrel Use)
We defined exposure to anti-PLT drugs (aspirin and clopidogrel) based on the prescription medication from the hospital or outpatient clinic in the NHIS database. The anti-PLT group included ECMO patients who were prescribed aspirin or clopidogrel continuously for at least 1 month before starting ECMO therapy, and the control group included the remaining patients. Therefore, even if they were prescribed anti-PLT drugs in the past, they were considered as the control group if they did not take aspirin 1 month before starting ECMO therapy. The anti-PLT therapy was continued during hospitalization in almost all the patients in the anti-PLT group who could take it orally or through the enteral route, using the Levin tube. However, anti-PLT drug use was sometimes interrupted in patients who could not take it through the oral or enteral routes.
2.4. Endpoints (Stroke)
The primary endpoint was the development of stroke within 7 days of initiating ECMO therapy. A case of stroke among the ECMO patients was identified by the codes of the International Classification of Diseases, 10th revision (ICD-10) (I60–I63) following diagnostic brain imaging using computed tomography or magnetic resonance imaging. Particularly, stroke was divided as hemorrhagic stroke (I60, I61, and I62) and ischemic stroke (I63). In South Korea, the ICD-10 codes of stroke are registered in the NHIS database by a physician for financial coverage of treatment or brain imaging test after the diagnosis of stroke.
2.5. Confounders
The following factors were considered as confounders: demographic information (age and sex), socioeconomic status-related information (area of residence and annual income level during ECMO therapy), and Charlson comorbidity index scores that were calculated using the registered ICD-10 codes assigned one year before the start date of ECMO therapy (Table S1). In addition, information on the following treatment-related variables was collected: length of hospital stay (days) and duration of ECMO therapy (days). The annual volume of cases involving ECMO treatment was examined to determine the ability of the ECMO centers in South Korea to administer treatment because this factor influences the outcomes of ECMO treatment [21]. It was divided into four groups using quartile ratios (Q1, <17; Q2, 17–36; Q3, 37–80; and Q4, >81). Finally, the prior use of novel oral anticoagulants (NOAC, such as apixaban, edoxaban, rivaroxaban, and dabigatran) and the use of low-molecular-weight heparin during hospitalization were investigated and considered as confounders because they can affect anticoagulation in critically ill patients who underwent ECMO.
2.6. Statistical Analysis
The clinicoepidemiological characteristics of patients who underwent ECMO are presented as mean values with standard deviation (SD) for continuous variables and as numbers with percentages for categorical variables. First, we fitted the data using a multivariable logistic regression model for the development of stroke in the entire ECMO cohort. All the covariates were included in the multivariable model for adjustment; however, the Charlson comorbidity index was included in a multivariable model separate from other underlying diseases used to calculate the Charlson comorbidity index to avoid multicollinearity within the model. Second, we performed subgroup analyses by constructing another multivariable logistic regression model, and the anti-PLT group was divided into three groups: aspirin monotherapy group, clopidogrel monotherapy group, and dual anti-PLT group. Third, we fitted the data using a multivariable logistic regression analysis for ischemic and hemorrhagic stroke among all ECMO patients to examine whether prior anti-PLT drug use affected the different types of stroke. Fourth, we performed subgroup analyses according to the main diagnosis at ECMO therapy considering the ECMO type (cardiovascular group, respiratory group, and other group). Fifth, we fitted the data using a multivariable logistic regression model for the development of stroke among ECMO patients who survived for ≥7 days because some ECMO patients did not experience stroke because of earlier mortality before 7 days after initiating ECMO therapy. Sixth, we fitted the data using a multivariable logistic regression analysis for the development of stroke within 30 days (contrary to the 7 days in the main analysis) to examine whether these associations would differ if the duration of stroke evaluation was prolonged to 30 days after initiating ECMO therapy. Lastly, to enhance the robustness of our findings, we performed 1:1 propensity score (PS) matching between the anti-PLT and control groups to reduce the risk of bias [22]. The nearest neighbor method was used without replacement and with a caliper of 0.25 for the PS matching. All covariates were included in the PS model, and a logistic regression analysis was performed to calculate the PSs. The absolute value of the standardized mean difference (ASD) was used to evaluate the balance between the groups before and after PS matching. The ASD was set at <0.1 to confirm adequate balance between the two groups, while t-test and chi-square test were used for comparing continuous variables and categorical variables, respectively. After confirming adequate balance between the two groups, we performed a logistic regression analysis for the development of stroke in the PS-matched cohort. The results of the logistic regression are presented as odds ratios (ORs) with 95% confidence intervals (CIs). We confirmed that there was no multicollinearity in all the multivariable models of the entire ECMO cohort with a variance inflation factor of <2.0. The Hosmer–Lemeshow test was used to test the goodness of fit of the multivariable models. Additionally, receiver operating characteristic (ROC) curve analysis was performed to identify the validity of the multivariable model for the development of stroke. All statistical analyses were performed using R software (version 4.0.3 with R packages, the R Project for Statistical Computing, Vienna, Austria). p < 0.05 was considered statistically significant.
3. Results
3.1. Study Population
Between 1 January 2009 and 31 December 2018, a total of 20,182 ECMO cases were registered in 127 hospitals in South Korea. After excluding 453 pediatric patients aged < 18 years and 2384 patients who received ECMO therapy for two or more times during the study period, a total of 17,345 adult patients were initially screened. Next, 108 patients who received ECMO therapy for stroke treatment were excluded, leaving a final sample of 17,237 ECMO patients. Among them, stroke occurred in 779 ECMO patients (4.5%) within 7 days of initiating ECMO therapy. There were 509 (3.0%) cases of ischemic stroke and 296 (1.7%) cases of hemorrhagic stroke; 26 patients (0.2%) experienced both ischemic and hemorrhagic stroke within 7 days of initiating ECMO therapy (Figure 1). The clinicoepidemiological characteristics of the patients are presented in Table 1. The mean duration of brain imaging from the date of starting ECMO therapy among ECMO patients with stroke was 2.4 (SD: 3.2) days. Among all the ECMO patients, the anti-PLT group had 3909 (22.7%) patients and the control group had 13,328 (77.3%) patients.
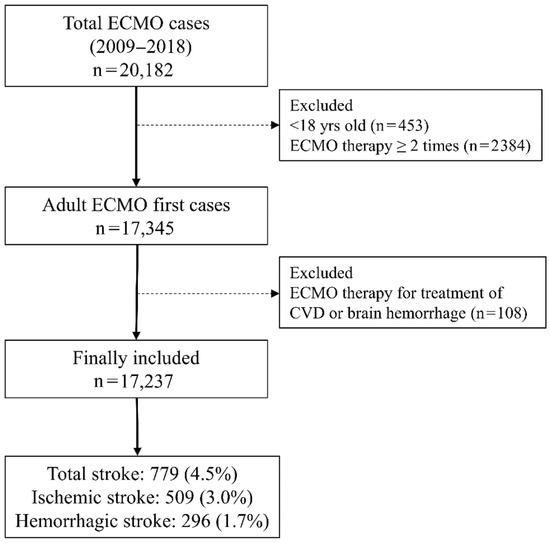
Figure 1.
Flowchart depicting the patient selection process. ECMO, extracorporeal membrane oxygenation; CVD, cardiovascular diseases.

Table 1.
Clinicoepidemiological characteristics of patients undergoing ECMO.
3.2. Incidence of Stroke in the Entire Cohort
Table 2 shows the results of the multivariable logistic regression model for the development of stroke in the entire cohort. In the multivariable model, the anti-PLT group showed a 33% lower incidence of stroke than the control group (OR: 0.67, 95% CI: 0.55–0.82; p < 0.001; Model 1). In addition, the anti-PLT group showed 37% (OR: 0.73, 95% CI: 0.55–0.92; p < 0.001; Model 2) and 44% (OR: 0.56, 95% CI: 0.40–0.80; p < 0.001; Model 3) lower incidences of ischemic and hemorrhagic stroke than the control group, respectively. In the subgroup analysis (Model 4), the aspirin monotherapy, clopidogrel monotherapy, and dual anti-PLT therapy groups showed 25% (OR: 0.75, 95% CI: 0.59–0.97; p = 0.025), 45% (OR: 0.55, 95% CI: 0.45–0.80; p = 0.007), and 37% (OR: 0.63, 95% CI: 0.44–0.88; p = 0.007) lower incidences of stroke, respectively, compared to the control group. Table S2 shows the results of the multivariable logistic regression model for stroke among ECMO patients who survived for ≥7 days (n = 11,975). Compared to the control group, the incidences of total stroke and ischemic stroke in the anti-PLT group were 26% lower (OR: 0.74, 95% CI: 0.58–0.95; p = 0.026) and 24% (OR: 0.78, 95% CI: 0.59–0.99; p = 0.048), respectively; the incidence of hemorrhagic stroke was not significantly different between the two groups (p = 0.238). Table S3 shows the results of the multivariable logistic regression analysis for stroke within 30 days (contrary to the 7 days in the main analysis) among all ECMO patients. Compared to the control group, the incidence of total stroke within 30 days in the anti-PLT group was 30% lower (OR: 0.70, 95% CI: 0.57–0.85; p < 0.001) than that in the control group.

Table 2.
Multivariable logistic regression model for stroke development in the entire cohort.
3.3. Subgroup Analyses According to Main Diagnosis at ECMO Therapy
Table 3 shows the results of the subgroup analysis according to main diagnosis at ECMO therapy, considering the ECMO type. In the cardiovascular group, the anti-PLT group shows 35% (OR: 0.65, 95% CI: 0.52–0.78; p < 0.001), 30% (OR: 0.70, 95% CI: 0.55–0.90; p = 0.006), and 50% (OR: 0.50, 95% CI: 0.35–0.75; p < 0.001) lower incidences of total, ischemic, and hemorrhagic stroke than the control group, respectively. However, the anti-PLT group in both the respiratory group and the other group did not show a significant association for total, ischemic, and hemorrhagic stroke (all p > 0.05).

Table 3.
Subgroup analyses according to main diagnosis at ECMO therapy considering ECMO type.
3.4. Sensitivity Analysis after PS Matching
Table 4 shows the results of the comparison of clinicoepidemiological characteristics between the anti-PLT and control groups before and after PS matching. Before PS matching, mean patient age in the anti-PLT group was 67.1 (SD: 10.9) years, which was higher than that of 57.2 (SD: 15.2) years in the control group (p < 0.001). After 1:1 PS matching, a total of 7818 patients who received ECMO (3909 in each group) were included in the sensitivity analysis. All the ASDs between the two groups were below 0.1 after PS matching, indicating that an adequate balance was achieved between the two groups. Table 5 shows the results of stroke incidence before and after PS matching. After PS matching, the incidences of total stroke in the anti-PLT and control groups were 4.4% (171/3909) and 6.0% (233/3909), respectively. In the logistic regression analysis of the PS-matched cohort, the anti-PLT group showed a 25% lower incidence of stroke than the control group (OR: 0.75, 95% CI: 0.60–0.90; p = 0.009). In addition, the anti-PLT group showed a 21% lower incidence of ischemic stroke (OR: 0.79, 95% CI: 0.60–0.98; p = 0.043) and a 35% lower incidence of hemorrhagic stroke (OR: 0.65, 95% CI: 0.50–0.94; p = 0.020) than the control group.

Table 4.
Comparison of clinicoepidemiological characteristics between the anti-PLT and control groups before and after PS matching.

Table 5.
Stroke incidence before and after PS matching.
4. Discussion
This population-based cohort study showed that prior anti-PLT therapy was associated with a lower incidence of stroke within 7 days of initiating ECMO therapy. Contrary to our hypothesis, the incidence of both ischemic and hemorrhagic stroke decreased in the anti-PLT group. However, in sensitivity analysis of ECMO patients who survived for ≥7 days, only ischemic stroke decreased in the anti-PLT group. Moreover, this association was most significant in the cardiovascular group than in the respiratory group and the other group. Our findings suggest that anti-PLT therapy can be a useful option in preventing stroke among patients undergoing ECMO in the ICU.
Using nationwide cohort claim data of South Korea, we reported a 4.5% incidence of total stroke among ECMO patients during the 7-day period after initiating ECMO therapy. The incidences of ischemic stroke, hemorrhagic stroke, and both ischemic and hemorrhagic stroke were 3.0%, 1.7%, and 0.2%, respectively. Previous retrospective cohort studies in single centers reported that the incidence of ischemic stroke ranged from 5.3% to 16.3% in patients undergoing ECMO [13,14,15,23]. By contrast, the incidence of hemorrhagic stroke ranged from 2.8% to 21% [13,24,25]. These previous studies used data obtained from single centers [13,14,15,23,24,25], whereas we obtained data from nationwide claims data. However, it was sometimes difficult to test critically ill patients requiring ECMO therapy for the presence of stroke because of hemodynamic instability. Thus, the actual incidence of stroke might have been underestimated in this study.
We focused on the development of stroke within 7 days of initiating ECMO therapy because of the recovery period of platelet function after discontinuation among ECMO patients in the anti-PLT group. Our results suggest that prior anti-PLT therapy might modify the risk of stroke during the 7-day period after initiating ECMO therapy by inhibiting blood clots and emboli formation [19]. Critically ill patients who undergo ECMO therapy require monitoring of coagulation function, and sometimes anticoagulation therapy is needed to prevent the development of stroke [26]. Although we did not evaluate the effect of coagulation function or anticoagulation on the risk of stroke among ECMO patients, it is possible that ECMO patients who had received anti-PLT therapy might have a lower risk of stroke than those who did not receive the anti-PLT therapy.
Interestingly, our study showed that a significant association between prior anti-PLT therapy and the occurrence of stroke was observed only in the cardiovascular group and not in the respiratory group or the other group. The cardiovascular group had a higher possibility of receiving VA ECMO than the respiratory group. In a recent cohort study, patients who underwent ECMO therapy with a primary diagnosis of acute respiratory failure constituted the VV ECMO group, whereas patients who underwent ECMO therapy with a primary diagnosis of cardiogenic shock or cardiac arrest constituted the VA ECMO group [27]. Furthermore, a recent study reported that 98% of ARDS patients associated with COVID-19 underwent VV ECMO therapy, whereas only 2% underwent VA ECMO therapy [28]. As recent studies reported that VA ECMO therapy is an independent predictor for a higher prevalence of ischemic stroke and hemorrhagic stroke [13,29], the impact of prior anti-PLT therapy might be more important in critically ill patients who underwent VA ECMO. However, we did not identify the ECMO type according to prescription codes, and this is a limitation of the present study; further studies are needed regarding this issue. Moreover, some confounding factors, other than ECMO itself, such as characteristics of the cardiovascular group might have played a role in the causation of stroke. Therefore, further study on the impact of prior anti-PLT therapy on ECMO patients considering the ECMO type is needed.
Among the covariates listed in Table 2, prior NOAC use, such as apixaban, edoxaban, rivaroxaban, and dabigatran, was associated with a 70% lower incidence of stroke among total ECMO patients in this study. NOAC use has been recommended for reducing the incidence of stroke [30]. However, the direct effect of NOAC use on reducing ischemic and hemorrhagic stroke, compared to anti-PLT drugs such as aspirin and clopidogrel, has not been identified clearly [31]. Therefore, the clinical efficacy of NOACs in preventing stroke among critically ill patients who underwent ECMO should be evaluated in the future.
Notably, the results regarding the protective effect of anti-PLT therapy against hemorrhagic stroke should be interpreted carefully because bleeding is one of the complications of anti-PLT therapy [32]. First, the pathophysiology of hemorrhagic stroke among ECMO patients is multifactorial; therefore, the impact of anti-PLT therapy on the risk of bleeding might not be an important factor [24]. Other pre-ECMO parameters, such as sequential organ failure assessment (SOFA) scores, septic shock, and platelet count, might affect the risk of hemorrhagic stroke [24]. Moreover, a rapid decrease in the partial pressure of carbon dioxide after initiating ECMO therapy can cause significant changes in cerebral blood flow because of vascular smooth muscle cell vasoconstriction, which can result in hemorrhagic stroke [33]. Second, the results of the sensitivity analysis of ECMO patients who survived for ≥7 days showed no significant association between prior anti-PLT therapy and the development of hemorrhagic stroke. A previous study reported that hemorrhagic stroke after ECMO therapy significantly increased the mortality of ECMO patients, whereas ischemic stroke was not associated with mortality [13]. This suggests that early mortality of ECMO patients might have affected the incidence of hemorrhagic stroke in our study because some ECMO patients who died due to hemorrhagic stroke had not been diagnosed with hemorrhagic stroke.
Our study has several limitations. First, some important variables, such as body mass index and lifestyle factors including history of smoking and alcohol use, were not included in this study because the NHIS database did not have a record of these data. Second, the disease severity of the patients who underwent ECMO was not evaluated and confirmed through objective methods such as the Acute Physiology and Chronic Health Disease Classification System II and SOFA scores. Third, as mentioned above, we did not distinguish between VA ECMO and VV ECMO because of limitations associated with the prescription codes for ECMO in South Korea. Therefore, it is impossible to know the proportions of patients who underwent VA ECMO and VV ECMO accurately. Fourth, the generalizability of our findings might have been limited because there were disparities between the Asian and non-Asian populations regarding risk factors for stroke [34]. Fifth, we also did not consider the incidence of ischemic stroke converting to hemorrhagic stroke. Twenty-six patients (0.2%) who experienced both ischemic and hemorrhagic stroke might have had the possibility of hemorrhagic conversion of ischemic stroke, but this issue cannot be identified accurately in this study. Sixth, there might have been some underdiagnosed stroke cases because of the use of neuromuscular blockers and sedatives, which might have affected our results. Lastly, we did not monitor coagulation function, platelet count, and anticoagulation effect among patients undergoing ECMO therapy. These three factors can influence the incidence of complications such as stroke.
5. Conclusions
In this population-based cohort study, we have shown that prior anti-PLT therapy (aspirin or clopidogrel) is associated with a lower incidence of stroke within 7 days of initiating ECMO therapy. This association was more evident in the cardiovascular group than in the respiratory group or in the other group. Therefore, prior anti-PLT therapy may not affect the risk of stroke in COVID-19 patients who underwent VV ECMO, and further study is needed to validate this finding. Although this association was observed in both ischemic and hemorrhagic stroke, the impact of anti-PLT therapy on hemorrhagic stroke should be carefully interpreted because it was not evident in ECMO patients who survived ≥7 days. Because of the retrospective study design, which is a limitation, further studies are needed to confirm these findings.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ijerph18168679/s1, Table S1. ICD-10 codes used by comorbidity to compute the Charlson comorbidity index, Table S2. Multivariable logistic regression analysis for the occurrence of stroke among patients undergoing ECMO who survived for ≥7 days (n = 11,975). Table S3. Multivariable logistic regression analysis for stroke within 30 days among total ECMO patients.
Author Contributions
Conceptualization: T.-K.O. and I.-A.S.; methodology: T.-K.O. and I.-A.S.; formal analysis: T.-K.O. and H.-R.C.; investigation: T.-K.O. and I.-A.S.; resources: S.-Y.L. and H.-R.C.; data curation: S.-Y.L. and H.-R.C.; writing—original draft preparation: T.-K.O. and H.-R.C.; writing—review and editing: S.-Y.L. and H.-R.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The Institutional Review Board of Seoul National University Bundang Hospital (X-2001-586-902) and the Health Insurance Review and Assessment Service approved the study protocol.
Informed Consent Statement
The need for informed consent was waived because the analyses were performed retrospectively with anonymized data, which were derived from the South Korean National Health Insurance Service (NHIS) database.
Data Availability Statement
Data will be available upon reasonable request to corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gattinoni, L.; Carlesso, E.; Langer, T. Clinical review: Extracorporeal membrane oxygenation. Crit. Care 2011, 15, 243. [Google Scholar] [CrossRef] [Green Version]
- Combes, A.; Hajage, D.; Capellier, G.; Demoule, A.; Lavoue, S.; Guervilly, C.; Da Silva, D.; Zafrani, L.; Tirot, P.; Veber, B.; et al. Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2018, 378, 1965–1975. [Google Scholar] [CrossRef]
- Lafc, G.; Budak, A.B.; Yener, A.U.; Cicek, O.F. Use of extracorporeal membrane oxygenation in adults. Heart Lung Circ. 2014, 23, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Tramm, R.; Ilic, D.; Davies, A.R.; Pellegrino, V.A.; Romero, L.; Hodgson, C. Extracorporeal membrane oxygenation for critically ill adults. Cochrane Database Syst. Rev. 2015, 1, CD010381. [Google Scholar] [CrossRef]
- Eckman, P.M.; Katz, J.N.; El Banayosy, A.; Bohula, E.A.; Sun, B.; van Diepen, S. Veno-Arterial Extracorporeal Membrane Oxygenation for Cardiogenic Shock: An Introduction for the Busy Clinician. Circulation 2019, 140, 2019–2037. [Google Scholar] [CrossRef] [PubMed]
- Brodie, D.; Slutsky, A.S.; Combes, A. Extracorporeal Life Support for Adults with Respiratory Failure and Related Indications: A Review. JAMA 2019, 322, 557–568. [Google Scholar] [CrossRef]
- Aneman, A.; Brechot, N.; Brodie, D.; Colreavy, F.; Fraser, J.; Gomersall, C.; McCanny, P.; Moller-Sorensen, P.H.; Takala, J.; Valchanov, K.; et al. Advances in critical care management of patients undergoing cardiac surgery. Intensive Care Med. 2018, 44, 799–810. [Google Scholar] [CrossRef]
- Cho, H.W.; Song, I.A.; Oh, T.K. Trends in extracorporeal membrane oxygenation treatment from 2005 to 2018 in South Korea. Perfusion 2021, 2676591211018130. [Google Scholar] [CrossRef] [PubMed]
- Murphy, D.A.; Hockings, L.E.; Andrews, R.K.; Aubron, C.; Gardiner, E.E.; Pellegrino, V.A.; Davis, A.K. Extracorporeal membrane oxygenation-hemostatic complications. Transfus. Med. Rev. 2015, 29, 90–101. [Google Scholar] [CrossRef]
- Parzy, G.; Daviet, F.; Puech, B.; Sylvestre, A.; Guervilly, C.; Porto, A.; Hraiech, S.; Chaumoitre, K.; Papazian, L.; Forel, J.M. Venous Thromboembolism Events Following Venovenous Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Syndrome Coronavirus 2 Based on CT Scans. Crit. Care Med. 2020, 48, e971–e975. [Google Scholar] [CrossRef]
- McCarthy, F.H.; McDermott, K.M.; Kini, V.; Gutsche, J.T.; Wald, J.W.; Xie, D.; Szeto, W.Y.; Bermudez, C.A.; Atluri, P.; Acker, M.A.; et al. Trends in U.S. Extracorporeal Membrane Oxygenation Use and Outcomes: 2002–2012. Semin. Thorac. Cardiovasc. Surg. 2015, 27, 81–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Millar, J.E.; Fanning, J.P.; McDonald, C.I.; McAuley, D.F.; Fraser, J.F. The inflammatory response to extracorporeal membrane oxygenation (ECMO): A review of the pathophysiology. Crit. Care 2016, 20, 387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Guennec, L.; Cholet, C.; Huang, F.; Schmidt, M.; Brechot, N.; Hekimian, G.; Besset, S.; Lebreton, G.; Nieszkowska, A.; Leprince, P.; et al. Ischemic and hemorrhagic brain injury during venoarterial-extracorporeal membrane oxygenation. Ann. Intensive Care 2018, 8, 129. [Google Scholar] [CrossRef] [Green Version]
- Omar, H.R.; Mirsaeidi, M.; Shumac, J.; Enten, G.; Mangar, D.; Camporesi, E.M. Incidence and predictors of ischemic cerebrovascular stroke among patients on extracorporeal membrane oxygenation support. J. Crit. Care 2016, 32, 48–51. [Google Scholar] [CrossRef]
- Saeed, O.; Jakobleff, W.A.; Forest, S.J.; Chinnadurai, T.; Mellas, N.; Rangasamy, S.; Xia, Y.; Madan, S.; Acharya, P.; Algodi, M.; et al. Hemolysis and Nonhemorrhagic Stroke during Venoarterial Extracorporeal Membrane Oxygenation. Ann. Thorac. Surg. 2019, 108, 756–763. [Google Scholar] [CrossRef] [PubMed]
- Aubron, C.; DePuydt, J.; Belon, F.; Bailey, M.; Schmidt, M.; Sheldrake, J.; Murphy, D.; Scheinkestel, C.; Cooper, D.J.; Capellier, G.; et al. Predictive factors of bleeding events in adults undergoing extracorporeal membrane oxygenation. Ann. Intensive Care 2016, 6, 97. [Google Scholar] [CrossRef] [Green Version]
- Xie, A.; Lo, P.; Yan, T.D.; Forrest, P. Neurologic Complications of Extracorporeal Membrane Oxygenation: A Review. J. Cardiothorac. Vasc. Anesth. 2017, 31, 1836–1846. [Google Scholar] [CrossRef]
- Tendera, M.; Wojakowski, W. Role of antiplatelet drugs in the prevention of cardiovascular events. Thromb. Res. 2003, 110, 355–359. [Google Scholar] [CrossRef]
- Oprea, A.D.; Popescu, W.M. Perioperative management of antiplatelet therapy. Br. J. Anaesth. 2013, 111 (Suppl. 1), i3–i17. [Google Scholar] [CrossRef] [Green Version]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gotzsche, P.C.; Vandenbroucke, J.P.; Initiative, S. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for reporting observational studies. Int. J. Surg. 2014, 12, 1495–1499. [Google Scholar] [CrossRef] [Green Version]
- Barbaro, R.P.; Odetola, F.O.; Kidwell, K.M.; Paden, M.L.; Bartlett, R.H.; Davis, M.M.; Annich, G.M. Association of hospital-level volume of extracorporeal membrane oxygenation cases and mortality. Analysis of the extracorporeal life support organization registry. Am. J. Respir. Crit. Care Med. 2015, 191, 894–901. [Google Scholar] [CrossRef] [Green Version]
- Rosenbaum, P.R.; Rubin, D.B. The central role of the propensity score in observational studies for causal effects. Biometrika 1983, 70, 41–55. [Google Scholar] [CrossRef]
- Malfertheiner, M.V.; Koch, A.; Fisser, C.; Millar, J.E.; Maier, L.S.; Zeman, F.; Poschenrieder, F.; Lubnow, M.; Philipp, A.; Muller, T. Incidence of early intra-cranial bleeding and ischaemia in adult veno-arterial extracorporeal membrane oxygenation and extracorporeal cardiopulmonary resuscitation patients: A retrospective analysis of risk factors. Perfusion 2020, 35, 8–17. [Google Scholar] [CrossRef]
- Fletcher-Sandersjoo, A.; Bartek, J., Jr.; Thelin, E.P.; Eriksson, A.; Elmi-Terander, A.; Broman, M.; Bellander, B.M. Predictors of intracranial hemorrhage in adult patients on extracorporeal membrane oxygenation: An observational cohort study. J. Intensive Care 2017, 5, 27. [Google Scholar] [CrossRef] [Green Version]
- Kasirajan, V.; Smedira, N.G.; McCarthy, J.F.; Casselman, F.; Boparai, N.; McCarthy, P.M. Risk factors for intracranial hemorrhage in adults on extracorporeal membrane oxygenation. Eur. J. Cardiothorac. Surg. 1999, 15, 508–514. [Google Scholar] [CrossRef]
- Koster, A.; Ljajikj, E.; Faraoni, D. Traditional and non-traditional anticoagulation management during extracorporeal membrane oxygenation. Ann. Cardiothorac. Surg. 2019, 8, 129–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, W.; Ma, X.D.; Su, L.X.; He, H.W.; Wang, L.; Tang, B.; Du, W.; Zhou, Y.K.; Wang, H.; Cui, N.; et al. Cross-sectional study for the clinical application of extracorporeal membrane oxygenation in Mainland China, 2018. Crit. Care 2020, 24, 554. [Google Scholar] [CrossRef]
- Schmidt, M.; Hajage, D.; Lebreton, G.; Monsel, A.; Voiriot, G.; Levy, D.; Baron, E.; Beurton, A.; Chommeloux, J.; Meng, P.; et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome associated with COVID-19: A retrospective cohort study. Lancet Respir. Med. 2020, 8, 1121–1131. [Google Scholar] [CrossRef]
- Iacobelli, R.; Fletcher-Sandersjoo, A.; Lindblad, C.; Keselman, B.; Thelin, E.P.; Broman, L.M. Predictors of brain infarction in adult patients on extracorporeal membrane oxygenation: An observational cohort study. Sci. Rep. 2021, 11, 3809. [Google Scholar] [CrossRef]
- Shrestha, S.; Coy, S.; Bekelis, K. Oral Antiplatelet and Anticoagulant Agents in the Prevention and Management of Ischemic Stroke. Curr. Pharm. Des. 2017, 23, 1377–1391. [Google Scholar] [CrossRef]
- Geisler, T.; Poli, S.; Meisner, C.; Schreieck, J.; Zuern, C.S.; Nagele, T.; Brachmann, J.; Jung, W.; Gahn, G.; Schmid, E.; et al. Apixaban for treatment of embolic stroke of undetermined source (ATTICUS randomized trial): Rationale and study design. Int. J. Stroke 2017, 12, 985–990. [Google Scholar] [CrossRef] [PubMed]
- Serebruany, V.L.; Malinin, A.I.; Eisert, R.M.; Sane, D.C. Risk of bleeding complications with antiplatelet agents: Meta-analysis of 338,191 patients enrolled in 50 randomized controlled trials. Am. J. Hematol. 2004, 75, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Luyt, C.-E.; Bréchot, N.; Demondion, P.; Jovanovic, T.; Hékimian, G.; Lebreton, G.; Nieszkowska, A.; Schmidt, M.; Trouillet, J.-L.; Leprince, P.J.I.c.m. Brain injury during venovenous extracorporeal membrane oxygenation. Intensive Care Med. 2016, 42, 897–907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Wang, X.; Chen, X.; Ouyang, M.; Sun, L.; Arima, H.; Robinson, T.; Lindley, R.I.; Chalmers, J.; Li, G. Disparities between Asian and Non-Asian Thrombolyzed Acute Ischemic Stroke Patients in the Enhanced Control of Hypertension and Thrombolysis Stroke Trial. Cerebrovasc. Dis. 2021, 1–7. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).