Physicochemical Properties of Indoor and Outdoor Particulate Matter 2.5 in Selected Residential Areas near a Ferromanganese Smelter
Abstract
:1. Introduction
2. Materials and Methods
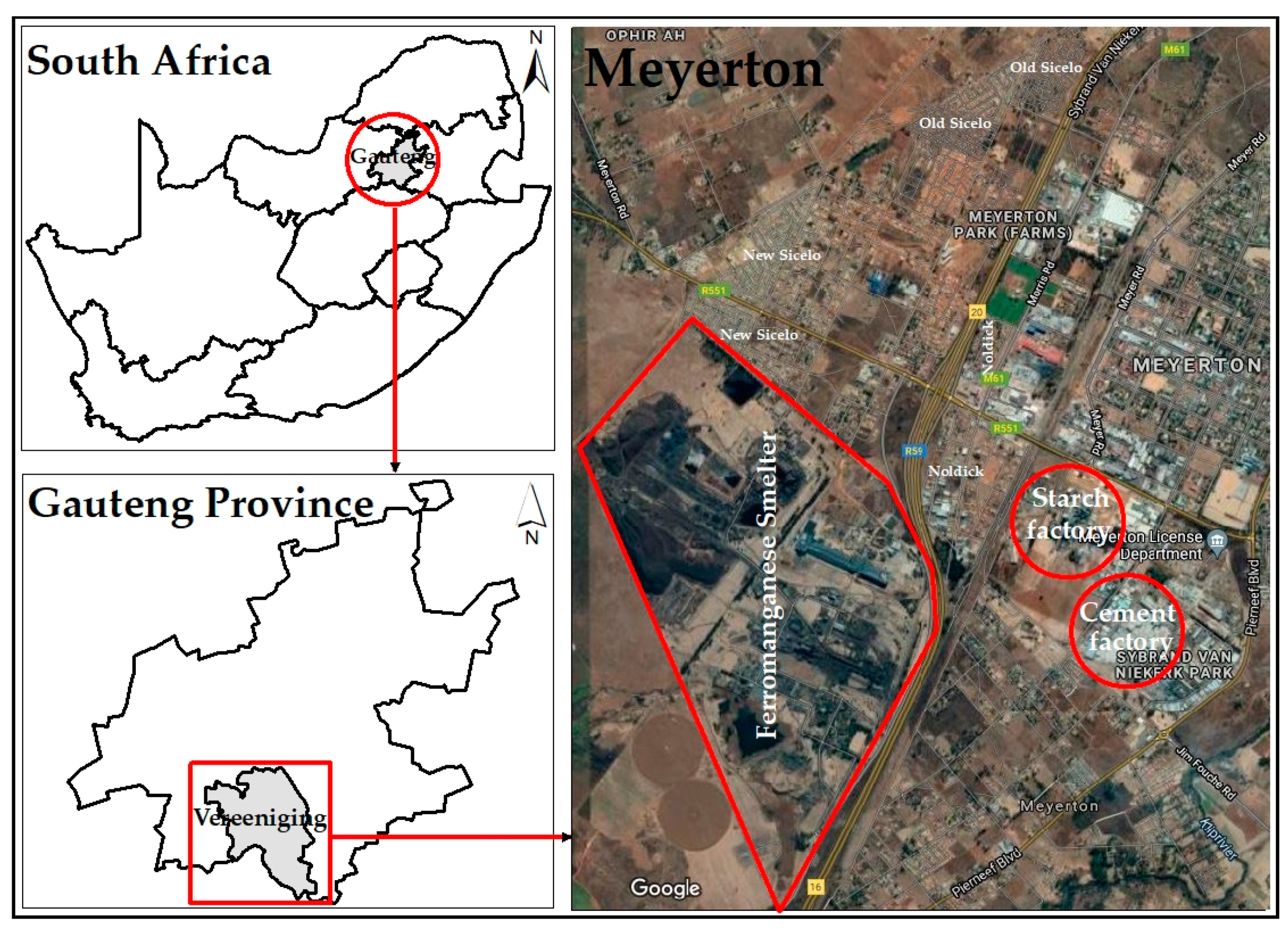
2.1. Description of Study Area
2.2. Sampling of Indoor and Outdoor PM2.5
2.3. Data Analysis
2.3.1. Indoor and Outdoor PM2.5 Mass Concentration
2.3.2. Indoor–Outdoor Ratio
2.3.3. Statistical Analysis
2.3.4. Scanning Electron Microscopy (SEM)
2.3.5. Inductively Coupled Mass Plasma Spectrometry (ICP-MS)
2.4. Quality Control
3. Results
3.1. Indoor and Outdoor PM2.5 Mass Concentration
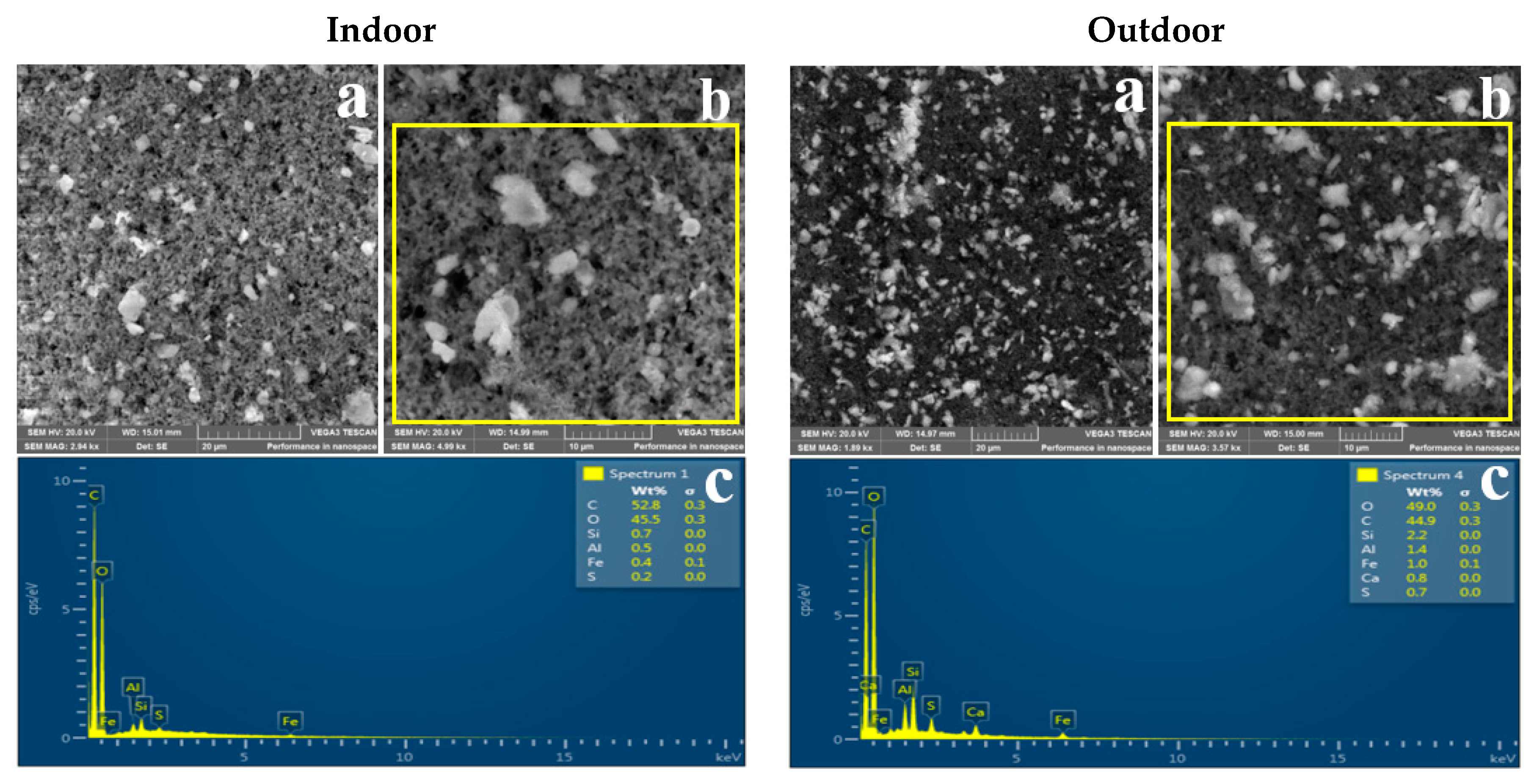
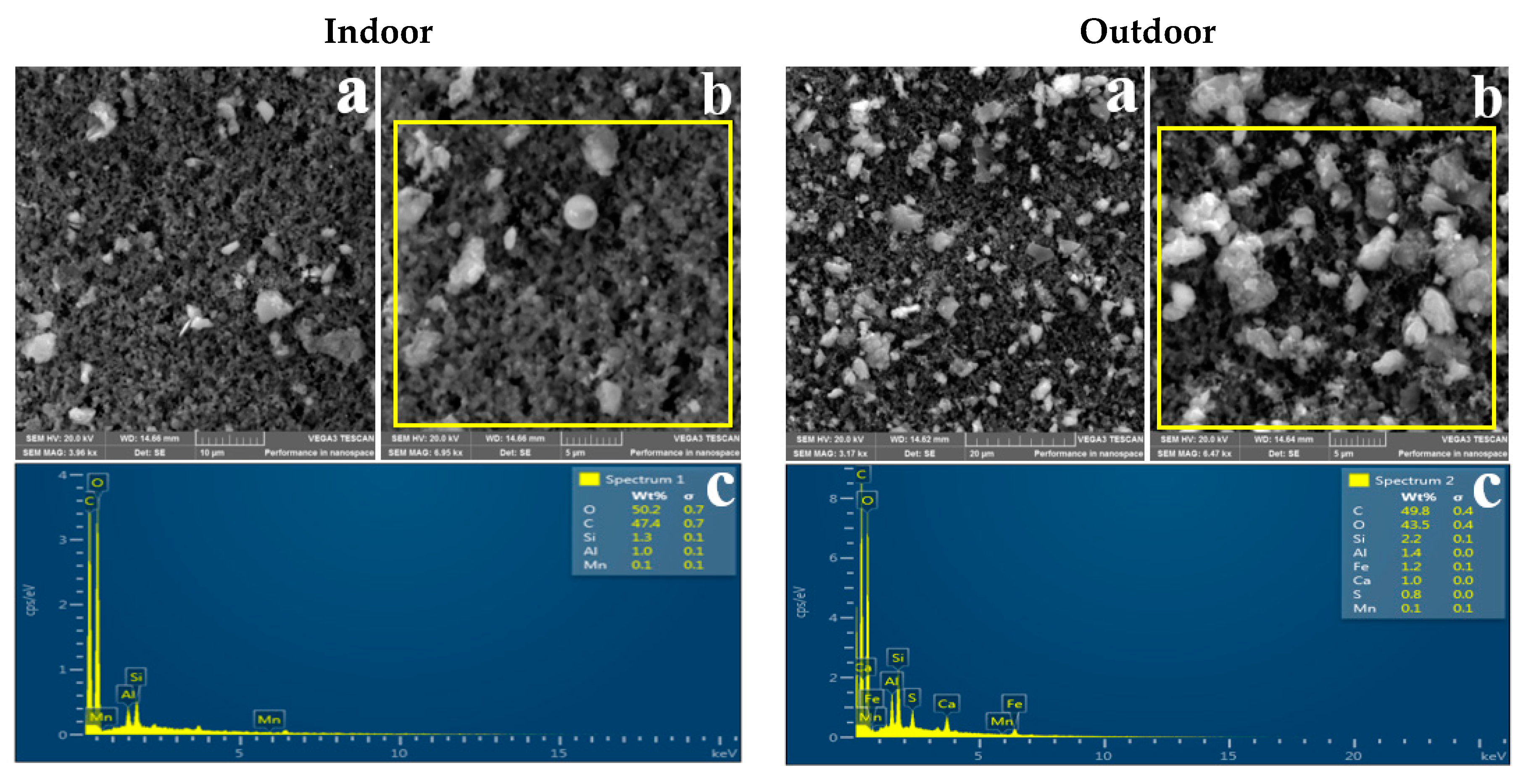
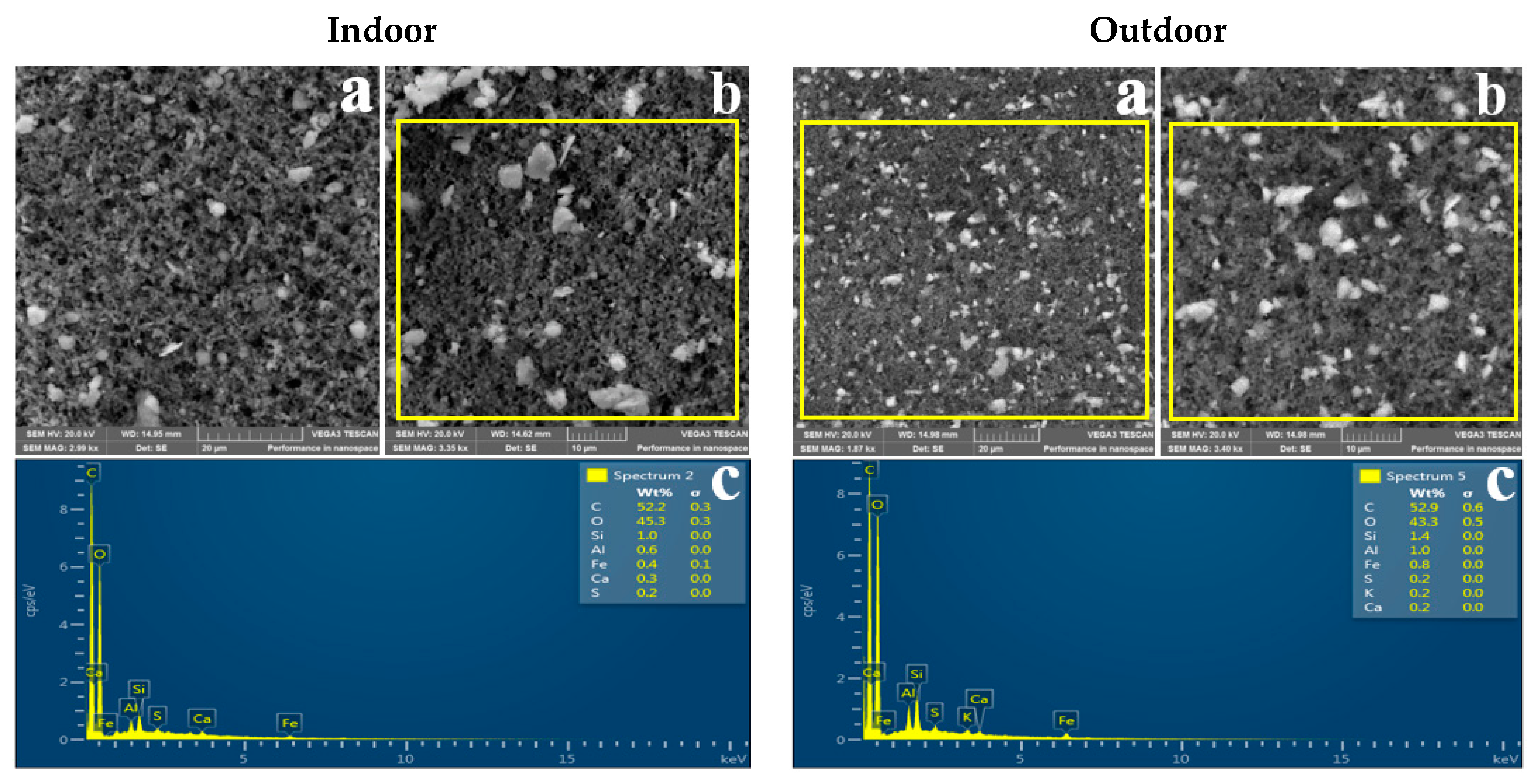
3.2. Morphology of Indoor and Outdoor PM2.5
3.3. Elemental Composition of Indoor and Outdoor PM2.5
4. Discussion
4.1. Indoor and Outdoor PM2.5 Mass Concentration
4.2. Relationship between Indoor and Outdoor PM2.5 Mass Concentrations
4.3. Morphology
4.4. Elemental Composition of Indoor and Outdoor PM2.5
4.5. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, X.; Sun, W.; Zhao, L.; Cai, J. Emission characterization of particulate matter in the ironmaking process. Environ. Technol. 2017, 40, 282–292. [Google Scholar] [CrossRef]
- Hänninen, O.; Goodman, P. Outdoor Air as a Source of Indoor Pollution. In Indoor Air Pollution; the Royal Society of Chemistry: London, UK, 2019; pp. 35–65. [Google Scholar]
- Goix, S.; Uzu, G.; Oliva, P.; Barraza, F.; Calas, A.; Castet, S.; Point, D.; Masbou, J.; Duprey, J.L.; Huayta, C.; et al. Metal concentration and bioaccessibility in different particle sizes of dust and aerosols to refine metal exposure assessment. J. Hazard. Mater. 2016, 317, 552–562. [Google Scholar] [CrossRef]
- Ahmed, M.; Guo, X.; Zhao, X.-M. Spectroscopic and microscopic characterization of atmospheric particulate matter. Instrum. Sci. Technol. 2017, 45, 659–682. [Google Scholar] [CrossRef]
- Galvão, E.S.; Santos, J.M.; Lima, A.T.; Reis, N.C.; Orlando, M.T.D.A.; Stuetz, R.M. Trends in analytical techniques applied to particulate matter characterization: A critical review of fundaments and applications. Chemosphere 2018, 199, 546–568. [Google Scholar] [CrossRef] [PubMed]
- Almeida, S.; Pio, C.; Freitas, M.; Reis, M.; Transcoso, M. Source apportionment of fine and coarse particulate matter in a sub-urban area at the Western European Coast. Atmos. Environ. 2005, 39, 3127–3138. [Google Scholar] [CrossRef]
- Hernández-Pellón, A.; Fernández-Olmo, I.; Ledoux, F.; Courcot, L.; Courcot, D. Characterization of manganese-bearing particles in the vicinities of a manganese alloy plant. Chemosphere 2017, 175, 411–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernández-Pellón, A.; Fernández-Olmo, I. Airborne concentration and deposition of trace metals and metalloids in an urban area downwind of a manganese alloy plant. Atmos. Pollut. Res. 2019, 10, 712–721. [Google Scholar] [CrossRef]
- Dos Santos, N.R.; Rodrigues, J.L.; Bandeira, M.J.; Anjos, A.L.d.S.; Araújo, C.d.F.S.; Adan, L.F.F.; Menezes-Filho, J.A. Manganese exposure and association with hormone imbalance in children living near a ferro-manganese alloy plant. Environ. Res. 2019, 172, 166–174. [Google Scholar] [CrossRef]
- Menezes-Filho, J.A.; Paes, C.R.; Ângela, Â.M.; Moreira, J.C.; Sarcinelli, P.N.; Mergler, D. High levels of hair manganese in children living in the vicinity of a ferro-manganese alloy production plant. Neurotoxicology 2009, 30, 1207–1213. [Google Scholar] [CrossRef] [Green Version]
- Colledge, M.A.; Julian, J.R.; Gocheva, V.V.; Beseler, C.L.; Roels, H.A.; Lobdell, D.T.; Bowler, R.M. Characterization of air manganese exposure estimates for residents in two Ohio towns. J. Air Waste Manag. Assoc. 2015, 65, 948–957. [Google Scholar] [CrossRef] [Green Version]
- Salma, I.; Füri, P.; Németh, Z.; Balásházy, I.; Hofmann, W.; Farkas, Á. Lung burden and deposition distribution of inhaled atmospheric urban ultrafine particles as the first step in their health risk assessment. Atmos. Environ. 2015, 104, 39–49. [Google Scholar] [CrossRef]
- Hasheminassab, S.; Daher, N.; Shafer, M.M.; Schauer, J.J.; Delfino, R.J.; Sioutas, C. Chemical characterization and source apportionment of indoor and outdoor fine particulate matter (PM 2.5 ) in retirement communities of the Los Angeles Basin. Sci. Total Environ. 2014, 490, 528–537. [Google Scholar] [CrossRef] [Green Version]
- Koivisto, A.J.; Kling, K.I.; Hänninen, O.; Jayjock, M.; Löndahl, J.; Wierzbicka, A.; Fonseca, A.S.; Uhrbrand, K.; Boor, B.E.; Jiménez, A.S.; et al. Source specific exposure and risk assessment for indoor aerosols. Sci. Total Environ. 2019, 668, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Bowler, R.M.; Beseler, C.L.; Gocheva, V.V.; Colledge, M.; Kornblith, E.S.; Julian, J.R.; Kim, Y.; Bollweg, G.; Lobdell, D.T. Environmental exposure to manganese in air: Associations with tremor and motor function. Sci. Total Environ. 2016, 541, 646–654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kornblith, E.S.; Casey, S.L.; Lobdell, D.T.; Colledge, M.A.; Bowler, R. Environmental exposure to manganese in air: Tremor, motor and cognitive symptom profiles. Neurotoxicology 2018, 64, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Solís-Vivanco, R.; Rodríguez-Agudelo, Y.; Riojas-Rodríguez, H.; Ríos, C.; Rosas, I.; Montes, S. Cognitive impairment in an adult Mexican population non-occupationally exposed to manganese. Environ. Toxicol. Pharmacol. 2009, 28, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Azcona, L.; Fernández-Olmo, I.; Expósito, A.; Markiv, B.; Paz-Zulueta, M.; Parás-Bravo, P.; Sarabia-Cobo, C.; Santibáñez, M. Impact of Environmental Airborne Manganese Exposure on Cognitive and Motor Functions in Adults: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2021, 18, 4075. [Google Scholar] [CrossRef]
- Rodrigues, J.L.; Araújo, C.F.S.; dos Santos, N.R.; Bandeira, M.J.; Anjos, A.L.S.; Carvalho, C.F.; Lima, C.S.; Abreu, J.N.S.; Mergler, D.; Menezes-Filho, J.A. Airborne manganese exposure and neurobehavior in school-aged children living near a ferro-manganese alloy plant. Environ. Res. 2018, 167, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Racette, B.A.; Nelson, G.; Dlamini, W.W.; Hershey, T.; Prathibha, P.; Turner, J.R.; Checkoway, H.; Sheppard, L.; Searles Nielsen, S. Depression and anxiety in a manganese-exposed community. Neurotoxicology 2021, 85, 222–233. [Google Scholar] [CrossRef]
- Hernández-Pellón, A.; Nischkauer, W.; Limbeck, A.; Fernández-Olmo, I. Metal(loid) bioaccessibility and inhalation risk assessment: A comparison between an urban and an industrial area. Environ. Res. 2018, 165, 140–149. [Google Scholar] [CrossRef]
- Moreno, T.; Pandolfi, M.; Querol, X.; Lavín, J.; Alastuey, A.; Viana, M.; Gibbons, W. Manganese in the urban atmosphere: Identifying anomalous concentrations and sources. Environ. Sci. Pollut. Res. 2011, 18, 173–183. [Google Scholar] [CrossRef]
- Haynes, E.N.; Sucharew, H.; Hilbert, T.J.; Kuhnell, P.; Spencer, A.; Newman, N.C.; Burns, R.; Wright, R.; Parsons, P.J.; Dietrich, K.N. Impact of air manganese on child neurodevelopment in East Liverpool, Ohio. Neurotoxicology 2018, 64, 94–102. [Google Scholar] [CrossRef]
- Sly, P.D.; Carpenter, D.O.; Van den Berg, M.; Stein, R.T.; Landrigan, P.J.; Brune-Drisse, M.-N.; Suk, W. Health Consequences of Environmental Exposures: Causal Thinking in Global Environmental Epidemiology. Ann. Glob. Health. 2016, 82, 3. [Google Scholar] [CrossRef]
- Haynes, E.N.; Ryan, P.; Chen, A.; Brown, D.; Roda, S.; Kuhnell, P.; Wittberg, D.; Terrell, M.; Reponen, T. Assessment of personal exposure to manganese in children living near a ferromanganese refinery. Sci. Total Environ. 2012, 427–428, 19–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Snyder, E.G.; Watkins, T.H.; Solomon, P.A.; Thoma, E.D.; Williams, R.W.; Hagler, G.S.W.; Shelow, D.; Hindin, D.A.; Kilaru, V.J.; Preuss, P.W. The Changing Paradigm of Air Pollution Monitoring. Environ. Sci. Technol. 2013, 47, 11369–11377. [Google Scholar] [CrossRef] [PubMed]
- Lowther, S.D.; Jones, K.C.; Wang, X.; Whyatt, J.D.; Wild, O.; Booker, D. Particulate Matter Measurement Indoors: A Review of Metrics, Sensors, Needs, and Applications. Environ. Sci. Technol. 2019, 53, 11644–11656. [Google Scholar] [CrossRef] [PubMed]
- West, J.J.; Cohen, A.; Dentener, F.; Brunekreef, B.; Zhu, T.; Armstrong, B.; Bell, M.L.; Brauer, M.; Carmichael, G.; Costa, D.L.; et al. What We Breathe Impacts Our Health: Improving Understanding of the Link between Air Pollution and Health. Environ. Sci. Technol. 2016, 50, 4895–4904. [Google Scholar] [CrossRef] [PubMed]
- Young, T.; Myers, J.E.; Thompson, M. Lou The Nervous System Effects of Occupational Exposure to Manganese-Measured as Respirable Dust-in a South African Manganese Smelter. Neurotoxicology 2005, 26, 993–1000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myers, J.E.; Tewaternaude, J.; Fourie, M.; Zogoe, H.B.A.; Naik, I.; Theodorou, P.; Tassel, H.; Daya, A.; Thompson, M. Lou Nervous System Effects of Occupational Manganese Exposure on South African Manganese Mineworkers. Neurotoxicology 2003, 24, 649–656. [Google Scholar] [CrossRef]
- Rodríguez-Agudelo, Y.; Riojas-Rodríguez, H.; Ríos, C.; Rosas, I.; Sabido Pedraza, E.; Miranda, J.; Siebe, C.; Texcalac, J.L.; Santos-Burgoa, C. Motor alterations associated with exposure to manganese in the environment in Mexico. Sci. Total Environ. 2006, 368, 542–556. [Google Scholar] [CrossRef]
- Zota, A.R.; Schaider, L.A.; Ettinger, A.S.; Wright, R.O.; Shine, J.P.; Spengler, J.D. Metal sources and exposures in the homes of young children living near a mining-impacted Superfund site. J. Expo. Sci. Environ. Epidemiol. 2011, 21, 495–505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morawska, L.; Afshari, A.; Bae, G.N.; Buonanno, G.; Chao, C.Y.H.; Hänninen, O.; Hofmann, W.; Isaxon, C.; Jayaratne, E.R.; Pasanen, P.; et al. Indoor aerosols: From personal exposure to risk assessment. Indoor Air 2013, 23, 462–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davourie, J.; Westfall, L.; Ali, M.; Mcgough, D. Evaluation of particulate matter emissions from manganese alloy production using life-cycle assessment. Neurotoxicology 2017, 58, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Kalisa, E.; Archer, S.; Nagato, E.; Bizuru, E.; Lee, K.; Tang, N.; Pointing, S.; Hayakawa, K.; Lacap-Bugler, D. Chemical and Biological Components of Urban Aerosols in Africa: Current Status and Knowledge Gaps. Int. J. Environ. Res. Public Health 2019, 16, 941. [Google Scholar] [CrossRef] [Green Version]
- Hermanus, M.A. Manganese-A Public Health Concern: Its Relevance for Occupational Health and Safety Policy and Regulation in South Africa. Int. J. Occup. Environ. Health 2000, 6, 151–160. [Google Scholar] [CrossRef]
- Hess, C.A.; Smith, M.J.; Trueman, C.; Schutkowski, H. Longitudinal and contemporaneous manganese exposure in apartheid-era South Africa: Implications for the past and future. Int. J. Paleopathol. 2015, 8, 1–9. [Google Scholar] [CrossRef]
- Crenn, V.; Chakraborty, A.; Fronval, I.; Petitprez, D.; Riffault, V. Fine particles sampled at an urban background site and an industrialized coastal site in Northern France—Part 2: Comparison of offline and online analyses for carbonaceous aerosols. Aerosol Sci. Technol. 2018, 52, 287–299. [Google Scholar] [CrossRef]
- Hopke, P.K. Review of receptor modeling methods for source apportionment. J. Air Waste Manag. Assoc. 2016, 66, 237–259. [Google Scholar] [CrossRef]
- Dong, Z.; Liu, Y.; Duan, L.; Bekele, D.; Naidu, R. Uncertainties in human health risk assessment of environmental contaminants: A review and perspective. Environ. Int. 2015, 85, 120–132. [Google Scholar] [CrossRef]
- Majestic, B.J.; Schauer, J.J.; Shafer, M.M. Development of a Manganese Speciation Method for Atmospheric Aerosols in Biologically and Environmentally Relevant Fluids. Aerosol Sci. Technol. 2007, 41, 925–933. [Google Scholar] [CrossRef]
- Statistics South Africa Statistics South Africa. Available online: http://www.statssa.gov.za/?page_id=4286&id=11181 (accessed on 20 October 2019).
- Steenkamp, J.D.; Basson, J. The manganese ferroalloys industry in southern Africa. J. S. Afr. Inst. Min. Metall. 2013, 113, 667–676. [Google Scholar]
- Boudissa, S.M.; Lambert, J.; Müller, C.; Kennedy, G.; Gareau, L.; Zayed, J. Manganese concentrations in the soil and air in the vicinity of a closed manganese alloy production plant. Sci. Total Environ. 2006, 361, 67–72. [Google Scholar] [CrossRef]
- Genga, A.; Siciliano, T.; Siciliano, M.; Aiello, D.; Tortorella, C. Individual particle SEM-EDS analysis of atmospheric aerosols in rural, urban, and industrial sites of Central Italy. Environ. Monit. Assess. 2018, 190, 456. [Google Scholar] [CrossRef]
- Yue, W.; Li, X.; Liu, J.; Li, Y.; Yu, X.; Deng, B.; Wan, T.; Zhang, G.; Huang, Y.; He, W.; et al. Characterization of PM2.5 in the ambient air of Shanghai city by analyzing individual particles. Sci. Total Environ. 2006, 368, 916–925. [Google Scholar] [CrossRef] [PubMed]
- Izhar, S.; Goel, A.; Chakraborty, A.; Gupta, T. Annual trends in occurrence of submicron particles in ambient air and health risk posed by particle bound metals. Chemosphere 2016, 146, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Hoek, G.; Kos, G.; Harrison, R.; de Hartog, J.; Meliefste, K.; ten Brink, H.; Katsouyanni, K.; Karakatsani, A.; Lianou, M.; Kotronarou, A.; et al. Indoor-outdoor relationships of particle number and mass in four European cities. Atmos. Environ. 2008, 42, 156–169. [Google Scholar] [CrossRef]
- Figueiredo Filho, D.B.; Paranhos, R.; Rocha, E.C.D.; Batista, M.; Silva, J.A.D., Jr.; Santos, M.L.W.D.; Marino, J.G. When is statistical significance not significant? Braz. Political Sci. Rev. 2013, 7, 31–55. [Google Scholar] [CrossRef] [Green Version]
- Pallarés, S.; Gómez, E.T.; Jordán, M.M. Typological characterisation of mineral and combustion airborne particles indoors in primary schools. Atmosphere 2019, 10, 209. [Google Scholar] [CrossRef] [Green Version]
- Panda, S.; Shiva Nagendra, S.M. Chemical and morphological characterization of respirable suspended particulate matter (PM10) and associated heath risk at a critically polluted industrial cluster. Atmos. Pollut. Res. 2018, 9, 791–803. [Google Scholar] [CrossRef]
- Laohaudomchok, W.; Cavallari, J.M.; Fang, S.C.; Lin, X.; Herrick, R.F.; Christiani, D.C.; Weisskopf, M.G. Assessment of occupational exposure to manganese and other metals in welding fumes by portable x-ray fluorescence spectrometer. J. Occup. Environ. Hyg. 2010, 7, 456–465. [Google Scholar] [CrossRef]
- Labrada-Delgado, G.; Aragon-Pina, A.; Campos-Ramos, A.; Castro-Romero, T.; Amador-Munoz, O.; Villalobos-Pietrini, R. Chemical and morphological characterization of PM2.5 collected during MILAGRO campaign using scanning electron microscopy. Atmos. Pollut. Res. 2012, 3, 289–300. [Google Scholar] [CrossRef] [Green Version]
- Pallarés, S.; Gómez, E.T.; Martínez, Á.; Miguel, J.M. Morphological Characterization of Indoor Airborne Particles in Seven Primary Schools. Int. J. Environ. Res. Public Health 2020, 17, 3183. [Google Scholar] [CrossRef] [PubMed]
- Kutchko, B.G.; Kim, A.G. Fly ash characterization by SEM-EDS. Fuel 2006, 85, 2537–2544. [Google Scholar] [CrossRef]
- Makonese, T.; Meyer, J.; von Solms, S. Characteristics of spherical organic particles emitted from fixed-bed residential coal combustion. Atmosphere 2019, 10, 441. [Google Scholar] [CrossRef] [Green Version]
- McDonald, R.; Biswas, P. A Methodology to Establish the Morphology of Ambient Aerosols. J. Air Waste Manage. Assoc. 2004, 54, 1069–1078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satsangi, P.G.; Yadav, S. Characterization of PM2.5 by X-ray diffraction and scanning electron microscopy–energy dispersive spectrometer: Its relation with different pollution sources. Int. J. Environ. Sci. Technol. 2014, 11, 217–232. [Google Scholar] [CrossRef] [Green Version]
- Stebounova, L.V.; Gonzalez-Pech, N.I.; Peters, T.M.; Grassian, V.H. Physicochemical properties of air discharge-generated manganese oxide nanoparticles: Comparison to welding fumes. Environ. Sci. Nano 2018, 5, 696–707. [Google Scholar] [CrossRef]
- Masekameni, M.D.; Makonese, T.; Rampedi, T.I.; Keretetse, G.S. Morphology and elemental analysis of freshly emitted particles from packed-bed domestic coal combustion. Clean Air J. 2020, 30, 1–10. [Google Scholar] [CrossRef]
- Arı, A.; Arı, P.E.; Gaga, E.O. Chemical characterization of size-segregated particulate matter (PM) by inductively coupled plasma–Tandem mass spectrometry (ICP-MS/MS). Talanta 2020, 208, 120350. [Google Scholar] [CrossRef]
- Zhang, S.; Broday, D.M.; Raz, R. Predictors of the indoor-to-outdoor ratio of particle number concentrations in israel. Atmosphere 2020, 11, 1074. [Google Scholar] [CrossRef]
- Abdel-Salam, M.M.M. Investigation of PM2.5 and carbon dioxide levels in urban homes. J. Air Waste Manag. Assoc. 2015, 65, 930–936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lv, Y.; Wang, H.; Wei, S.; Zhang, L.; Zhao, Q. The Correlation between Indoor and Outdoor Particulate Matter of Different Building Types in Daqing, China. Procedia Eng. 2017, 205, 360–367. [Google Scholar] [CrossRef]
- Martins, V.; Faria, T.; Diapouli, E.; Manousakas, M.I.; Eleftheriadis, K.; Viana, M.; Almeida, S.M. Relationship between indoor and outdoor size-fractionated particulate matter in urban microenvironments: Levels, chemical composition and sources. Environ. Res. 2020, 183, 109203. [Google Scholar] [CrossRef] [PubMed]
- Urso, P.; Cattaneo, A.; Garramone, G.; Peruzzo, C.; Cavallo, D.M.; Carrer, P. Identification of particulate matter determinants in residential homes. Build. Environ. 2015, 86, 61–69. [Google Scholar] [CrossRef]
- Perrino, C.; Tofful, L.; Canepari, S. Chemical characterization of indoor and outdoor fine particulate matter in an occupied apartment in Rome, Italy. Indoor Air 2016, 26, 558–570. [Google Scholar] [CrossRef] [PubMed]
- Board on Population Health and Public Health Practice; Health and Medicine Division; National Academies of Sciences, Engineering, and Medicine. Health Risks of Indoor Exposure to Particulate Matter; National Academies Press: Washington, DC, USA, 2016; pp. 1–159. [Google Scholar]
- Kim, H.; Kang, K.; Kim, T. Measurement of Particulate Matter (PM2.5) and Health Risk Assessment of Cooking-Generated Particles in the Kitchen and Living Rooms of Apartment Houses. Sustainability 2018, 10, 843. [Google Scholar] [CrossRef] [Green Version]
- Lazaridis, M.; Aleksandropoulou, V.; Smolík, J.; Hansen, J.E.; Glytsos, T.; Kalogerakis, N.; Dahlin, E. Physico-chemical characterization of indoor/outdoor particulate matter in two residential houses in Oslo, Norway: Measurements overview and physical properties-Urban-Aerosol project. Indoor Air 2006, 16, 282–295. [Google Scholar] [CrossRef]
- Nadali, A.; Arfaeinia, H.; Asadgol, Z.; Fahiminia, M. Indoor and outdoor concentration of PM 10, PM 2.5 and PM 1 in residential building and evaluation of negative air ions (NAIs) in indoor PM removal. Environ. Pollut. Bioavailab. 2020, 32, 47–55. [Google Scholar] [CrossRef] [Green Version]
- Bozlaker, A.; Peccia, J.; Chellam, S. Indoor/Outdoor Relationships and Anthropogenic Elemental Signatures in Airborne PM 2.5 at a High School: Impacts of Petroleum Refining Emissions on Lanthanoid Enrichment. Environ. Sci. Technol. 2017, 51, 4851–4859. [Google Scholar] [CrossRef]
- Martuzevicius, D.; Grinshpun, S.A.; Lee, T.; Hu, S.; Biswas, P.; Reponen, T.; LeMasters, G. Traffic-related PM2.5 aerosol in residential houses located near major highways: Indoor versus outdoor concentrations. Atmos. Environ. 2008, 42, 6575–6585. [Google Scholar] [CrossRef]
- Abdel-Salam, M.M.M. Outdoor and indoor factors influencing particulate matter and carbon dioxide levels in naturally ventilated urban homes. J. Air Waste Manag. Assoc. 2021, 71, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Mbengue, S.; Alleman, L.Y.; Flament, P. Bioaccessibility of trace elements in fine and ultrafine atmospheric particles in an industrial environment. Environ. Geochem. Health 2015, 37, 875–889. [Google Scholar] [CrossRef]
- Expósito, A.; Markiv, B.; Ruiz-Azcona, L.; Santibáñez, M.; Fernández-Olmo, I. Personal inhalation exposure to manganese and other trace metals in an environmentally exposed population: Bioaccessibility in size-segregated particulate matter samples. Atmos. Pollut. Res. 2021, 12, 101123. [Google Scholar] [CrossRef]
- Massey, D.; Kulshrestha, A.; Masih, J.; Taneja, A. Seasonal trends of PM10, PM5.0, PM2.5 & PM1.0 in indoor and outdoor environments of residential homes located in North-Central India. Build. Environ. 2012, 47, 223–231. [Google Scholar]
- Allen, R.W.; Adar, S.D.; Avol, E.; Cohen, M.; Curl, C.L.; Larson, T.; Liu, L.-J.S.; Sheppard, L.; Kaufman, J.D. Modeling the Residential Infiltration of Outdoor PM 2.5 in the Multi-Ethnic Study of Atherosclerosis and Air Pollution (MESA Air). Environ. Health Perspect. 2012, 120, 824–830. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.; Gao, W.; Sun, X.; Jiang, W.; Wang, Z.; Li, W. Measurements of indoor and outdoor fine particulate matter during the heating period in Jinan, in North China: Chemical composition, health risk, and source apportionment. Atmosphere 2020, 11, 885. [Google Scholar] [CrossRef]
- Zhao, J.; Birmili, W.; Wehner, B.; Daniels, A.; Weinhold, K.; Wang, L.; Merkel, M.; Kecorius, S.; Tuch, T.; Franck, U.; et al. Particle Mass Concentrations and Number Size Distributions in 40 Homes in Germany: Indoor-to-outdoor Relationships, Diurnal and Seasonal Variation. Aerosol. Air Qual. Res. 2020, 20, 576–589. [Google Scholar] [CrossRef]
- Jenkins, N.T.; Pierce, M.-G.; Eagar, T.W. Particle Size Distribution of Gas Metal and Flux Cored Arc Welding Fumes. Weld. J. 2005, 84, 156–163. [Google Scholar]
- Park, R.M.; Baldwin, M.; Bouchard, M.F.; Mergler, D. Airborne manganese as dust vs. fume determining blood levels in workers at a manganese alloy production plant. Neurotoxicology 2014, 45, 267–275. [Google Scholar] [CrossRef] [Green Version]
- Gjønnes, K.; Skogstad, A.; Hetland, S.; Ellingsen, D.G.; Thomassen, Y.; Weinbruch, S. Characterisation of workplace aerosols in the manganese alloy production industry by electron microscopy. Anal. Bioanal. Chem. 2011, 399, 1011–1020. [Google Scholar] [CrossRef]
- Smichowski, P.; Gómez, D.R. Atmospheric Aerosols, Analysis of. In Encyclopedia of Analytical Chemistry; John Wiley & Sons, Ltd.: Chichester, UK, 2012; ISBN 9780470027318. [Google Scholar]
- Ervik, T.K.; Benker, N.; Weinbruch, S.; Skogstad, A.; Thomassen, Y.; Ellingsen, D.G.; Berlinger, B. Phase identification of individual crystalline particles by combining EDX and EBSD: Application to workplace aerosols. Anal. Bioanal. Chem. 2018, 410, 2711–2721. [Google Scholar] [CrossRef]
- Gholampour, A.; Nabizadeh, R.; Hassanvand, M.S.; Taghipour, H.; Rafee, M.; Alizadeh, Z.; Faridi, S.; Mahvi, H. Characterization and source identification of trace elements in airborne particulates at urban and suburban atmospheres of Tabriz, Iran. Environ. Sci. Pollut. Res. 2016, 23, 1703–1713. [Google Scholar] [CrossRef]
- Lu, S.; Liu, D.; Zhang, W.; Liu, P.; Fei, Y.; Gu, Y.; Wu, M.; Yu, S.; Yonemochi, S.; Wang, X.; et al. Physico-chemical characterization of PM2.5 in the microenvironment of Shanghai subway. Atmos. Res. 2015, 153, 543–552. [Google Scholar] [CrossRef]
- Ledoux, F.; Laversin, H.; Courcot, D.; Courcot, L.; Zhilinskaya, E.A.; Puskaric, E.; Aboukaïs, A. Characterization of iron and manganese species in atmospheric aerosols from anthropogenic sources. Atmos. Res. 2006, 82, 622–632. [Google Scholar] [CrossRef]
- Walsh, M.P. The global experience with lead in gasoline and the lessons we should apply to the use of MMT. Am. J. Ind. Med. 2007, 50, 853–860. [Google Scholar] [CrossRef]
- Röllin, H.; Mathee, A.; Levin, J.; Theodorou, P.; Wewers, F. Blood manganese concentrations among first-grade schoolchildren in two South African cities. Environ. Res. 2005, 97, 93–99. [Google Scholar] [CrossRef]
- Sanderson, P.; Su, S.S.; Chang, I.T.H.; Delgado, S.J.M.; Kepaptsoglou, D.M.; Weber, R.J.M.; Harrison, R.M. Characterisation of iron-rich atmospheric submicrometre particles in the roadside environment. Atmos. Environ. 2016, 140, 167–175. [Google Scholar] [CrossRef]
- Batterman, S.; Su, F.-C.; Jia, C.; Naidoo, R.N.; Robins, T.; Naik, I. Manganese and lead in children’s blood and airborne particulate matter in Durban, South Africa. Sci. Total Environ. 2011, 409, 1058–1068. [Google Scholar] [CrossRef]
- Moreno, T.; Jones, T.P.; Richards, R.J. Characterisation of aerosol particulate matter from urban and industrial environments: Examples from Cardiff and Port Talbot, South Wales, UK. Sci. Total Environ. 2004, 334–335, 337–346. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, Y.; Wei, C.; Yao, B.; Zheng, C. Mineralogy and microstructure of ash deposits from the Zhuzhou coal-fired power plant in China. Int. J. Coal Geol. 2010, 81, 309–319. [Google Scholar] [CrossRef]
- Steinle, S.; Reis, S.; Sabel, C.E.; Semple, S.; Twigg, M.M.; Braban, C.F.; Leeson, S.R.; Heal, M.R.; Harrison, D.; Lin, C.; et al. Personal exposure monitoring of PM 2.5 in indoor and outdoor microenvironments. Sci. Total Environ. 2015, 508, 383–394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucchini, R.G.; Guazzetti, S.; Zoni, S.; Donna, F.; Peter, S.; Zacco, A.; Salmistraro, M.; Bontempi, E.; Zimmerman, N.J.; Smith, D.R. Tremor, olfactory and motor changes in Italian adolescents exposed to historical ferro-manganese emission. Neurotoxicology 2012, 33, 687–696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Residential Area | Pairings | Min | Max | Mean | SD | %Difference | I/O | CI (95%) | |
|---|---|---|---|---|---|---|---|---|---|
| Q1 | Q3 | ||||||||
| Old Sicelo | Indoor | 5.9475 | 18.840 | 12.2516 | 4.3295 | 8.2864 | 16.1538 | ||
| vs. | 48.5095 | 0.5040 | |||||||
| Outdoor | 15.2314 | 33.9638 | 23.7939 | 6.1595 | 18.2963 | 28.8151 | |||
| New Sicelo | Indoor | 8.3359 | 19.1895 | 12.9288 | 3.290 | 10.4902 | 15.9271 | ||
| vs. | 48.0221 | 0.5441 | |||||||
| Outdoor | 11.680 | 40.4385 | 24.8737 | 9.0046 | 18.4296 | 31.4255 | |||
| Noldick | Indoor | 2.8828 | 16.9837 | 7.7841 | 6.0794 | 3.6197 | 15.9553 | ||
| vs. | 70.3243 | 0.2732 | |||||||
| Outdoor | 19.470 | 35.0946 | 26.2305 | 5.0204 | 21.8400 | 30.2382 | |||
| Residential Area | r-Value | Intercept | Slope | R-Square | p-Value | Adjusted p-Value |
|---|---|---|---|---|---|---|
| Old Sicelo | 0.9688 | −3.9517 | 0.6810 | 0.9386 | 0.0307 | 0.0038 |
| New Sicelo | 0.8932 | 4.8116 | 0.3263 | 0.7978 | 0.0136 | 0.0025 |
| Noldick | 0.8703 | −19.8615 | 1.0539 | 0.7575 | 0.0077 | 0.0010 |
| Parameter | Old Sicelo | New Sicelo | Noldick | |||
|---|---|---|---|---|---|---|
| Indoor | Outdoor | Indoor | Outdoor | Indoor | Outdoor | |
| Minimum | 0.09 | 0.11 | 0.10 | 0.10 | 0.10 | 0.14 |
| Mean | 0.49 | 0.37 | 0.38 | 0.36 | 0.40 | 0.30 |
| Maximum | 1.06 | 0.67 | 0.66 | 0.70 | 0.70 | 0.48 |
| Elements | Old Sicelo | New Sicelo | Noldick | |||
|---|---|---|---|---|---|---|
| Indoor | Outdoor | Indoor | Outdoor | Indoor | Outdoor | |
| Si | 4.7417 | 11.3543 | 6.2718 | 15.3786 | 5.3376 | 7.5356 |
| Fe | 3.8665 | 10.5339 | 7.0881 | 12.0622 | 4.8544 | 6.1707 |
| Mg | 1.5450 | 1.9122 | 0.6943 | 1.1227 | 0.8178 | 0.9574 |
| Mn | 1.2045 | 3.9739 | 1.0346 | 5.5495 | 1.6712 | 6.5604 |
| Na | 0.9193 | 2.9715 | 1.1837 | 2.0935 | 0.8950 | 0.7115 |
| Zn | 0.1422 | 1.7705 | 0.3307 | 0.7567 | 0.3446 | 0.5178 |
| Cr | 0.0586 | 0.3284 | 0.0540 | 0.0631 | 0.0610 | 0.5136 |
| Pb | 0.0258 | 0.2958 | 0.0656 | 0.1037 | 0.0494 | 0.5098 |
| Cu | 0.0241 | 0.3270 | 0.0317 | 0.2742 | 0.0866 | 0.2819 |
| Ni | 0.0123 | 0.0882 | 0.0074 | 0.0510 | 0.0222 | 0.0800 |
| Co | 0.0046 | 0.0667 | 0.0073 | 0.0091 | 0.0074 | 0.0080 |
| V | 0.0024 | 0.2045 | 0.0225 | 0.0268 | 0.0225 | 0.0544 |
| K | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ |
| Cd | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mbazima, S.J.; Masekameni, M.D.; Nelson, G. Physicochemical Properties of Indoor and Outdoor Particulate Matter 2.5 in Selected Residential Areas near a Ferromanganese Smelter. Int. J. Environ. Res. Public Health 2021, 18, 8900. https://doi.org/10.3390/ijerph18178900
Mbazima SJ, Masekameni MD, Nelson G. Physicochemical Properties of Indoor and Outdoor Particulate Matter 2.5 in Selected Residential Areas near a Ferromanganese Smelter. International Journal of Environmental Research and Public Health. 2021; 18(17):8900. https://doi.org/10.3390/ijerph18178900
Chicago/Turabian StyleMbazima, Setlamorago Jackson, Masilu Daniel Masekameni, and Gill Nelson. 2021. "Physicochemical Properties of Indoor and Outdoor Particulate Matter 2.5 in Selected Residential Areas near a Ferromanganese Smelter" International Journal of Environmental Research and Public Health 18, no. 17: 8900. https://doi.org/10.3390/ijerph18178900






