Systematic Review and Meta-Analysis of Tocilizumab Therapy versus Standard of Care in over 15,000 COVID-19 Pneumonia Patients during the First Eight Months of the Pandemic
Abstract
:1. Introduction
2. Material and Methods
2.1. Systematic Review Study Protocol and Systematic Review Findings Reporting
2.2. Search Strategy
2.3. Inclusion and Exclusion Criteria
- P—(patients): subjects with COVID-19 (either suspected or confirmed);
- I—(intervention): treated with tocilizumab;
- C—(comparator/comparison/control): any kind of comparison possible (such as tocilizumab versus tocilizumab plus standard care, one versus multiple doses, intravenous versus subcutaneous injection, earlier versus later administration, administration in hospital ward versus in ICU setting, in ventilated versus not ventilated patients, and use of other concomitant therapy);
- O—(outcomes): mortality rate, admission to the ICU, need for mechanical ventilation, impact of concomitant therapy use on survival, with an emphasis on corticosteroids, and side-effects;
- S—(study design): investigations designed as case-control studies, ether matched or unmatched, and those investigations that, even if not explicitly devised as case-control studies, provided information for each treatment cohort.
2.4. Data Extraction
2.5. Study Quality Appraisal
2.6. Statistical Analysis
3. Results
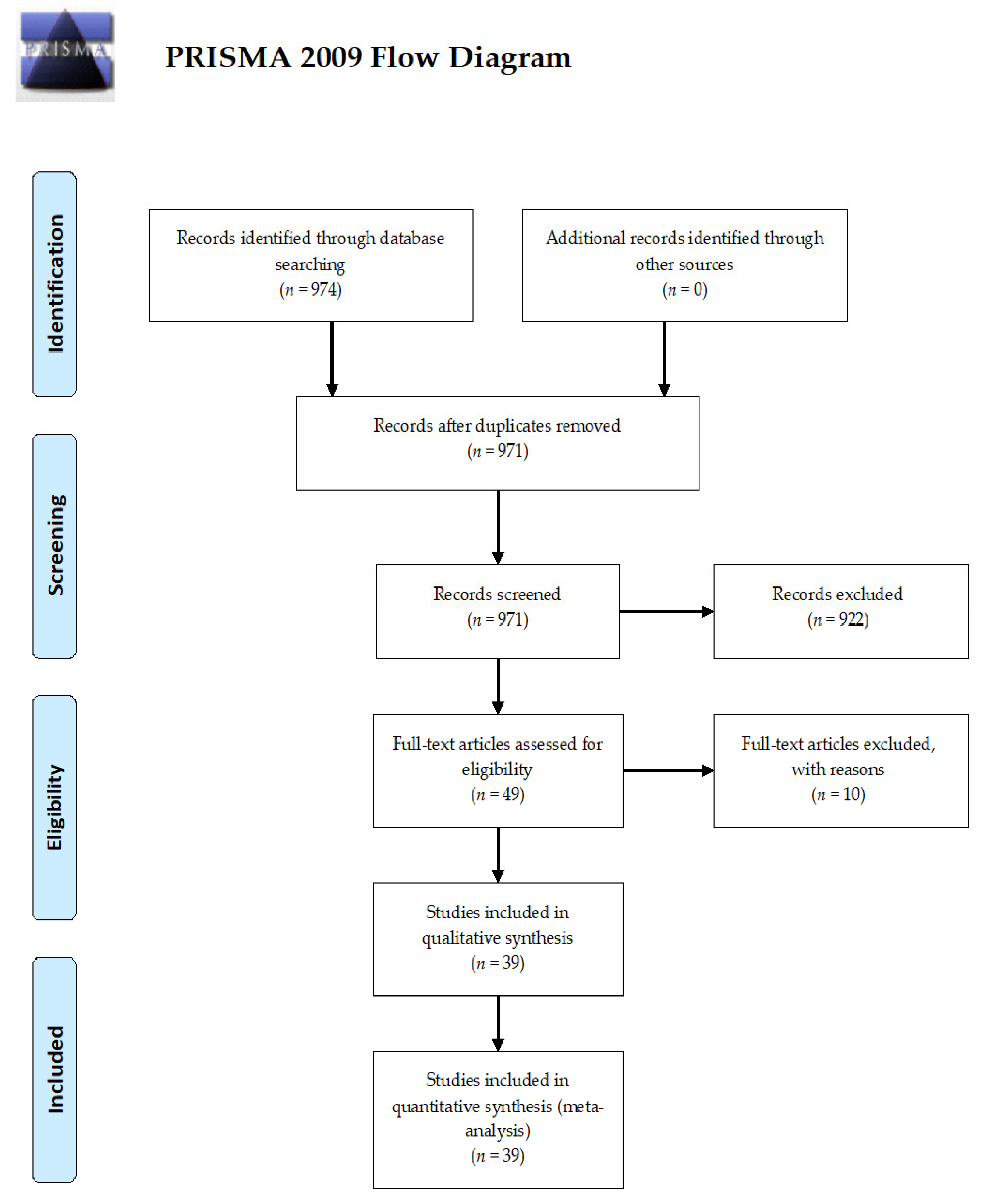
3.1. Search Strategy and Study Setting
3.2. Age and Gender in Tocilizumab Treated Cases and Controls
3.3. Underlying Co-Morbidities
3.4. Laboratory Parameters and Oxygen Saturation
3.5. Concomitant Therapies
3.6. Major Outcomes
3.6.1. Overall Impact of Tocilizumab on Death Rate
3.6.2. Impact of Concomitant Corticosteroid Use on Survival
3.6.3. Impact of Tocilizumab on Preventing Mechanical Ventilation
3.6.4. Studies Specifically Reporting the Impact of Tocilizumab on ICU Admission
3.6.5. Tocilizumab Side-Effects
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Habibzadeh, P.; Stoneman, E.K. The Novel Coronavirus: A Bird’s Eye View. Int. J. Occup. Environ. Med. 2020, 11, 65–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, Z.J.; Shan, J. 2019 Novel coronavirus: Where we are and what we know. Infection 2020, 48, 155–163. [Google Scholar] [CrossRef] [Green Version]
- Peeri, N.C.; Shrestha, N.; Rahman, M.S.; Zaki, R.; Tan, Z.; Bibi, S.; Baghbanzadeh, M.; Aghamohammadi, N.; Zhang, W.; Haque, U. The SARS, MERS and novel coronavirus (COVID-19) epidemics, the newest and biggest global health threats: What lessons have we learned? Int. J. Epidemiol. 2020, 22, dyaa033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, G.; Fan, Y.; Lai, Y.; Han, T.; Li, Z.; Zhou, P.; Pan, P.; Wang, W.; Hu, D.; Liu, X.; et al. Coronavirus infections and immune responses. J. Med. Virol. 2020, 92, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Birra, D.; Benucci, M.; Landolfi, L.; Merchionda, A.; Loi, G.; Amato, P.; Licata, G.; Quartuccio, L.; Triggiani, M.; Moscato, P. COVID 19: A clue from innate immunity. Immunol. Res. 2020, 10, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.B.; June, C.H. Cytokine release syndrome in severe COVID-19. Science 2020, 368, 473–474. [Google Scholar] [CrossRef] [Green Version]
- McGonagle, D.; Sharif, K.; O’Regan, A.; Bridgewood, C. The Role of Cytokines including Interleukin-6 in COVID-19 induced Pneumonia and Macrophage Activation Syndrome-Like Disease. Autoimmun. Rev. 2020, 19, 102537. [Google Scholar] [CrossRef] [PubMed]
- Hay, K.A. Cytokine release syndrome and neurotoxicity after CD19 chimeric antigen receptor-modified (CAR-) T cell therapy. Br. J. Haematol. 2018, 183, 364–374. [Google Scholar] [CrossRef] [Green Version]
- Castañeda, S.; Martínez-Quintanilla, D.; Martín-Varillas, J.L.; García-Castañeda, N.; Atienza-Mateo, B.; González-Gay, M.A. Tocilizumab for the treatment of adult-onset Still’s disease. Expert Opin. Biol. Ther. 2019, 19, 273–286. [Google Scholar] [CrossRef]
- Scheinecker, C.; Smolen, J.; Yasothan, U.; Stoll, J.; Kirkpatrick, P. Tocilizumab. Nat. Rev. Drug Discov. 2009, 8, 273–274. [Google Scholar] [CrossRef]
- Mihara, M.; Nishimoto, N.; Ohsugi, Y. The therapy of autoimmune diseases by anti-interleukin-6 receptor antibody. Expert Opin. Biol. Ther. 2005, 5, 683–690. [Google Scholar] [CrossRef]
- Paul-Pletzer, K. Tocilizumab: Blockade of interleukin-6 signaling pathway as a therapeutic strategy for inflammatory disorders. Drugs Today 2006, 42, 559–576. [Google Scholar] [CrossRef]
- Quartuccio, L.; Sonaglia, A.; Pecori, D.; Peghin, M.; Fabris, M.; Tascini, C.; De Vita, S. Higher levels of IL-6 early after tocilizumab distinguish survivors from non-survivors in COVID-19 pneumonia: A possible indication for deeper targeting IL-6. J. Med. Virol. 2020, 92, 2852–2856. [Google Scholar] [CrossRef]
- Xu, X.; Han, M.; Li, T.; Sun, W.; Wang, D.; Fu, B.; Zhou, Y.; Zheng, X.; Yang, Y.; Li, X.; et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc. Natl. Acad. Sci. USA 2020, 117, 10970–10975. [Google Scholar] [CrossRef]
- Fu, B.; Xu, X.; Wei, H. Why tocilizumab could be an effective treatment for severe COVID-19? J. Transl. Med. 2020, 18, 164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horby, P.; Lim, W.S.; Emberson, J.; Mafham, M.; Bell, J.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; Elmahi, E.; et al. Effect of Dexamethasone in Hospitalized Patients with COVID-19: Preliminary Report. medRxiv 2020. [Google Scholar] [CrossRef]
- Fadel, R.; Morrison Austin, R.; Vahia, A.; Smith, Z.R.; Chaudhry, Z.; Bhargava, P.; Miller, J.; Kenney, R.M.; Alangaden, G.; Mayur, S.R.; et al. Early Short Course Corticosteroids in Hospitalized Patients with COVID-19. Clin. Infect. Dis. 2020, 71, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [Green Version]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA Statement. Open Med. 2009, 3, e123–e130. [Google Scholar] [PubMed]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. Br. Med. J. 2003, 327, 557–560. [Google Scholar] [CrossRef] [Green Version]
- Allenbach, Y.; Saadoun, D.; Maalouf, G.; Vieira, M.; Hellio, A.; Boddaert, J.; Gros, H.; Salem, J.E.; Resche-Rigon, M.; Biard, L.; et al. Multivariable prediction model of intensive care unit transfer and death: A French prospective cohort study of COVID-19 patients. medRxiv 2020. [Google Scholar] [CrossRef]
- Ayerbe, L.; Risco, C.; Ayis, S. The association between treatment with heparin and survival in patients with Covid-19. J. Thromb. Thrombolysis 2020, 50, 298–301. [Google Scholar] [CrossRef] [PubMed]
- Campochiaro, C.; Della-Torre, E.; Cavalli, G.; De Luca, G.; Ripa, M.; Boffini, N.; Tomelleri, A.; Baldissera, E.; Rovere-Querini, P.; Ruggeri, A.; et al. Efficacy and safety of tocilizumab in severe COVID-19 patients: A single-centre retrospective cohort study. Eur. J. Intern Med. 2020, 76, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Capra, R.; De Rossi, N.; Mattioli, F.; Romanelli, G.; Scarpazza, C.; Sormani, M.P.; Cossi, S. Impact of low dose tocilizumab on mortality rate in patients with COVID-19 related pneumonia. Eur. J. Intern Med. 2020, 76, 31–35. [Google Scholar] [CrossRef]
- Carvalho, V.; Turon, R.; Goncalves, B.; Ceotto, V.; Kurtz, P.; Righy, C. Effects of Tocilizumab in Critically Ill Patients With COVID-19: A Quasi-Experimental Study. medRxiv 2020. [Google Scholar] [CrossRef]
- Colaneri, M.; Bogliolo, L.; Valsecchi, P.; Sacchi, P.; Zuccaro, V.; Brandolino, F.; Montecucco, C.; Mojoli, F.; Giusti, E.M.; Bruno, R.; et al. The Covid Irccs San Matteo Pavia Task Force. Microorganisms 2020, 8, 695. [Google Scholar] [CrossRef]
- Crotty, M.P.; Akins, R.L.; Nguyen, A.T.; Slika, R.; Rahmanzadeh, K.; Wilson, M.H.; Dominguez, E.A. Investigation of subsequent and co-infections associated with SARS-CoV-2 (COVID-19) in hospitalized patients. medRxiv 2020. [Google Scholar] [CrossRef]
- De la Rica, R.; Marcio, B.; Maria, A.; del Castillo, A.; Antonia, S.; Antoni, P.; Gemma, R.; Lorenzo, S.; Lluis, M.; Gonzalez-Freire, M. Low albumin levels are associated with poorer outcomes in a case series of COVID-19 patients in Spain: A retrospective cohort study. Microorganisms 2020, 8, 1106. [Google Scholar] [CrossRef]
- Edwards, D.; McGrail, D.E. COVID-19 Case Series at UnityPoint Health St. Lukes Hospital in Cedar Rapids, IA. medRxiv 2020. [Google Scholar] [CrossRef]
- Fernandez-Cruz, A.; Ruiz-Antoran, B.; Munoz-Gomez, A.; Sancho-Lopez, A.; Mills-Sanchez, P.; Centeno-Soto, G.A.; Blanco-Alonso, S.; Javaloyes-Garachana, L.; Galan-Gomez, A.; Valencia-Alijo, A.; et al. Impact of glucocorticoid treatment in SARS-CoV-2 infection mortality: A retrospective controlled cohort study. medRxiv 2020. [Google Scholar] [CrossRef]
- Garibaldi, B.T.; Fiksel, J.; Muschelli, J.; Robinson, M.L.; Rouhizadeh, M.; Nagy, P.; Gray, J.H.; Malapati, H.; Ghobadi-Krueger, M.; Niessen, T.M.; et al. Patient trajectories and risk factors for severe outcomes among persons hospitalized for COVID-19 in the Maryland/DC region. medRxiv 2020. [Google Scholar] [CrossRef]
- Gokhale, Y.; Mehta, R.; Karnik, N.; Kulkarni, U.; Gokhale, S. Tocilizumab improves survival in patients with persistent hypoxia in severe COVID-19 pneumonia. EClinical Med. 2020, 24, 100467. [Google Scholar] [CrossRef] [PubMed]
- Guaraldi, G.; Meschiari, M.; Cozzi-Lepri, A.; Milic, J.; Tonelli, R.; Menozzi, M.; Franceschini, E.; Gianluca, C.; Orlando, G.; Vanni, B.; et al. Tocilizumab in patients with severe COVID-19: A retrospective cohort study. Lancet Rheumatol. 2020, 2, e474–e484. [Google Scholar] [CrossRef]
- Holt, G.E.; Batra, M.; Murthi, M.; Kambali, S.; Santos, K.; Bastidas, M.V.P.; Huda, A.; Haddadi, S.; Arias, S.; Mehdi, M. Lack of Tocilizumab effect on mortality in COVID19 patients. Sci. Rep. 2020, 10, 17100. [Google Scholar] [CrossRef] [PubMed]
- Ip, A.; Berry, D.A.; Hansen, E.; Goy, A.H.; Pecora, A.L.; Sinclaire, B.A.; Bednarz, U.; Marafelias, M.; Berry, S.M.; Berry, N.S.; et al. Hydroxychloroquine and Tocilizumab Therapy in COVID-19 Patients—An Observational Study. medRxiv 2020. [Google Scholar] [CrossRef]
- Kewan, T.; Covut, F.; Al–Jaghbeer, M.J.; Rose, L.; Gopalakrishna, K.V.; Akbik, B. Tocilizumab for treatment of patients with severe COVID–19: A retrospective cohort study. EClinical Med. 2020, 24, 100418. [Google Scholar] [CrossRef]
- Kimmig, L.M.; Wu, D.; Gold, M.; Pettit, N.N.; Pitrak, D.; Mueller, J.; Husain, A.N.; Mutlu, E.A.; Mutlu, G.M. IL6 inhibition in critically ill COVID-19 patients is associated with increased secondary infections. medRxiv 2020. [Google Scholar] [CrossRef]
- Klopfenstein, T.; Zayet, S.; Lohse, A.; Balblanc, J.-C.; Badie, J.; Royer, P.-Y.; Toko, L.; Mezher, C.; Kadiane-Oussou, N.J.; Bossert, M.; et al. Tocilizumab therapy reduced intensive care unit admissions and/or mortality in COVID-19 patients. Med. Mal. Infect. 2020, 50, 397–400. [Google Scholar] [CrossRef]
- Martinez-Sanz, J.; Muriel, A.; Ron, R.; Herrera, S.; Ron, R.; Perez-Molina, J.A.; Moreno, S.; Serrano-Villar, S. Effects of Tocilizumab on Mortality in Hospitalized Patients with COVID-19: A Multicenter Cohort Study. medRxiv 2020. [Google Scholar] [CrossRef]
- Mikulska, M.; Nicolini, L.A.; Signori, A.; Di Biagio, A.; Sepulcri, C.; Russo, C.; Dettori, S.; Berruti, M.; Sormani, M.P.; Giacobbe, D.R.; et al. Tocilizumab and steroid treatment in patients with severe Covid-19 pneumonia. medRxiv 2020. [Google Scholar] [CrossRef]
- Moreno Garcia, E.; Caballero, V.R.; Albiach, L.; Aguero, D.; Ambrosioni, J.; Bodro, M.; Cardozo, C.; Chumbita, M.; De la Mora, L.; Pouton, N.G.; et al. Tocilizumab is associated with reduction of the risk of ICU admission and mortality in patients with SARS-CoV-2 infection. medRxiv 2020. [Google Scholar] [CrossRef]
- Moreno-Pérez, O.; Andres, M.; Leon-Ramirez, J.M.; Sánchez-Payá, J.; Rodríguez, J.C.; Sánchez, R.; García-Sevila, R.; Boix, V.; Gil, J.; Merino, E. Experience with tocilizumab in severe COVID-19 pneumonia after 80 days of follow-up: A retrospective cohort study. J. Autoimmun. 2020, 16, 102523. [Google Scholar] [CrossRef] [PubMed]
- Narain, S.; Stefanov, D.; Chau, A.S.; Weber, A.G.; Marder, G.S.; Kaplan, B.; Malhotra, P.; Bloom, O.; Liu, A.; Lesser, M.; et al. Comparative Survival Analysis of Immunomodulatory Therapy for COVID-19 ‘Cytokine Storm’: A Retrospective Observational Cohort Study. medRxiv 2020. [Google Scholar] [CrossRef]
- Ibrahim, T.B.H.; Ahmed, Z.; Dana, B.; Abdelrauof, A.; Anas, B.; Bassem, A.; Reem, E.; Ahmed, H.; Mohamed, N.B.; Fatma, B.A.; et al. First Consecutive 5000 Patients with Coronavirus Disease 2019 from Qatar: A Nation-wide Cohort Study. medRxiv 2020. [Google Scholar] [CrossRef]
- Pandolfi, L.; Fossali, T.; Frangipane, V.; Bozzini, S.; Morosini, M.; D’Amato, M.; Lettieri, S.; Urtis, M.; Di Toro, A.; Saracino, L.; et al. Broncho-alveolar inflammation in COVID-19 patients: A correlation with clinical out come. medRxiv 2020. [Google Scholar] [CrossRef]
- Patel, M.; Gangemi, A.; Marron, R.; Chowdhury, J.; Yousef, I.; Zheng, M.; Mills, N.; Tragesser, L.; Giurintano, J.; Gupta, R.; et al. Use of High Flow Nasal Therapy to Treat Moderate to Severe Hypoxemic Respiratory Failure in COVID-19. medRxiv 2020. [Google Scholar] [CrossRef]
- Patel, M.; Chowdhury, J.; Mills, N.; Marron, R.; Gangemi, A.; Dorey-Stein, Z.; Yousef, I.; Zheng, M.; Tragesser, L.; Giurintano, J.; et al. ROX Index Predicts Intubation in Patients with COVID-19 Pneumonia and Moderate to Severe Hypoxemic Respiratory Failure Receiving High Flow Nasal Therapy. medRxiv 2020. [Google Scholar] [CrossRef]
- Perez Tanoira, R.; Perez Garcia, F.; Romanyk, J.; Gomez-Herruz, P.; Arroyo, T.; Gonzalez, R.; Lledo Garcia, L.; Verdu Exposito, C.; Sanz Moreno, J.; Gutierrez, I.; et al. Prevalence and risk factors for mortality related to COVID-19 in a severely affected area of Madrid, Spain. medRxiv 2020. [Google Scholar] [CrossRef]
- Perrone, F.; Piccirillo, M.C.; Ascierto, P.A.; Salvarani, C.; Parrella, R.; Marata, A.M.; Popoli, P.; Ferraris, L.; Marrocco Trischitta, M.M.; Ripamonti, D.; et al. Tocilizumab for patients with COVID-19 pneumonia. The TOCIVID-19 phase 2 trial. medRxiv 2020. [Google Scholar] [CrossRef]
- Potere, N.; Di Nisio, M.; Cibelli, D.; Scurti, R.; Frattari, A.; Porreca, E.; Abbate, A.; Parruti, G. Interleukin-6 receptor blockade with subcutaneous tocilizumab in severe COVID-19 pneumonia and hyperinflammation: A case-control study. Ann. Rheum. Dis. 2020, 80, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Quartuccio, L.; Sonaglia, A.; McGonagle, D.; Fabris, M.; Peghin, M.; Pecori, D.; De Monte, A.; Bove, T.; Curcio, F.; Bassi, F.; et al. Profiling COVID-19 pneumonia progressing into the cytokine storm syndrome: Results from a single Italian Centre study on tocilizumab versus standard of care. J. Clin. Virol. 2020, 129, 104444. [Google Scholar] [CrossRef] [PubMed]
- Ramaswamy, M.; Mannam, P.; Comer, R.; Sinclair, E.; McQuaid, D.B.; Schmidt, M.L. Off-Label Real World Experience Using Tocilizumab for Patients Hospitalized with COVID-19 Disease in a Regional Community Health System: A Case-Control Study. medRxiv 2020. [Google Scholar] [CrossRef]
- Rodríguez Molinero, A.; Pérez-López, C.; Gálvez-Barrón, C.; Miñarro, A.; Macho, O.; López, G.F.; Robles, M.T.; Dapena, M.D.; Martínez, S.; Rodríguez, E.; et al. Matched Cohort Study on the Efficacy of Tocilizumab in Patients with COVID-19. Res. Sq. 2020. preprint. [Google Scholar] [CrossRef]
- Rojas-Marte, G.R.; Khalid, M.; Mukhtar, O.; Hashmi, A.T.; Waheed, M.A.; Ehrlich, S.; Aslam, A.; Siddiqui, S.; Agarwal, C.; Malyshev, Y.; et al. Outcomes in Patients with Severe COVID-19 Disease Treated with Tocilizumab—A Case-Controlled Study. QJM 2020, 22, hcaa206. [Google Scholar] [CrossRef]
- Rossi, B.; Nguyen, L.S.; Zimmermann, P.; Boucenna, F.; Baucher, L.; Dubret, L.; Guillot, H.; Bouldouyre, M.-A.; Allenbach, Y.; Salem, J.-E.; et al. Effect of tocilizumab in hospitalized patients with severe pneumonia COVID-19: A cohort study. medRxiv 2020. [Google Scholar] [CrossRef]
- Roumier, M.; Paule, R.; Groh, M.; Vallee, A.; Ackermann, F. Interleukin-6 blockade for severe COVID-19. medRxiv 2020. [Google Scholar] [CrossRef]
- Sisó-Almirall, A.; Kostov, B.; Mas-Heredia, M.; Vilanova-Rotllan, S.; Sequeira-Aymar, E.; Sans-Corrales, M.; Sant-Arderiu, E.; Cayuelas-Redondo, L.; Martínez-Pérez, A.; García-Plana, N.; et al. Prognostic factors in Spanish COVID-19 patients: A case series from Barcelona. medRxiv 2020. [Google Scholar] [CrossRef]
- Somers, E.C.; Eschenauer, G.A.; Troost, J.P.; Golob, J.L.; Gandhi, T.N.; Wang, L.; Zhou, N.; Petty, L.A.; Baang, J.H.; Dillman, N.O.; et al. Tocilizumab for treatment of mechanically ventilated patients with COVID-19. medRxiv 2020. [Google Scholar] [CrossRef]
- Wadud, N.; Ahmed, N.; Shergil, M.M.; Khan, M.; Krishna, M.G.; Gilani, A.; El Zarif, S.; Galaydick, J.; Linga, K.; Koor, S.; et al. Improved survival outcome in SARs-CoV-2 (COVID-19) Acute Respiratory Distress Syndrome patients with Tocilizumab administration. medRxiv 2020. [Google Scholar] [CrossRef]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J. HLH Across Speciality Collaboration, UK. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef]
- Ragab, D.; Salah Eldin, H.; Taeimah, M.; Khattab, R.; Salem, R. The COVID-19 Cytokine Storm; What We Know So Far. Front. Immunol. 2020, 11, 1446. [Google Scholar] [CrossRef]
- McGonagle, D.; O’Donnell, J.S.; Sharif, K.; Emery, P.; Bridgewood, C. Immune mechanisms of pulmonary intravascular coagulopathy in COVID-19 pneumonia. Lancet Rheumatol. 2020, 2, e437–e445. [Google Scholar] [CrossRef]
- Klopfenstein, T.; Gendrin, V.; Gerazime, A.; Conrozier, T.; Balblanc, J.C.; Royer, P.Y.; Lohse, A.; Mezher, C.; Toko, L.; Guillochon, C.; et al. Systematic Review and Subgroup Meta-analysis of Randomized Trials to Determine Tocilizumab’s Place in COVID-19 Pneumonia. Infect. Dis. Ther. 2021, 10, 1195–1213. [Google Scholar] [CrossRef]
- WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group; Shankar-Hari, M.; Vale, C.L.; Godolphin, P.J.; Fisher, D.; Higgins, J.P.T.; Spiga, F.; Savovic, J.; Tierney, J.; Baron, G.; et al. Association Between Administration of IL-6 Antagonists and Mortality Among Patients Hospitalized for COVID-19: A Meta-analysis. JAMA 2021, 326, 499–518. [Google Scholar] [CrossRef] [PubMed]
- Matthay, M.A.; Luetkemeyer, A.F. IL-6 Receptor Antagonist Therapy for Patients Hospitalized for COVID-19: Who, When, and How? JAMA 2021, 326, 483–485. [Google Scholar] [CrossRef] [PubMed]









| Systematic Review Search Strategy Item | Details |
|---|---|
| International scholarly electronic databases searched | PubMed/MEDLINE, Scopus, pre-print servers (medRxiv, SSRN, Research Square) |
| Keywords | (“SARS-CoV-2” OR “novel coronavirus” OR “emerging coronavirus” OR “Wuhan coronavirus” OR “2019-nCoV” OR “COVID-19”) AND (tocilizumab OR Actemra OR “interleukin 6-blockade treatment” OR “interleukin 6-blockade therapy” OR “IL-6-blockade treatment” OR “IL-6-blockade therapy” OR “IL-6 blocker”) |
| Time filter | None |
| Language filter | None |
| Inclusion criteria |
P (patients): laboratory- and/or radiologically suspected or confirmed COVID-19 patients
I (intervention): treated with tocilizumab C (comparisons/comparators/controls): pharmacological treatment (tocilizumab + standard care versus standard care); dose, route, timing, and setting of tocilizumab administration, and use of concomitant therapy O (outcomes): death rate, need for mechanical ventilation; ICU admission, side-effects S (study design): any original paper (designed as case report) |
| Exclusion criteria |
P (patients): patients without a suspected/confirmed diagnosis of COVID-19
I (intervention): other treatments rather than tocilizumab C (comparisons/comparators/controls): comparisons different from those previously stated (for example, disease severity) O (outcomes): outcomes different from those previously stated or reporting those outcomes without sufficient details S (study design): any kind of review paper (including systematic reviews and meta-analyses if available), letter to editor, editorial, commentary, expert opinion; original studies designed as case reports, case series, cohort studies |
| Hand-searched target journals | Any journal potentially related to intensive care medicine, infectious disorders, virology, microbiology, epidemiology, global and public health, hygiene |
| Reference | Country | Sample Size | Inclusion Criteria | Age | Sex | Treatment | Tocilizumab Dosage | Starting of Tocilizumab | Admission to ICU | Parameters/Outcomes Evaluated |
|---|---|---|---|---|---|---|---|---|---|---|
| Allenbach et al., 2020 [22] | France | 147 consecutive patients out of an initial list of 152 (6 cases and 141 controls) | Laboratory-confirmed cases (positive SARS-CoV-2 RT-PCR assay from nasal swabs) | NA | NA | Tocilizumab + standard care (hydroxychloroquine, antibiotics and steroids) | NA | At admission | Hospitalized; with some admitted to the ICU | Composite index (mortality rate and/or ICU admission) |
| Ayerbe et al., 2020 [23] | Spain | 2019 consecutive patients (421 cases and 1598 controls); all severe | Laboratory-confirmed cases | 66.1 ± 13.11; younger than controls (p = 0.0267) | n = 304 (72.21%); less females among cases than among controls | Tocilizumab + standard care (hydroxychloroquine, azithromycin, steroids, lopinavir/ritonavir, or oseltamivir, heparin) | NA | NA (at any time during admission) | All hospitalized; none admitted to the ICU | Mortality rate (after 8 days of follow–up) |
| Campochiaro et al., 2020 [24] | Italy | 65 consecutive patients (32 cases versus 33 controls), with severe disease | Laboratory-and radiologically-confirmed cases | 64 [range 53–75]; no difference with controls | n = 29 (91%); no difference with controls | Tocilizumab + standard care (hydroxychloroquine, lopinavir/ritonavir, ceftriaxone, azithromycin, anti-coagulation prophylaxis with enoxaparin) | Single dose of i.v. 400 mg followed by a dose of 400 mg 24 h after in case of respiratory worsening. A second dose was administered in 9 (28%) patients (seven of which were under non-invasive ventilation) | 24 h prior to ICU admission and/or intubation | Hospitalized and all admitted to the ICU; 25 (78%) under non-invasive ventilation, 4 (13%) under mechanical ventilation | Mortality rate (at 28 days) Need for ventilation |
| Capra et al., 2020 [25] | Italy | 85 consecutive patients (62 cases versus 23 controls) | Laboratory- and radiologically-confirmed cases; critically ill patients requiring mechanic ventilation, with abnormal platelets and transaminases values were exclusion criteria | 63 [range 54–73]; younger than controls | 45(73%); less males among cases than among controls (73% versus 83%) | Tocilizumab + standard care versus standard care (hydroxychloroquine and lopinavir plus ritonavir) | 33 (53%) 400 mg i.v. once, 27 (43.5%) subcutaneous 324 mg once; 2 (3.5%) 800 mg i.v. | As soon as tocilizumab was available (within 4 days from admission) | Hospitalized, no one admitted to ICU, 5 under mechanical ventilation | Mortality |
| Carvalho et al., 2020 [26] | Brazil | 53 consecutive patients (29 cases and 24 controls); all critically ill | Suspected or laboratory-confirmed cases | 55 [range 44–65] | 62% | Tocilizumab + standard care (hydroxychloroquine, azythromycin, steroids) | 400 mg i.v., two doses | At admission | Admitted to the ICU | Mortality Positive cultures |
| Colaneri et al., 2020 [27] | Italy | 112 patients from the SMACORE study (21 cases and 91 controls) | Laboratory-confirmed cases | 62.33 | 19/21 (90.5%); less females among cases than among controls | Tocilizumab + standard care versus standard care (hydroxychloroquine, azithromycin and low weight heparin, and methylprednisolone) | 8 mg/kg (up to a maximum 800 mg per dose) i.v., repeated after 12 h | NA | Hospitalized, 3 admitted to the ICU | ICU admission Mortality |
| Crotty et al., 2020 [28] | USA | 289 patients (18 cases and 271 controls) | Laboratory-confirmed cases | NA | NA | Tocilizumab + standard care (antibiotics, hydroxychloroquine, remdesivir, steroids) | NA | NA | Hospitalized patients | Infections |
| de la Rica et al., 2020 [29] | Spain | 58 patients (11 and 47 controls) | Laboratory-confirmed cases (nasal and pharyngeal swabs) | NA | NA | Tocilizumab + standard care (chloroquine or hydroxychloroquine, Remdesivir, lopinavir + ritovanir, steroids, antibiotics, interferon beta) | NA | NA | Hospitalized patients | ICU admission |
| Edwards and McGrail, 2020 [30] | USA | 35 consecutive patients (11 cases and 24 controls), all critically ill | Laboratory-confirmed cases | NA | NA | Tocilizumab + standard care (hydroxychloroquine, azithromycin and convalescent plasma; remdesivir only for 1 patient) | 4/11 receiving a second dose | NA | Admitted to the ICU; 8/11 requiring mechanical ventilation | Mortality |
| Fernández-Cruz et al., 2020 [31] | Spain | 463 (180 cases and 283 controls) | Laboratory-confirmed cases | NA | NA | Tocilizumab + standard care (hydroxychloroquine, lopinavir/ritonavir, antibiotics, interferon) | NA | NA | NA | Mortality rate |
| Garibaldi et al., 2020 [32] | USA | 832 patients (39 cases and 793 controls) | Laboratory-confirmed cases | NA | NA | Tocilizumab + standard care (antibiotics, hydroxychloroquine, corticosteroids, antivirals) | NA | NA | NA | Mortality rate |
| Gokhale et al., 2020 [33] | India | 161 consecutive patients (70 cases and 91 controls) | Laboratory-confirmed cases | 52 [range 44–57], younger than controls (p = 0.001) | 67.1% | Tocilizumab + standard care (antibiotics, hydroxychloroquine, ivermectin, oseltamivir, low molecular weight heparin s.c., methylprednisolone i.v.) | 70 received a single i.v. dose of 400 mg while 91 did not | NA | Hospitalized, 2 (2.9%) requiring mechanical ventilation | Mortality rate |
| Guaraldi et al., 2020 [34] | Italy | 544 patients (179 cases and 365 controls) | Laboratory-confirmed cases | 64 [range 54–72], younger than controls (p = 0.0064) | 127 (71%); comparable in terms of gender | Tocilizumab + steroids, hydroxychloroquine, azithromycin, antivirals and antiretrovirals, such as darunavir–cobicistat or lopinavir/ritonavir, anticoagulants | 8 mg/kg i.v. up to a maximum of 800 mg administered twice, 12 h apart; 162 mg administered s.c. in two simultaneous doses; n = 91 s.c., n = 88, i.v. | At the time of hospital admission | Hospitalized patients | Survival rate Need for mechanical ventilation |
| Holt et al., 2020 [35] | USA | 62 patients (32 cases and 30 controls) | Laboratory-confirmed cases | NA | NA | Tocilizumab + standard care (NA) | NA | NA | Hospitalized patients | Mortality |
| Ip et al., 2020 [36] | USA | 547 patients (134 cases and 413 controls) | Laboratory-confirmed cases | 62 [range 53–70], younger than controls (p < 0.0001) | 99 (73.9%), less females among cases than among controls | Tocilizumab + steroids, hydroxychloroquine, azithromycin | 104 (78%) receiving 400 mg (96%), followed by 800 mg (1%), 8 mg/kg (1%), 4 mg/kg (1%), and missing dosing (1%) | After entering the ICU | All admitted to the ICU (29 admitted on first day to the ICU) | Survival rate |
| Kewan et al., 2020 [37] | USA | 51 patients (28 cases and 23 controls) | Laboratory-confirmed cases | 62 [range 53–71], younger than controls | 20 (71%), less females among cases than among controls | Tocilizumab + standard care (azythromicin, hydroxychloroquine, steroids) | 8 mg/kg up to 400 mg as a 60 min single i.v. infusion | Following admission based on clinical parameters | Hospitalized/admitted to the CU | Mortality rate Length of stay ICU admission Need for mechanical ventilation Bacterial infection |
| Kimmig et al., 2020 [38] | USA | 60 patients (28 cases versus 32 controls) | Laboratory-confirmed cases | 63.86 ± 16.04 | 20 (71.4%), less females among cases than males among controls | Tocilizumab + standard care (NA) | 400–800 mg (n = 3 patients received a second dose; n = 1 patient received a single dose of 800 mg) | NA | All admitted to the ICU | Mortality rate Infection rate |
| Klopfenstein et al., 2020 [39] | France | 45 patients (20 cases versus 25 controls), with severe disease | Laboratory-confirmed cases and clinical suspicion, exclusion of patients receiving non standard care treatment (such as IVIG), exclusion of patients with moderate disease | 76.8 ± 11 [range 52–93]; no differences with controls | NA | Tocilizimab + standard care (hydroxychloroquine, lopinavir-ritonavir, antibiotics, and corticosteroids) | At least 1 or 2 doses | 13 days after symptoms onset, 7 days after admission/hospitalization | Hospitalized, none admitted to the ICU | ICU admission and death (composite clinical outcome) |
| Martínez-Sanz et al., 2020 [40] | Spain | 1229 patients (260 cases and 969 controls) | Laboratory-confirmed cases (nasopharyngeal swabs or other valid respiratory samples) | 65 [range 55–76] (younger than controls, p = 0.017) | n = 191 (73%); less females among cases than among controls (p < 0.001) | Tocilizumab + standard care (steroids, hydroxychloroquine, azithromycin, lopinavir/ritonavir) | 600 mg (IQR 600–800 mg) | After 4 (IQR 3–5) days since admission | Hospitalized, 50 (19%) admitted to the ICU | ICU admission Death rate |
| Mikulska et al., 2020 [41] | Italy | 196 patients (85 cases and 111 controls) | Laboratory-confirmed cases | 32–85 | 59 | Tocilizumab + standard care (hydroxychloroquine, darunavir/ritonavir, methylprednisolone) | 8 mg/kg i.v. or 162 mg s.c. | After 3 days from admission | Hospitalized | Mortality rate |
| Moreno-Garcia et al., 2020 [42] | Spain | 171 patients (77 cases versus 94 controls) | Laboratory-confirmed cases (n = 68, 88.3%) | 61.5 ± 12.4 | 53 (68.8%) | Tocilizumab + standard care (lopinavir/ritonavir plus hydroxychloroquine, azithromycin, steroids such as methylprednisolone, heparin) | 400–600 mg i.v. | After admission based on clinical course | 8 (10.3%) admitted to the ICU | Oxygen therapy |
| Moreno-Pérez et al., 2020 [43] | Spain | 236 patients (77 cases and 159 controls) | Laboratory-confirmed cases | 62.0 [range 53.0–72.0], slightly older than controls but borderline significant | 64.9 (50/77) | Tocilizumab + standard care (hydroxychloroquine, lopinavir/ritonavir, and azithromycin) | Initial dose of 600 mg, with a second or third dose (400 mg) in case of persistent or progressive disease | 2.0 days [range 1.0–4.0] after admission | Hospitalized, forty-two patients (54.5%) admitted to the ICU | Mortality |
| Narain et al., 2020 [44] | USA | 3098 patients (364 cases and 2734 controls) | Laboratory confirmed cases | 64.91, comparable with controls | 267/364, less females among cases than among controls | Tocilizumab + standard care (hydroxychloroquine, remdesivir, ritonavir/lopinavir, steroids) | NA | 52.66 h after admission (approximately 2 days after admission) | Hospitalized | Mortality |
| Omrani et al., 2020 [45] | Qatar | 1409 patients (111 cases and 1298 controls) | Laboratory confirmed cases | NA | NA | Tocilizumab + standard care (hydroxychloroquine, antibiotics, ribavirin, interferon, lopinavir/ritonavir) | NA | NA | Hospitalized | ICU admission |
| Pandolfi et al., 2020 [46] | Italy | 28 patients (8 cases and 20 controls) | Laboratory confirmed cases | NA | NA | Tocilizumab + standard care (hydroxychloroquine, remdesivir, ritonavir/lopinavir, steroids) | NA | NA | Admitted to the ICU | Mortality |
| Patel et al., 2020a [47] | USA | 129 patients (24 cases and 105 controls) | Laboratory confirmed cases | NA | NA | Tocilizumab + standard care (Azithromycin, hydroxychloroquine and steroids) | NA | NA | NA | Progression to invasive ventilation |
| Patel et al., 2020b [48] | USA | 104 patients (6 cases and 98 controls) | Laboratory confirmed cases | NA | NA | Tocilizumab + standard care (remdesivir, steroids, hydroxychloroquine antibiotics) | NA | NA | NA | Need of invasive ventilation |
| Pérez-Tanoira et al., 2020 [49] | Spain | 382 patients (36 cases and 346 controls) | Laboratory confirmed cases | NA | NA | Tocilizumab + standard care (darunavir/cobicistat, lopinavir/ritonavir, chloroquine/hydroxychloroquine, interferon β-1B, antibiotics) | NA | NA | NA | Mortality rate |
| Perrone et al., 2020 [50] | Italy | 1158 patients (708 cases, 180 from ITT phase 2 trial and 528 from ITT validation trial, and 450 controls) | Laboratory confirmed cases | NA, younger than controls (p = 0.04) | 82.8% and 79.5%, less females among cases than among controls | Tocilizumab + standard care: antivirals/antiretroviral, Lopinavir/Ritonavir, Remdesivir, (hydroxy)chloroquine, colchicine, immune suppressor, antibiotics, azythromicin, ceftriaxone, linezolid, steroids, heparin | 400–800 mg | 138 (76.7%) and 404 (76.5%) within 3 days from registration | Admitted to the ICU (NA) | Mortality rate |
| Potere et al., 2020 [51] | Italy | 80 patients (40 cases and 40 controls) | Laboratory-confirmed cases | 56.0 [range 50.3–73.2]; age-matched with controls | 26 (65.0%); gender-matched with control | Tocilizumab + standard care | 324 mg, given as two concomitant subcutaneous injection | NA | NA | Infection Mortality rate Need for mechanical ventilation and/or death |
| Quartuccio et al., 2020 [52] | Italy | 111 consecutive patients (42 cases versus 69 controls) | Laboratory-confirmed cases | 58.5 ± 13.6, older than controls | 77 (69.4%) | Tocilizumab + standard care (antiviral therapy, anticoagulants, hydroxychloroquine, antibiotics and glucocorticoids) | 8 mg/kg i.v. as a single infusion; 2 patients received 200 mg/day s.c. for 3 consecutive days | 8.4 ± 3.7 days after symptoms onset | 27 transferred to ICU (3 before receiving Tocilizumab and 24 after hospital admission); 26 intubated and 1 with non invasive ventilation | Mortality rate |
| Ramaswamy et al., 2020 [53] | USA | 86 patients (21 cases versus 65 controls) | Laboratory-confirmed cases | 63.2 ± 15.6, age-matched | 13 (61.9%), gender-matched | Tocilizumab + standard care: azithromycin, hydroxychloroquine, corticosteroids | 400–800 mg; seven receiving a single dose of 800 mg | 10 prior to mechanical ventilation, 11 after mechanical ventilation | Admitted to the ICU (n= 10, 47.6%), with 13 requiring mechanical ventilation (61.9%) | Mortality rate |
| Rodríguez Molinero et al., 2020 [54] | Spain | 418 consecutive patients (96 cases and 322 controls) | Laboratory-confirmed cases | NA, age-matched sub-analysis | NA, gender-matched sub-analysis | Tocilizumab + standard care (hydroxychloroquine, lopinavir/ritonavir, azithromycin, steroids such as metilpredinosolone) | An initial dose of 600 mg i.v., a second dose of 400–600 mg at 12 h, and a third optional dose of 400 mg | NA | Hospitalized patients | Mortality rate Time to discharge |
| Rojas-Marte et al., 2020 [56] | USA | 193 patients (96 cases versus 97 controls) | Laboratory-confirmed cases | 58.8 ± 13.6, age-matched | n = 74 (77.1%), gender-marched | Tocilizumab + standard care (hydroxychloroquine, azithromycin, steroids, anticoagulants, remdesivir, vitamin C, zinc, antibiotics for suspected bacterial infections) | NA | NA | Hospitalized patients, 61 (63.5%) requiring invasive ventilation | Mortality rate |
| Rossi et al., 2020 [56] | France | 246 patients (106 cases versus 140 controls) | Laboratory-confirmed cases | 64.3 ± 13, younger than controls | 66%, less females among cases than among controls | Tocilizumab + standard care (antibiotics, betalactamin, macrolides, antivirals, hydroxychloroquine, lopinavir/ritonavir, immunosuppressants and/or corticosteroids, Baricitinib) | A single dose of 8 mg/kg (400 mg) | Within 1 ± 1 day after hospitalization | Hospitalized patients | Mortality rate and all-cause mortality rate Need for mechanical ventilation |
| Roumier et al., 2020 [57] | France | 59 patients (30 cases versus 29 controls) with severe disease | Laboratory-confirmed cases (n = 29) | Mean 58.8 ± 12.4; median 50; younger than controls (p = 0.001) | n = 24 80% | Tocilizumab + standard care (hydroxychloroquine and azithromycin); 2 controls received steroids | 8 mg/kg i.v. | 14.1 ± 3.5 days after symptoms onset | Admitted to the ICU (n = 7, 23%), 10 under invasive ventilation (33.3%) | Need for mechanical ventilation Mortality rate ICU admission |
| Sisó-Almirall et al., 2020 [58] | Spain | 322 patients (27 cases and 295 controls) | Laboratory-confirmed cases | NA | NA | Tocilizumab + standard care (remdesivir, hydroxychloroquine, steroids, anti-coagulation, antibiotics) | NA | NA | Hospitalized, with some admitted to the ICU | Mortality and/or ICU admission |
| Somers et al., 2020 [59] | USA | 154 patients (78 cases and 76 controls) with severe disease, requiring mechanical ventilation | Laboratory-confirmed cases | 55 ± 14.9, younger than controls (p = 0.05) | n = 53, 68% | Tocilizumab + standard care (remdesivir, hydroxychloroquine, steroids, anti-coagulation) | Single dose of 8 mg/kg up to a maximum of 800 mg | 24 h prior to intubation, in 26% patients > 48 h after intubation | All on mechanical ventilation; 40 transferred on mechanical ventilation; all hospitalized, no one admitted to the ICU | Mortality rate |
| Wadud et al., 2020 [60] | USA | 94 patients (44 cases versus 50 controls) | Laboratory-confirmed cases | 55.5 | NA | Tocilizumab + standard care (hydroxychloroquine, azithromycin, steroids-hydrocortisone/methylprednisolone/dexamethasone)) | NA | NA | Admitted to the ICU (not specified how many subjects); all requiring mechanical ventilation | Mortality rate |
| Reference | Main Findings | Adjustment of the Outcome(s) | Effects of Tocilizumab on Clinical and Lab Parameters | Publication Status | Quality Appraisal |
|---|---|---|---|---|---|
| Allenbach et al., 2020 [22] | 2 (2.1%) ICU-free and/or alive versus 4 (8.7%) ICU-admitted and/or died (p = 0.087) among those receiving tocilizumab | No | NA | Pre-print | Selection = 2, comparability = 0, exposure = 2 |
| Ayerbe et al., 2020 [23] | 89/421 (21.14%) versus 197/1598 (12.33%) | No | NA | Peer-reviewed | Selection = 2, comparability = 0, exposure = 2 |
| Campochiaro et al., 2020 [24] |
5/32 deaths; mortality rate among cases (16%) and among controls (n = 11/33, 33%) was not different (p = 0.150) for the first outcome
4/32 (13%) versus 2/33 (6%) (p = 0.43) for the second outcome | No | Age predicted survival and PaO2/FiO2 ratio predicted clinical improvement | Peer-reviewed | Selection = 2, comparability = 2 (no difference with controls in terms of co-morbidities and respiratory parameters), exposure = 3 |
| Capra et al., 2020 [25] | Overall, 2/62 (3.22%) versus 11/23 (47.8%) with HR 0.035 ([95%CI 0.004–0.347], p = 0.004) Among those with a concluded outcome 2/25 (8%) versus 11/19 (57.9%) | Age, co-morbidities and PCR baseline levels | None (no changes in procalcitonin levels) | Peer-reviewed | Selection = 2, comparability = 2, exposure = 3 |
| Carvalho et al., 2020 [26] | 17.2% (5/29) versus 16.7% (4/24); adjusted OR 3.97 ([95%CI 0.28–57.2], p = 0.3) for the first outcome 11 (38%) versus 4 (17%); adjusted OR 1.73 ([95%CI 0.22–13.82], p = 0.6) for the second outcome | Yes (multivariate analysis) Yes (multivariate analysis) | None Mechanical ventilation (p = 0.006) | Pre-print | Selection = 1, comparability = 1 (use of steroids 83% versus 37%, p = 0.001; differences in terms of biochemical parameters), exposure = 3 |
| Colaneri et al., 2020 [27] | 3/21 versus 12/91, no significant effect, with OR 0.11 ([95%CI 0.00–3.38], p = 0.22) | Yes (nearest neighbor propensity score matching) | None | Peer-reviewed | Selection = 2, comparability = 2, exposure = 3 |
| 5/21 versus 19/91 with OR 0.78 ([95%CI 0.06–9.34], p = 0.84) | Yes | ||||
| Crotty et al., 2020 [28] | 20% versus 4.9%, (p = 0.013) | No | NA | Pre-print | Selection = 2, comparability = 0, exposure = 2 |
| de la Rica et al., 2020 [29] | 10/11 versus 11/47 | No | NA | Pre-print | Selection = 2, comparability = 0, exposure = 2 |
| Edwards and McGrail, 2020 [30] | 2/11 versus 6/24 | No | NA | Pre-print | Selection = 2, comparability = 0, exposure = 2 |
| Fernández-Cruz et al., 2020 [31] | 24/180 versus 47/283 | No | NA | Peer-reviewed | Selection = 2, comparability = 0, exposure = 2 |
| Garibaldi et al., 2020 [32] | 6/39 among cases versus 107/793 among controls | No | NA | Pre-print | Selection = 2, comparability = 0, exposure = 2 |
| Gokhale et al., 2020 [33] | 33 (47.1%) versus 61 (67%) (p = 0.011) | No | NA | Peer-reviewed | Selection = 2, comparability = 0, exposure = 3 |
| Guaraldi et al., 2020 [34] | 13 (7%) versus 73 (20%), p = 0.0007; unadjusted HR 0.60 ([95%CI 0.43–0.84], p = 0.0030) 33 (18%) among cases versus 57 (16%) among controls | Yes (adjusted; also, inverse probability weighting model is presented) | NA | Peer-reviewed | Selection = 2, comparability = 2, (not comparable in terms of co-morbidities, clinical, biochemical and respiratory parameter) exposure = 3 |
| Holt et al., 2020 [35] | 10/32 versus 9/30 | Yes (matched) | None | Pre-print | Selection = 2, comparability = 2, exposure = 3 |
| Ip et al., 2020 [36] | Adjusted HR 0.76 ([95%CI 0.57–1.00], p = 0.053), 46% versus 56% | Yes (propensity-score model) | None | Pre-print | Selection = 2, comparability = 2 (differences in terms of co-morbidities, respiratory parameters and use o antibiotics and hydroxychloroquine), exposure = 3 |
| Kewan et al., 2020 [37] | 3 (11) 2 (9) | No | NA | Peer-reviewed | Selection = 2, comparability = 0, exposure = 3 |
| 11 [6–22.25] versus 7 [5–13.5] | |||||
| 86% versus 70%, p = 0.19 | |||||
| 75% versus 48%, p = 0.046 | |||||
| Five (18%) versus five (22%) (p = 0.74) | |||||
| Kimmig et al., 2020 [38] | 12 (42.9%) versus 8 (25%) | No | None | Pre-print | Selection = 2, comparability = 1, exposure = 3 |
| 18/28 versus 10/32 | |||||
| Klopfenstein et al., 2020 [39] | 25% (cases; n = 5/20) versus 72% (controls; n = 18/25) overall (p = 0.002); ICU admission n = 0 versus n = 11 (p < 0.0001); deaths n = 5 versus n = 12 (p = 0.066), mechanical ventilation n = 0 versus n = 8 (p = 0.006), hospitalization n = 3 versus n = 2 (p = 0.642), discharge n = 11 versus n = 11 (p = 0.463). | No | NA | Peer-reviewed | Selection = 2, comparability = 1 (differences in terms of biochemical and respiratory parameters), exposure = 3 |
| Martínez-Sanz et al., 2020 [40] | 50 (19%) among cases versus 32 (3%) among controls (p < 0.001) | Yes, using inverse probability treatment weighting | High CRP values | Pre-print | Selection = 2, comparability = 2 (non comparable in terms of co-morbidities, respiratory and laboratory parameters), exposure = 3 |
| 61 (23%) among cases versus 120 (12%) among controls (p < 0.001); unadjusted HR 1.53, ([95%CI 1.20–1.96], p = 0.001), adjusted HR 0.34 ([95%CI 0.16–0.72], p = 0.005), stratifying according to CRP levels; composite index adjusted HR 0.39 ([95%CI 0.19–0.80, p = 0.011) stratifying according to CRP levels | |||||
| Mikulska et al., 2020 [41] | 9/85 versus 36/111 | No (adjustment is done but not for this specific outcome) | NA | Pre-print | Selection = 2, comparability = 0, exposure = 3 |
| Moreno-Garcia et al., 2020 [42] | ICU admission (10.3% versus 27.6%, p = 0.005) Need of invasive ventilation (0% versus 13.8%, p = 0.001); ICU admission and/or death composite outcome OR 0.03 ([95%CI 0.007–0.1], p = 0.0001) | Yes | Co-morbidities (hypertension, heart diseases and lymphoma), need of oxygen at day 1, CRP > 16 mg/dL and cardiovascular, renal or respiratory (ARDS, invasive ventilation) complications predicted ICU admission and/or death | Pre-prrint | Selection = 1, comparability = 1 (age-, gender-matched, differences in use f steroids) exposure = 2 |
| Moreno-Pérez et al., 2020 [43] | 10/77 versus 3/159 | No | None | Peer-reviewed | Selection = 2, comparability = 1 (differences in terms of clinical, biochemical and respiratory parameters), exposure = 3 |
| Narain et al., 2020 [44] | Adjusted HR 0.718 ([95%CI 0.403–1.280, p = 0.2615) for tocilizumab only, adjusted HR 0.459 ([95%CI 0.399–0.622), p < 0.0001) for tocilizumab plus steroids | Yes | None | Pre-print | Selection = 2, comparability = 2, exposure = 3 |
| Omrani et al., 2020 [45] | 99/111 versus 12/1298 | No | NA | Pre-print | Selection = 2, comparability = 0, exposure = 2 |
| Pandolfi et al., 2020 [46] | 4/8 versus 8/20 | No | NA | Pre-print | Selection = 2, comparability = 0, exposure = 2 |
| Patel et al., 2020a [47] | 14 (15.7%) versus 10 (25.0%) (p = 0.211) | No | NA | Pre-print | Selection = 2, comparability = 0, exposure = 2 |
| Patel et al., 2020b [48] | 5 (13.89%) versus 1 (1.52%) (p = 0.011) | No | NA | Pre-print | Selection = 2, comparability = 0, exposure = 2 |
| Pérez-Tanoira et al., 2020 [49] | 10/36 versus 95/346 | No | NA | Pre-print | Selection = 2, comparability = 0, exposure = 2 |
| Perrone et al., 2020 [50] | 67 and 158 overall deaths; 36/180 versus 31/119; 99/495 versus 59/331 | No | Older age and low PaO2/FiO2 ratio predicted mortality rate | Pre-print | Selection = 2, comparability = 0 (differences in terms of respiratory parameters), exposure = 2 |
| Potere et al., 2020 [51] | 1 (2.5%) among cases developed bacterial pneumonia versus 3 (7.5%) among controls | No | NA | Peer-reviewed | Selection = 2, comparability = 2, exposure = 3 |
| 2 (5%) versus 11 (27.5% (p = 0.006) | |||||
| IMV or death (2 (5%) vs 12 (30%), p = 0.003 | |||||
| Quartuccio et al., 2020 [52] | 9.5% among cases versus 0.0% among controls | No | Co-morbidities and superinfections | Peer-reviewed | Selection = 2, comparability = 0 (differences in terms of use of drugs, clinical and biochemical parameters)), exposure = 3 |
| Ramaswamy et al., 2020 [53] | 3/21 deaths versus 8/65 deaths, HR 0.25 [95%CI 0.07–0.90], RR 0.472 [95%CI 0.449–0.497] | Yes | Being treated with tocilizumab and age at admission predicted survival rate | Pre-print | Selection = 2, comparability = 2, exposure = 3 |
| Rodríguez Molinero et al., 2020 [54] | Adjusted OR 0.99 ([95%CI 0.30–3.27], p = 0.990) | Yes (brute-force matching algorithm refined by propensity score) | None | Pre-print | Selection = 2, comparability = 2, exposure = 3 |
| p = 0.472 | |||||
| Rojas-Marte et al., 2020 [56] | 43 (44.8%) deaths versus 55 (56.7%) (p = 0.09); excluding intubated patients, 2 (6.1%) versus 9 (26.5%) (p = 0.024) | No | NA | Pre-print | Selection = 2, comparability = 1, exposure = 3 |
| Rossi et al., 2020 [56] | Adjusted HR 0.34 ([95%CI 0.22–0.52], p < 0.0001); adjusted HR 0.29 ([95%CI 0.17–0.53], p < 0.0001)/ HR 0.42 ([95%CI 0.22–0.82], p = 0.008) | Yes | SpO2/FiO2 ratio and CKD predicted mortality rate | Pre-print | Selection = 2, comparability = 2 (differences in terms of use of antibiotics, respiratory parameters), exposure = 3 |
| HR 0.49 ([95%CI 0.30–0.81], p = 0.00) | |||||
| Roumier et al., 2020 [57] | OR 0.42 ([95%CI 0.20–0.89], p = 0.025) | Yes (age, gender, disease severity) | NA | Pre-print | Selection = 1, comparability = 2 (differences in terms of co-morbidities), exposure = 3 |
| 3 versus 9 deaths (p = 0.041), 4 discharged from the ICU and 6 from hospital; at the univariate analysis, OR 0.25 ([95%CI 0.05–0.95], p = 0.04); at the multivariate analysis, no statistical significance; considering those treated outside the ICU tocilizumab resulted protective | Yes (age, gender, disease severity) | ||||
| OR 0.17 ([95%CI 0.06–0.48], p = 0.001) | Yes (age, gender, disease severity) | None | |||
| Sisó-Almirall et al., 2020 [58] | Adjusted OR 3.17 ([95%CI 1.22–7.88], p = 0.013) | Yes (multivariate analysis) | None | Pre-print | Selection = 2, comparability = 2, exposure = 3 |
| Somers et al., 2020 [59] | 14 (18%) versus 27 (36%), p = 0.01; p = 0.0189 at the Kaplan–Meyer analysis; adjusted HR 0.55 [95%CI 0.33–0.90]; when stratifying into patients with super-infections, no difference in 28-day case fatality rate (22% versus 15%, p = 0.42) | Yes (propensity score-based inverse probability treatment weighting) | None | Peer-reviewed | Selection = 2, comparability = 2 (differences n terms of clinical, respiratory and biochemical parameters), exposure = 3 |
| Wadud et al., 2020 [60] | 61.36 % versus 48 % in the control group (17 deaths versus 26) | Yes (cases and controls matched in terms of age, sex, BMI and HS score- calculated using inflammatory markers- ferritin, triglycerides, AST and fibrinogen) | NA | Pre-print | Selection = 2, comparability = 2, exposure = 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahroum, N.; Watad, A.; Bridgewood, C.; Mansour, M.; Nasr, A.; Hussein, A.; Khamisy-Farah, R.; Farah, R.; Gendelman, O.; Lidar, M.; et al. Systematic Review and Meta-Analysis of Tocilizumab Therapy versus Standard of Care in over 15,000 COVID-19 Pneumonia Patients during the First Eight Months of the Pandemic. Int. J. Environ. Res. Public Health 2021, 18, 9149. https://doi.org/10.3390/ijerph18179149
Mahroum N, Watad A, Bridgewood C, Mansour M, Nasr A, Hussein A, Khamisy-Farah R, Farah R, Gendelman O, Lidar M, et al. Systematic Review and Meta-Analysis of Tocilizumab Therapy versus Standard of Care in over 15,000 COVID-19 Pneumonia Patients during the First Eight Months of the Pandemic. International Journal of Environmental Research and Public Health. 2021; 18(17):9149. https://doi.org/10.3390/ijerph18179149
Chicago/Turabian StyleMahroum, Naim, Abdulla Watad, Charlie Bridgewood, Muhammad Mansour, Ahmad Nasr, Amr Hussein, Rola Khamisy-Farah, Raymond Farah, Omer Gendelman, Merav Lidar, and et al. 2021. "Systematic Review and Meta-Analysis of Tocilizumab Therapy versus Standard of Care in over 15,000 COVID-19 Pneumonia Patients during the First Eight Months of the Pandemic" International Journal of Environmental Research and Public Health 18, no. 17: 9149. https://doi.org/10.3390/ijerph18179149
APA StyleMahroum, N., Watad, A., Bridgewood, C., Mansour, M., Nasr, A., Hussein, A., Khamisy-Farah, R., Farah, R., Gendelman, O., Lidar, M., Shoenfeld, Y., Amital, H., Kong, J. D., Wu, J., Bragazzi, N. L., & McGonagle, D. (2021). Systematic Review and Meta-Analysis of Tocilizumab Therapy versus Standard of Care in over 15,000 COVID-19 Pneumonia Patients during the First Eight Months of the Pandemic. International Journal of Environmental Research and Public Health, 18(17), 9149. https://doi.org/10.3390/ijerph18179149











