Bioaccumulation of Macronutrients in Edible Mushrooms in Various Habitat Conditions of NW Poland—Role in the Human Diet
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Fungal and Soil Materials
- BCF = Cm/Cs
- BCF—coefficient of bioconcentration
- Cm—is the concentration of macronutrient in mushroom
- Cs—is the concentration of macronutrient in mushroom substrate (soil)
2.3. Statistical Analysis
3. Results and Discussion
3.1. Soil Properties and Macronutrients Concentrations
3.2. Macronutrient Concentrations in Mushrooms
3.2.1. Phosphorus
3.2.2. Potassium
3.2.3. Magnesium
3.2.4. Calcium
3.2.5. Sodium
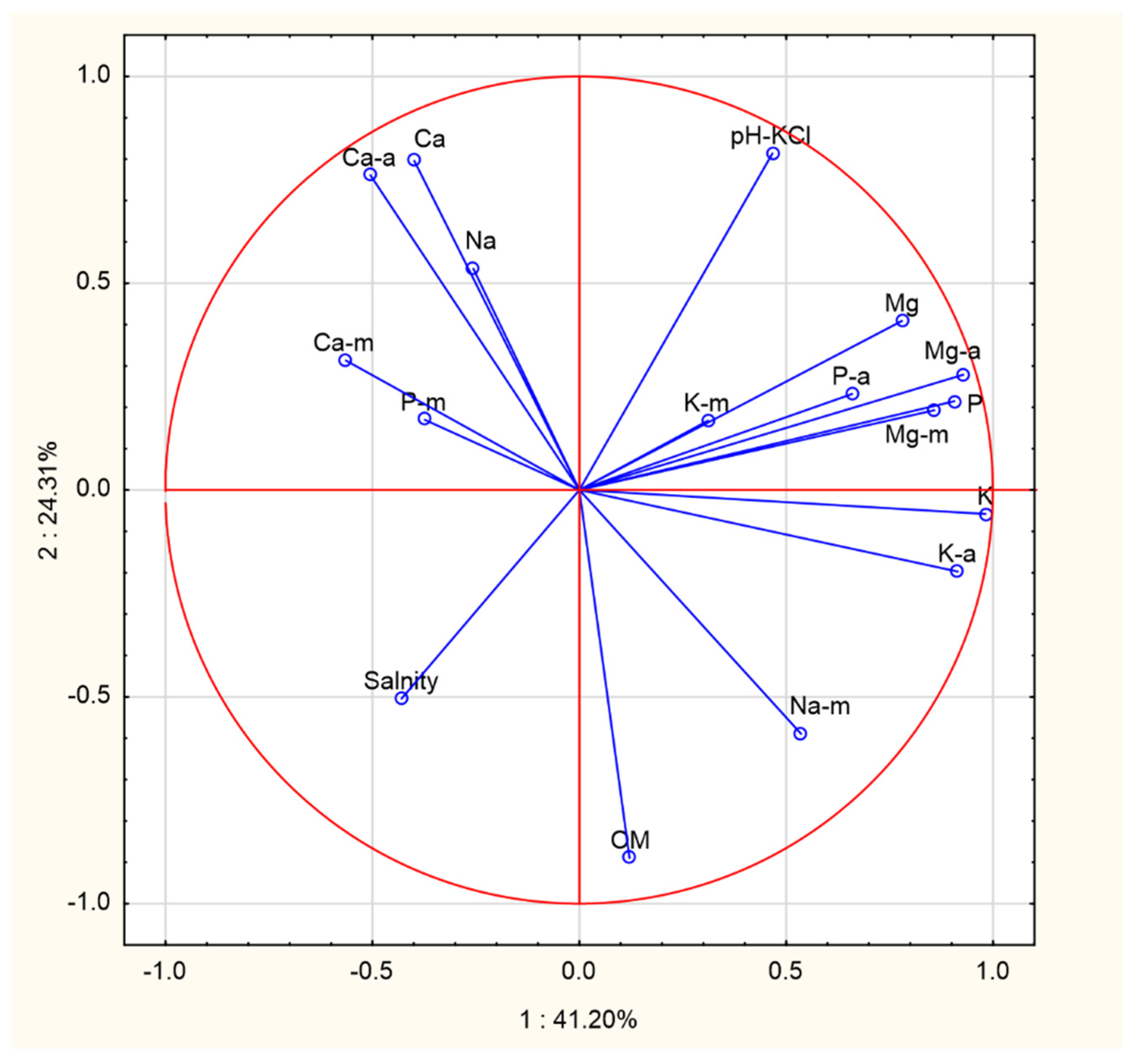
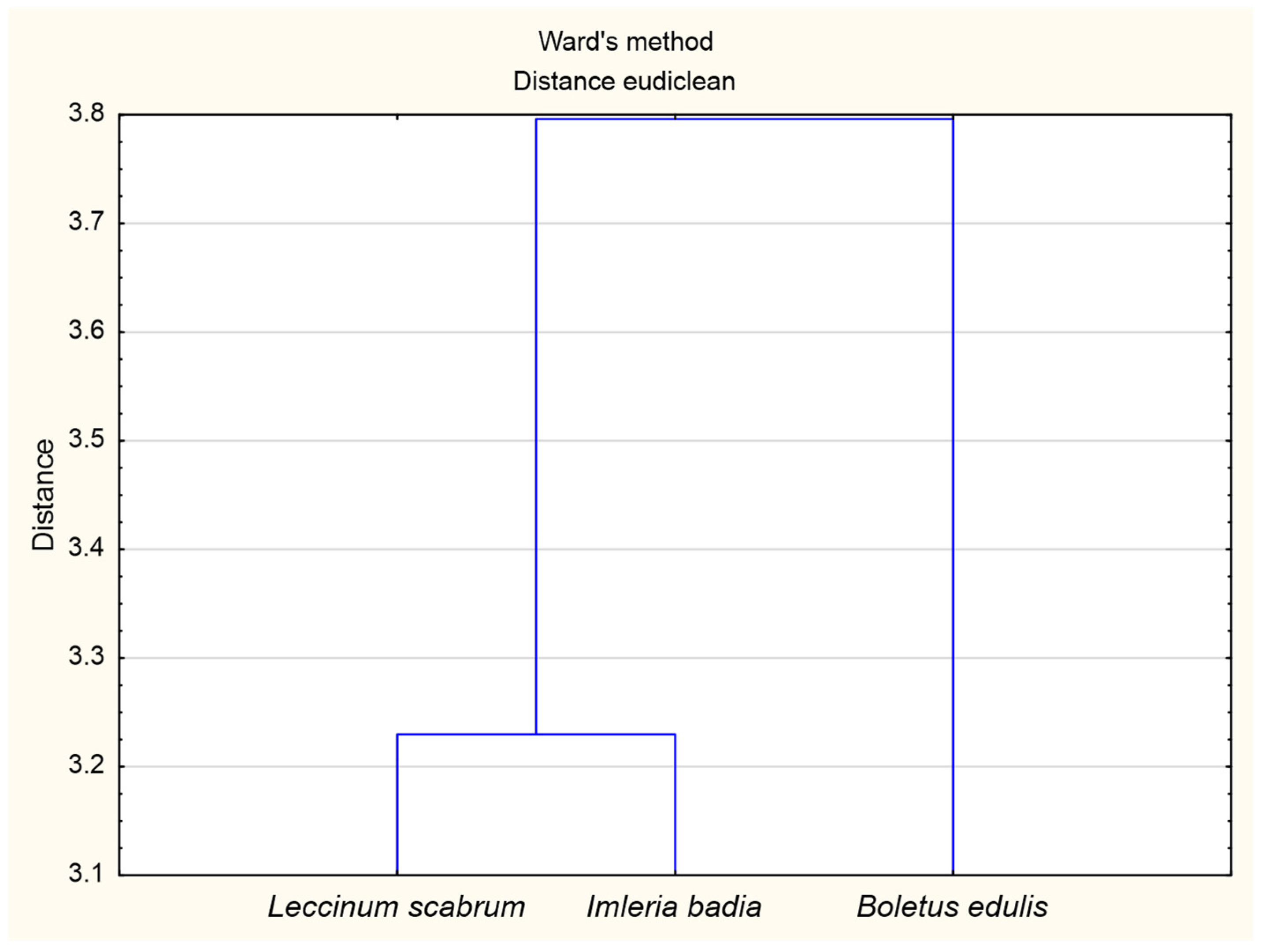
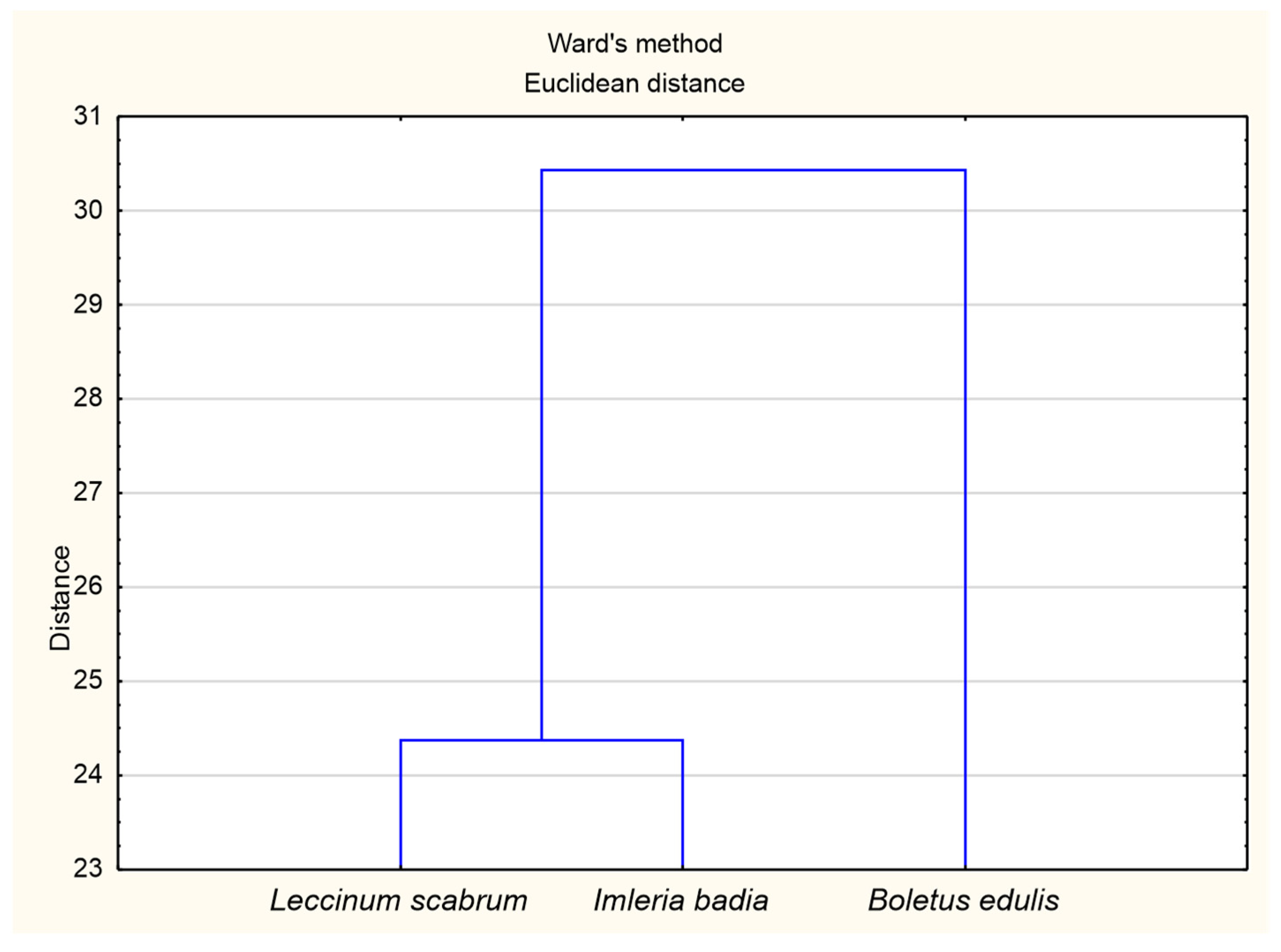
3.3. The Principal Component Analysis (PCA) for Soil and Mushroom Chemical Composition and Ward’s Cluster Analysis for Macronutrients Content in Soils and Mushrooms
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Agrahar-Murugkar, D.; Subbulakshmi, G. Nutritional value of edible wild mushrooms collected from the Khasi hills of Meghalaya. Food Chem. 2005, 89, 599. [Google Scholar] [CrossRef]
- Siwulski, M.; Sobieralski, K.; Sas-Golak, I. Nutritive and health-promoting value of mushrooms. Food Sci. Technol. Qual. 2014, 1, 16–28. [Google Scholar] [CrossRef]
- Cheung, P.C.K. The nutritional and health benefits of mushrooms. Nutr. Bull. 2010, 35, 292–299. [Google Scholar] [CrossRef]
- Kalač, P. Chemical composition and nutritional value of European species of wild growing mushrooms: A review. Food Chem. 2009, 113, 9–16. [Google Scholar] [CrossRef]
- Grangeia, C.; Heleno, S.A.; Barros, L.; Martins, A.; Ferreira, I.C. Effects of trophism on nutritional and nutraceutical potential of wild edible mushrooms. Food Res. Int. 2011, 44, 1029–1035. [Google Scholar] [CrossRef]
- Manzi, P.; Aguzzi, A.; Pizzoferrato, L. Nutritional value of mushrooms widely consumed in Italy. Food Chem. 2001, 73, 321–325. [Google Scholar] [CrossRef]
- Manzi, P.; Marconi, S.; Aguzzi, A.; Pizzoferrato, L. Commercial mushrooms: Nutritional quality and effect of cooking. Food Chem. 2004, 84, 201–206. [Google Scholar] [CrossRef]
- Pelkonen, R.; Alfthan, G.; Järvinen, O. Cadmium, Lead, Arsenic and Nickel in Wild Edible Mushrooms; Finnish Environment Institute: Helsinki, Finland, 2006. [Google Scholar]
- Ouzouni, P.; Riganakos, K.A. Nutritional value and metal content profile of Greek wild edible fungi. Acta Aliment. 2007, 36, 99–110. [Google Scholar] [CrossRef]
- Reis, F.S.; Barros, L.; Martins, A.; Ferreira, I.C. Chemical composition and nutritional value of the most widely appreciated cultivated mushrooms: An inter-species comparative study. Food Chem. Toxicol. 2012, 50, 191–197. [Google Scholar] [CrossRef] [Green Version]
- Heleno, S.A.; Barros, L.; Sousa, M.J.; Martins, A.; Ferreira, I.C.F.R. Tocopherols composition of Portuguese wild mushrooms with antioxidant capacity. Food Chem. 2010, 119, 1443–1450. [Google Scholar] [CrossRef] [Green Version]
- Pereira, E.; Barros, L.; Martins, A.; Ferreira, I.C.F.R. Towards chemical and nutritional inventory of Portuguese wild edible mushrooms in different habitats. Food Chem. 2012, 130, 394–403. [Google Scholar] [CrossRef] [Green Version]
- Sanmee, R.; Dell, B.; Lumyong, P.; Izumori, K.; Lumyong, S. Nutritive value of popular wild edible mushrooms from northern Thailand. Food Chem. 2003, 82, 527–532. [Google Scholar] [CrossRef]
- Faik, A.A.; Hülya, T.; Ahmet, C.; Ertugrul, S.; Mark, M.; Robert, H.G. Macro- and microelement contents of fruiting bodies of wild-edible mushrooms growing in the East Black Sea Region of Turkey. Food Nutr. Sci. 2011, 2, 53–59. [Google Scholar] [CrossRef] [Green Version]
- Chudzyński, K.; Falandysz, J. Multivariate analysis of elements content of Larch Bolete (Suillus grevillei) mushroom. Chemosphere 2008, 73, 1230–1239. [Google Scholar] [CrossRef]
- Mattila, P.; Könkö, K.; Eurola, M.; Pihlava, J.M.; Astola, J.; Vahteristo, L.; Piironen, V. Contents of vitamins, mineral elements, and some phenolic compounds in cultivated mushrooms. J. Agric. Food Chem. 2001, 49, 2343–2348. [Google Scholar] [CrossRef]
- García, M.Á.; Alonso, J.; Melgar, M.J. Lead in edible mushrooms: Levels and bioaccumulation factors. J. Hazard. Mater. 2009, 167, 777–783. [Google Scholar] [CrossRef]
- Nikkarinen, M.; Mertanen, E. Impact of geological origin on trace element composition of edible mushrooms. J. Food Comp. Anal. 2004, 17, 301–310. [Google Scholar] [CrossRef]
- Falandysz, J.; Kunito, T.; Kubota, R.; Bielawski, L.; Frankowska, A.; Falandysz, J.J.; Tanabe, S. Multivariate characterization of elements accumulated in King Bolete Boletus edulis mushroom at lowland and high mountain regions. J. Environ. Sci. Health Part A 2008, 43, 1692–1699. [Google Scholar] [CrossRef] [PubMed]
- Falandysz, J.; Frankowska, A. Biokumulacja pierwiastkow i radionuklidow przez grzyby wielkoowocnikowe. Przeglad bibliograficzny dla ziem polskich. Rocz. Państwowego Zakładu Hig. 2000, 4, 321–344. [Google Scholar]
- Kalač, P.; Svoboda, L. A review of trace element concentrations in edible mushrooms. Food Chem. 2000, 69, 273–281. [Google Scholar] [CrossRef]
- Falandysz, J.; Borovička, J. Macro and trace mineral constituents and radionuclides in mushrooms: Health benefits and risks. Appl. Microbiol. Biotechnol. 2013, 97, 477–501. [Google Scholar] [CrossRef] [Green Version]
- Kalač, P. A review of chemical composition and nutritional value of wild-growing and cultivated mushrooms. J. Sci. Food Agric. 2013, 93, 209–218. [Google Scholar] [CrossRef]
- Perez-Moreno, J.; Read, D.J. Mobilization and transfer of nutrients from litter to tree seedlings via the vegetative mycelium of ectomycorrhizal plants. New Phytol. 2000, 145, 301–309. [Google Scholar] [CrossRef]
- Andreson, I.; Cairney, J. Ectomycorrhizal fungi: Exploring the mycelial frontier. FEMS Microbiol. Rev. 2007, 31, 388–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gadd, G.M. Geomycology: Biogeochemical transformations of rocks, minerals, metals and radionuclides by fungi, bioweathering and bioremediation. Mycol. Res. 2007, 111, 3–49. [Google Scholar] [CrossRef] [PubMed]
- Statistics Poland. Statistical Yearbook of Forestry, Warsaw Poland 2019. Available online: https://bdl.stat.gov.pl/BDL/metadane/cechy/2804 (accessed on 1 June 2021).
- Kondracki, J. Geografia Regionalna Polski; Wydawnictwo Naukowe PWN: Warszawa, Poland, 2002. [Google Scholar]
- Knudsen, H.; Vesterholt, J. Funga Nordica: Agaricoid, Boletoid, Cyphelloid and Gastroid Genera; Nordsvamp: Copenhagen, Denmark, 2012. [Google Scholar]
- Ostrowska, A.; Gawliński, S.; Szczubiałka, Z. Metody Analizy Gleb I Roślin [Procedures for Soil and Plants Analysis]; IUNG: Warsaw, Poland, 1991. [Google Scholar]
- WRB. World reference base for soil resources 2014. International soil classification system for naming soils and creating legends for soil maps. In World Soil Resources Report 106; FAO: Rome, Italy, 2014. [Google Scholar]
- Thompson, C.H. Genesis of podzols on coastal dunes in southern Queensland. I. Field relationships and profile morphology. Soil Res. 1992, 30, 593–613. [Google Scholar] [CrossRef]
- Mokma, D.L.; Yli-Halla, M.; Lindqvist, K. Podzol formation in sandy soils of Finland. Geoderma 2004, 120, 259–272. [Google Scholar] [CrossRef]
- Buurman, P.; Jongmans, A.G. Podzolisation and soil organic matter dynamics. Geoderma 2005, 125, 71–83. [Google Scholar] [CrossRef]
- Sauer, D.; Sponagel, H.; Sommer, M.; Giani, L.; Jahn, R.; Stahr, K. Podzol: Soil of the year 2007. A review on its genesis, occurrence, and functions. J. Plant Nutr. Soil Sci. 2007, 170, 581–597. [Google Scholar] [CrossRef]
- Pollmann, T.; Tsukamoto, S.; Frechen, M.; Giani, L. Rate of soil formation in Arenosols of dunes on Spiekeroog Island (Germany). Geoderma Reg. 2020, 20, 1–53. [Google Scholar] [CrossRef]
- Mleczek, M.; Siwulski, M.; Kaczmarek, Z.; Rissmann, I.; Goliński, P.; Sobieralski, K.; Magdziak, Z. Nutritional elements and aluminium accumulation in Xerocomus badius mushrooms. Acta Sci. Pol. Technol. Aliment. 2013, 12, 411–420. [Google Scholar]
- IUNG (Institute of Soil Science and Plant Cultivation). Fertiliser Recommendations Part I. Limits for Estimating Soil Macro- and Microelement Content; Series P. (44); Institute of Soil Science and Plant Cultivation: Puławy, Poland, 1990. [Google Scholar]
- Frankowska, A.; Ziółkowska, J.; Bielawski, L.; Falandysz, J. Profile and bioconcentration of minerals by King Bolete (Boletus edulis) from the Płocka Dale in Poland. Food Addit. Contam. 2010, 3, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Malinowska, E.; Szefer, P.; Falandysz, J.J.F.C. Metals bioaccumulation by bay bolete, Xerocomus badius, from selected sites in Poland. Food Chem. 2004, 84, 405–416. [Google Scholar] [CrossRef]
- Vogt, K.A.; Edmonds, R.L. Patterns of nutrient concentration in basidiocarps in western Washington. Can. J. Bot. 1980, 58, 694–698. [Google Scholar] [CrossRef]
- Rudawska, M.; Leski, T. Macro-and microelement contents in fruiting bodies of wild mushrooms from the Notecka forest in west-central Poland. Food Chem. 2005, 92, 499–506. [Google Scholar] [CrossRef]
- Kowalewska, I.; Bielawski, L.; Falandysz, J. Niektóre metale i fosfor oraz ich współczynniki nagromadzania w koźlarzu czerwonym Leccinum rufum z terenu Wyżyny Lubelskiej. Some metals, phosphorus, and their bioconcentration factors in red aspen bolete [Leccinum rufum] from the Lubelska Upland. Bromatol. Toxicol. Chem. 2007, 2, 153–158. [Google Scholar]
- Kojta, A.K.; Jarzyńska, G.; Falandysz, J. Mineral composition and heavy metal accumulation capacity of Bay Bolete (Xerocomus badius) fruiting bodies collected near a former gold and copper mining area. J. Geochem. Explor. 2012, 121, 76–82. [Google Scholar] [CrossRef]
- Falandysz, J.; Szymczyk, K.; Ichihashi, H.; Bielawski, L.; Gucia, M.; Frankowska, A.; Yamasaki, S.I. ICP/MS and ICP/AES elemental analysis (38 elements) of edible wild mushrooms growing in Poland. Food Addit. Contam. 2001, 18, 503–513. [Google Scholar] [CrossRef]
- Yoshida, S.; Muramatsu, Y. Determination of major and trace elements in mushroom, plant and soil samples collected from Japanese forests. Int. J. Environ. Anal. Chem. 1997, 67, 49–58. [Google Scholar] [CrossRef]
- Gençcelep, H.; Uzun, Y.; Tunçtürk, Y.; Demirel, K. Determination of mineral contents of wild-grown edible mushrooms. Food Chem. 2009, 113, 1033–1036. [Google Scholar] [CrossRef]
- Kuziemska, B.; Wysokiński, A.; Pakuła, K.; Jaremko, D.; Czapliński, K. Macronutrient content in selected edible mushroom species. Ecol. Eng. 2019, 20, 1–4. [Google Scholar] [CrossRef]
- Vetter, J.; Hajdú, C.S.; Gyorfi, J.; Maszlavér, P. Mineral composition of the cultivated mushrooms Agaricus bisporus, Pleurotus ostreatus and Lentinula edodes. Acta Aliment. 2005, 34, 441–451. [Google Scholar] [CrossRef]
- Entry, J.A.; Rose, C.L.; Cromack Jr, K. Litter decomposition and nutrient release in ectomycorrhizal mat soils of a Douglas fir ecosystem. Soil Biol. Biochem. 1991, 23, 285–290. [Google Scholar] [CrossRef]
- Andersson, S.; Jensen, P.; Söderström, B. Effects of mycorrhizal colonization by Paxillus involutus on uptake of Ca and P by Picea abies and Betula pendula grown in unlimed and limed peat. New Phytol. 1996, 133, 695–704. [Google Scholar] [CrossRef]
- Chudzynski, K.; Bielawski, L. Falandysz, J. Mineral elements and their bioconcentration factors in the fruiting bodies of larch bolete (Suillus grevillei) from the area of Beskid Zachodni. Bromatol. Toxicol. Chem. 2007, 2, 159–166. [Google Scholar]
- Falandysz, J.; Treu, R.; Meloni, D. Distribution and bioconcentration of some elements in the edible mushroom Leccinum scabrum from locations in Poland. J. Environ. Sci. Health Part B 2021, 56, 396–414. [Google Scholar] [CrossRef] [PubMed]
- Bučinová, K.; Janík, R.; Jamnická, G.; Kuklová, M. Accumulation and bioconcentration factors of mineral macronutrients in representative species of macrofungi prevailing in beech-dominated forests affected by air pollution. Czech Mycol. 2014, 66, 193–207. [Google Scholar] [CrossRef]
- Dighton, J. Acquisition of nutrients from organic resources by mycorrhizal autotrophic plants. Experientia 1991, 47, 362–369. [Google Scholar] [CrossRef]
- McElhinney, C.; Mitchell, D.T. Phosphatase activity of four ectomycorrhizal fungi found in a Sitka spruce-Japanese larch plantation in Ireland. Mycol. Res. 1993, 97, 725–732. [Google Scholar] [CrossRef]
- Jarosz, M.; Rychlik, E.; Stoś, K.; Wierzejska, R.; Wojtasik, A.; Charzewska, J.; Cichocka, A. Nutrition Standards for the Polish Population; Institute of Food and Nutrition: Warsaw, Poland, 2017. [Google Scholar]
- Falandysz, J.; Kunito, T.; Kubota, R.; Bielawski, L.; Mazur, A.; Falandysz, J.J.; Tanabe, S. Selected elements in brown birch scaber stalk Leccinum scabrum. J. Environ. Sci. Health 2007, 42, 2081–2088. [Google Scholar] [CrossRef]
- Zhang, D.; Frankowska, A.; Jarzyńska, G.; Kojta, A.K.; Drewnowska, M.; Wydmańska, D. Metals of King Bolete (Boletus edulis) Bull.: Fr. collected at the same site over two years. Afr. J. Agric. Res. 2010, 5, 3050–3055. [Google Scholar] [CrossRef]
- Kojta, A.K.; Falandysz, J. Metallic elements (Ca, Hg, Fe, K, Mg, Mn, Na, Zn) in the fruiting bodies of Boletus badius. Food Chem. 2016, 200, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Demirbaş, A. Concentrations of 21 metals in 18 species of mushrooms growing in the East Black Sea region. Food Chem. 2001, 75, 453–457. [Google Scholar] [CrossRef]
- Kabata-Pendias, A.; Pendias, H. Biochemistry of Trace Elements; Państwowe Wydawnictwo Naukowe: Warsaw, Poland, 1999. (In Polish) [Google Scholar]
- WHO. Potassium Intake for Adults and Children; World Health Organization (WHO): Geneva, Switzerland, 2012. [Google Scholar]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA), Scientific opinion on dietary reference values for potassium. EFSA J. 2016, 14, 4592. [CrossRef]
- Michelot, D.; Siobud, E.; Doré, J.C.; Viel, C.; Poirier, F. Update on metal content profiles in mushrooms—Toxicological implications and tentative approach to the mechanisms of bioaccumulation. Toxicon 1998, 36, 1997–2012. [Google Scholar] [CrossRef]
- Gast, C.H.; Jansen, E.; Bierling, J.; Haanstra, L. Heavy metals in mushrooms and their relationship with soil characteristics. Chemosphere 1988, 17, 789–799. [Google Scholar] [CrossRef]
- Tyler, G. Accumulation and exclusion of metals in Collybia peronata and Amanita rubescens. Trans. Br. Mycol. Soc. 1982, 79, 239–245. [Google Scholar] [CrossRef]
| Localization | Organic Matter | pH in KCl | Salinity | K | Mg | P | Ca | ||
|---|---|---|---|---|---|---|---|---|---|
| % | µS/cm | mg/kg DM | |||||||
| B. edulis | |||||||||
| Soil layer (cm) | |||||||||
| 0–5 | 5–20 | 0–5 | 5–20 | 5–20 | 0–5 | ||||
| UW | 46.59 ab | 9.17 a | 3.25 abc | 3.17 ab | 132 ab | 366.75 a | 497.83 ab | 1411.67 a | 2560.58 ab |
| DP | 67.83 ab | 5.06 a | 3.54 c | 3.39 b | 92 a | 1082.91 b | 982.95 b | 2827.94 a | 889.38 a |
| IL | 64.38 ab | 14.98 a | 2.80 ab | 3.00 ab | 105 a | 397.45 a | 474.91 a | 2834.31 a | 1052.81 ab |
| x | 62.05A | 9.74A | 3.21A | 3.19B | 109.89A | 684.64A | 697.55B | 2526.73A | 1305.86A |
| I. badia | |||||||||
| Soil layer (cm) | |||||||||
| 0–10 | 10–20 | 0–10 | 10–20 | 10–20 | 0–10 | ||||
| UW | 59.53 ab | 21.24 a | 3.19 ac | 2.98 ab | 117 a | 406.46 a | 414.42 a | 2802.97 a | 2831.79 b |
| DP | 75.98 ab | 31.54 a | 2.65 ab | 2.63 ab | 277 c | 437.53 a | 344.93 ab | 1338.33 a | 1091.92 ab |
| IL | 80.42 a | 16.43 a | 2.64 b | 2.77 a | 112 a | 467.00 a | 351.02 a | 1458.74 a | 1262.08 ab |
| x | 71.18A | 21.37AB | 2.87A | 2.83A | 146.68A | 436.89A | 375.16A | 1972.35A | 1855.93A |
| L. scabrum | |||||||||
| Soil layer (cm) | |||||||||
| 0–10 | 10–20 | 0–10 | 10–20 | 10–20 | 0–10 | ||||
| UW | 42.06 b | 20.80 a | 3.25 ac | 3.08 ab | 128 ab | 305.25 a | 424.92 a | 1250.04 a | 2036.09 ab |
| DP | 86.87 a | 77.62 b | 2.65 ab | 2.58 a | 245 bc | 570.17 ab | 378.42 a | 1325.87 a | 1760.42 ab |
| IL | 79.52 ab | 16.86 a | 2.53 ab | 2.80 ab | 128 ab | 633.63 ab | 388.73 a | 1732.64 a | 1257.63 ab |
| x | 64.50A | 35.22B | 2.89A | 2.86AB | 159.81A | 467.06A | 402.37A | 1402.34A | 1748.60A |
| Localization | K | Mg | P | Ca | Na | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| g/kg DM | ||||||||||
| B. edulis | ||||||||||
| Soil layer (cm) | ||||||||||
| 0–5 | 5–20 | 0–5 | 5–20 | 0–5 | 5–20 | 0–5 | 5–20 | 0–5 | 5–20 | |
| UW | 0.54 a | 0.24 a | 1.57 a | 0.31 a | 3.16 ab | 0.88 ab | 3.01 ab | 1.18 abc | 0.18 a | 0.10 a |
| DP | 1.51 b | 0.31 a | 2.01 a | 1.27 b | 4.20 b | 1.04 ab | 1.24 a | 0.27 a | 0.12 a | 0.03 b |
| IL | 0.65 a | 0.62 b | 1.65 a | 0.50 a | 3.34 ab | 1.350 b | 1.19 a | 0.37 ab | 0.12 a | 0.10 a |
| x | 1.00B | 0.39A | 1.79B | 0.69B | 3.67B | 1.09A | 1.60A | 0.61A | 0.13A | 0.08A |
| I. badia | ||||||||||
| Soil layer (cm) | ||||||||||
| 0–10 | 10–20 | 0–10 | 10–20 | 0–10 | 10–20 | 0–10 | 10–20 | 0–10 | 10–20 | |
| UW | 0.54 a | 0.31 a | 1.39 a | 0.34 a | 3.33 ab | 0.86 a | 3.53 b | 1.08 abc | 0.15 a | 0.10 a |
| DP | 0.58 a | 0.43 ab | 1.17 a | 0.25 a | 2.75 ab | 1.26 ab | 1.19 ab | 0.63 abc | 0.12 a | 0.09 ab |
| IL | 0.56 a | 0.31 a | 1.23 a | 0.24 a | 3.13 b | 1.11 ab | 1.30 a | 0.47 ab | 0.10 a | 0.08 ab |
| x | 0.56A | 0.33A | 1.28A | 0.28A | 3.13AB | 1.04A | 2.17A | 0.75A | 0.12A | 0.09A |
| L. scabrum | ||||||||||
| Soil layer (cm) | ||||||||||
| 0–10 | 10–20 | 0–10 | 10–20 | 0–10 | 10–20 | 0–10 | 10–20 | 0–10 | 10–20 | |
| UW | 0.44 a | 0.29 a | 1.54 a | 0.33 a | 2.60 a | 0.97 ab | 2.38 ab | 2.03 c | 0.12 a | 0.09 ab |
| DP | 0.62 a | 0.46 ab | 1.13 a | 0.31 a | 2.67 ab | 1.23 ab | 2.11 ab | 2.33 bc | 0.16 a | 0.14 a |
| IL | 0.73 ab | 0.29 a | 1.66 a | 0.31 a | 3.10 ab | 1.01 ab | 1.33 ab | 0.35 abc | 0.12 a | 0.10 ab |
| x | 0.57A | 0.34A | 1.46AB | 0.32A | 2.76A | 1.05A | 2.02A | 1.66B | 0.13A | 0.11A |
| Localization | P | K | Mg | Ca | Na |
|---|---|---|---|---|---|
| g/kg DM | |||||
| B. edulis | |||||
| UW | 9.96 a | 9.63 a | 0.97 a | 0.14 ab | 0.41 a |
| DP | 6.98 a | 9.61 a | 2.20 b | 0.11 a | 0.89 a |
| IL | 10.00 a | 3.40 a | 0.85 a | 0.11 a | 0.75 a |
| x | 8.92A | 7.79A | 1.37B | 0.12A | 0.68A |
| I. badia | |||||
| UW | 8.16 a | 6.04 a | 0.99 a | 0.13 ab | 0.47 a |
| DP | 8.38 a | 9.49 a | 0.94 a | 0.14 ab | 0.70 a |
| IL | 8.12 a | 0.69 a | 0.76 a | 0.14 ab | 0.61 a |
| x | 8.19A | 4.59A | 0.89A | 0.14A | 0.57A |
| L. scabrum | |||||
| UW | 7.50 a | 5.40 a | 0.95 a | 0.20 b | 0.34 a |
| DP | 8.08 a | 4.21 a | 1.05 a | 0.14 ab | 0.89 a |
| IL | 7.66 a | 12.14 a | 0.68 a | 0.10 a | 0.49 a |
| x | 7.70A | 6.91A | 0.91A | 0.16A | 0.53A |
| Mushroom Species | K | Mg | P | Ca | Na | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| B. edulis | Soil layer (cm) | |||||||||
| 0–5 | 5–20 | 0–5 | 5–20 | 0–5 | 5–20 | 0–5 | 5–20 | 0–5 | 5–20 | |
| 7.79 | 19.97 | 0.77 | 1.99 | 2.43 | 8.18 | 0.08 | 0.20 | 5.23 | 8.50 | |
| I. badia | Soil layer (cm) | |||||||||
| 0–10 | 10–20 | 0–10 | 10–20 | 0–10 | 10–20 | 0–10 | 10–20 | 0–10 | 10–20 | |
| 8.20 | 2.70 | 3.59 | 3.18 | 2.62 | 7.88 | 0.06 | 0.19 | 4.75 | 6.33 | |
| L. scabrum | Soil layer (cm) | |||||||||
| 0–10 | 10–20 | 0–10 | 10–20 | 0–10 | 10–20 | 0–10 | 10–20 | 0–10 | 10–20 | |
| 12.12 | 20.32 | 0.62 | 2.84 | 2.79 | 7.33 | 0.08 | 0.10 | 4.08 | 4.82 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malinowski, R.; Sotek, Z.; Stasińska, M.; Malinowska, K.; Radke, P.; Malinowska, A. Bioaccumulation of Macronutrients in Edible Mushrooms in Various Habitat Conditions of NW Poland—Role in the Human Diet. Int. J. Environ. Res. Public Health 2021, 18, 8881. https://doi.org/10.3390/ijerph18168881
Malinowski R, Sotek Z, Stasińska M, Malinowska K, Radke P, Malinowska A. Bioaccumulation of Macronutrients in Edible Mushrooms in Various Habitat Conditions of NW Poland—Role in the Human Diet. International Journal of Environmental Research and Public Health. 2021; 18(16):8881. https://doi.org/10.3390/ijerph18168881
Chicago/Turabian StyleMalinowski, Ryszard, Zofia Sotek, Małgorzata Stasińska, Katarzyna Malinowska, Patrycja Radke, and Alicja Malinowska. 2021. "Bioaccumulation of Macronutrients in Edible Mushrooms in Various Habitat Conditions of NW Poland—Role in the Human Diet" International Journal of Environmental Research and Public Health 18, no. 16: 8881. https://doi.org/10.3390/ijerph18168881
APA StyleMalinowski, R., Sotek, Z., Stasińska, M., Malinowska, K., Radke, P., & Malinowska, A. (2021). Bioaccumulation of Macronutrients in Edible Mushrooms in Various Habitat Conditions of NW Poland—Role in the Human Diet. International Journal of Environmental Research and Public Health, 18(16), 8881. https://doi.org/10.3390/ijerph18168881






