Identification of the Prodromal Symptoms and Pre-Ataxic Stage in Cerebellar Disorders: The Next Challenge
Abstract
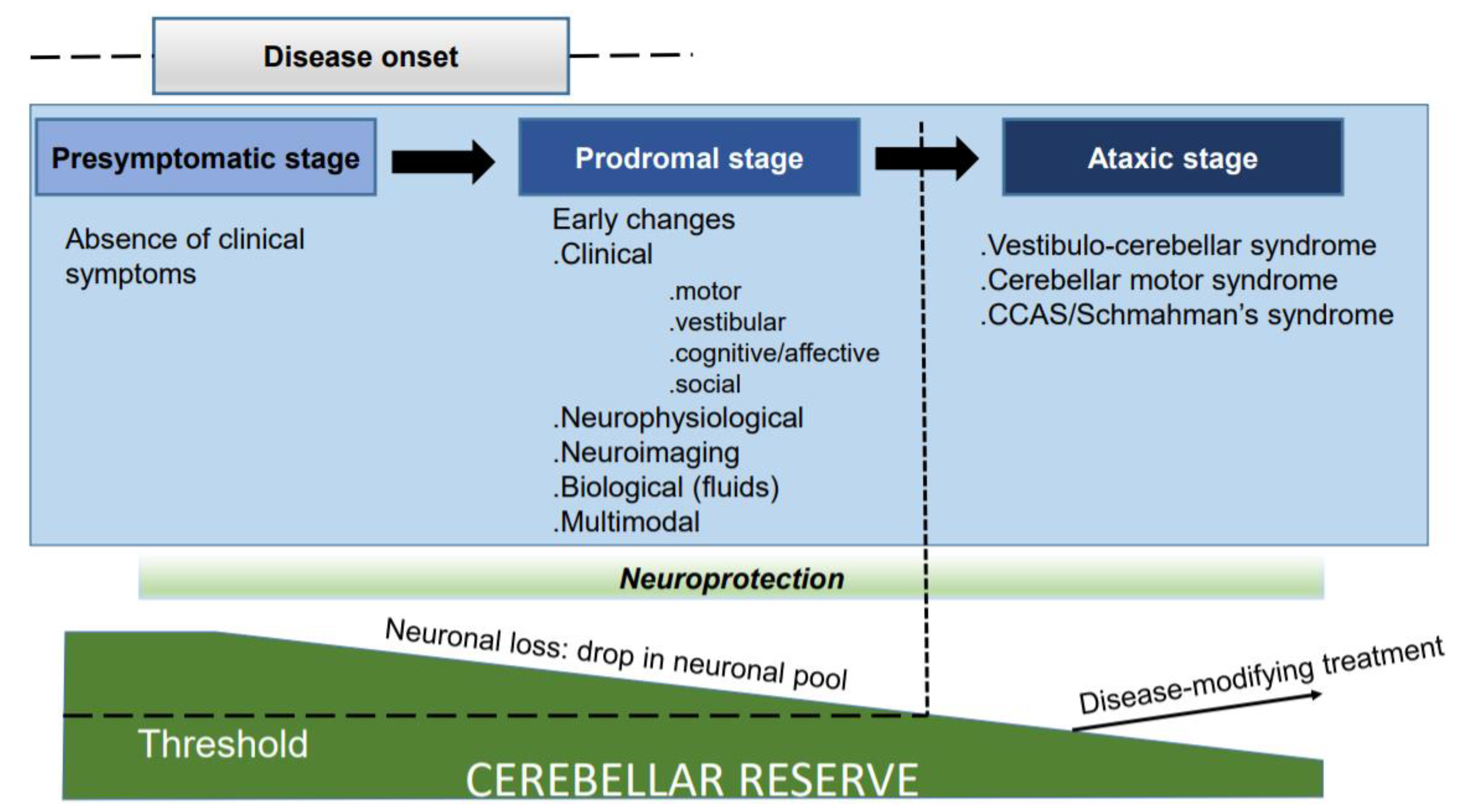
:1. Turning Cerebellar Ataxias from Incurable to Treatable Diseases
2. Unravelling Early Predictors: Opening the Box of Novel Biomarkers to Complement Neuroimaging Tools
3. Preserving and Potentiating the Cerebellar Reserve
4. Neuromodulation to Restore Cerebellar Function
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Manto, M.; Gruol, D.; Schmahmann, J.D.; Koibuchi, N.; Sillitoe, R. Handbook of the Cerebellum and Cerebellar Disorders, 2nd ed.; Springer: New York, NY, USA, 2013. [Google Scholar]
- Klockgether, T.; Mariotti, C.; Paulson, H.L. Spinocerebellar ataxia. Nat. Rev. Dis. Primers 2019, 5, 24. [Google Scholar] [CrossRef]
- Paulson, H.L.; Shakkottai, V.G.; Clark, H.B.; Orr, H.T. Polyglutamine spinocerebellar ataxias—From genes to potential treatments. Nat. Rev. Neurosci. 2017, 18, 613–626. [Google Scholar] [CrossRef]
- McLoughlin, H.S.; Moore, L.R.; Chopra, R.; Komlo, R.; McKenzie, M.; Blumenstein, K.G.; Zhao, H.; Kordasiewicz, H.B.; Shakkottai, V.G.; Paulson, H.L. Oligonucleotide therapy mitigates disease in spinocerebellar ataxia type 3 mice. Ann. Neurol. 2018, 84, 64–77. [Google Scholar] [CrossRef]
- Wilke, C.; Haas, E.; Reetz, K.; Faber, J.; Garcia-Moreno, H.; Santana, M.M.; van de Warrenburg, B.; Hengel, H.; Lima, M.; Filla, A.; et al. Neurofilaments in spinocerebellar ataxia type 3: Blood biomarkers at the preataxic and ataxic stage in humans and mice. EMBO Mol. Med. 2020, 12, e11803. [Google Scholar] [CrossRef]
- Bushart, D.D.; Zalon, A.J.; Zhang, H.; Morrison, L.M.; Guan, Y.; Paulson, H.L.; Shakkottai, V.G.; McLoughlin, H.S. Antisense Oligonucleotide Therapy Targeted Against ATXN3 Improves Potassium Channel-Mediated Purkinje Neuron Dysfunction in Spinocerebellar Ataxia Type 3. Cerebellum 2021, 20, 41–53. [Google Scholar] [CrossRef]
- Moore, L.R.; Keller, L.; Bushart, D.D.; Delatorre, R.G.; Li, D.; McLoughlin, H.S.; do Carmo Costa, M.; Shakkottai, V.G.; Smith, G.D.; Paulson, H.L. Antisense oligonucleotide therapy rescues aggresome formation in a novel spinocerebellar ataxia type 3 human embryonic stem cell line. Stem Cell Res. 2019, 39, 101504. [Google Scholar] [CrossRef]
- O’Keefe, J.A.; Bang, D.; Robertson, E.E.; Biskis, A.; Ouyang, B.; Liu, Y.; Pal, G.; Berry-Kravis, E.; Hall, D.A. Prodromal Markers of Upper Limb Deficits in FMR1 Premutation Carriers and Quantitative Outcome Measures for Future Clinical Trials in Fragile X-associated Tremor/Ataxia Syndrome. Mov. Disord. Clin. Pract. 2020, 7, 810–819. [Google Scholar] [CrossRef]
- Velázquez-Pérez, L.C.; Rodríguez-Labrada, R.; Fernandez-Ruiz, J. Spinocerebellar Ataxia Type 2: Clinicogenetic Aspects, Mechanistic Insights, and Management Approaches. Front. Neurol. 2017, 8, 472. [Google Scholar] [CrossRef] [Green Version]
- Velázquez-Pérez, L.; Rodriguez-Labrada, R.; González-Garcés, Y.; Arrufat-Pie, E.; Torres-Vega, R.; Medrano-Montero, J.; Ramirez-Bautista, B.; Vazquez-Mojena, Y.; Auburger, G.; Horak, F.; et al. Prodromal Spinocerebellar Ataxia Type 2 Subjects Have Quantifiable Gait and Postural Sway Deficits. Mov. Disord. 2021, 36, 471–480. [Google Scholar] [CrossRef]
- Smith, M.A.; Brandt, J.; Shadmehr, R. Motor disorder in Huntington’s disease begins as a dysfunction in error feedback control. Nature 2000, 403, 544–549. [Google Scholar] [CrossRef]
- Lemos, J.; Novo, A.; Duque, C.; Cunha, I.; Ribeiro, J.; Castelhano, J.; Januário, C. Static and dynamic ocular motor abnormalities as potential biomakers in spinocerebellar ataxia type 3. Cerebellum 2021, 20, 402–409. [Google Scholar] [CrossRef]
- Prudencio, M.; Garcia-Moreno, H.; Jansen-West, K.R.; Al-Shaikh, R.H.; Gendron, T.F.; Heckman, M.G.; Spiegel, M.R.; Carlomagno, Y.; Daughrity, L.M.; Song, Y.; et al. Toward allele-specific targeting therapy and pharmacodynamic marker for spinocerebellar ataxia type3. Sci. Transl. Med. 2020, 12, eabb708612. [Google Scholar] [CrossRef]
- Li, Q.F.; Dong, Y.; Yang, L.; Xie, J.J.; Ma, Y.; Du, Y.C.; Cheng, H.L.; Ni, W.; Wu, Z.Y. Neurofilament light chain is a promising serum biomarker in spinocerebellar ataxia type 3. Mol. Neurodegener. 2019, 1, 39. [Google Scholar] [CrossRef]
- Khalil, M.; Teunissen, C.E.; Otto, M.; Piehl, F.; Sormani, M.P.; Gattringer, T.; Barro, C.; Kappos, L.; Comabella, M.; Fazekas, F.; et al. Neurofilaments as biomarkers in neurological disorders. Nat. Rev. Neurol. 2018, 14, 577–589. [Google Scholar] [CrossRef]
- Peng, Y.; Zhang, Y.; Chen, Z.; Peng, H.; Wan, N.; Zhang, J.; Tang, J.; Wang, P.; Xie, Y.; Cai, Q.; et al. Association of serum neurofilament light and disease severity in patients with spinocerebellar ataxia type 3. Neurology 2020, 95, e2977–e2987. [Google Scholar] [CrossRef]
- Donath, H.; Woelke, S.; Schubert, R.; Kieslich, M.; Theis, M.; Auburger, G.; Duecker, R.P.; Zielen, S. Neurofilament light chain is a biomarker of neurodegeneration in ataxia telangiectasia. Cerebellum 2021. [Google Scholar] [CrossRef]
- de Assis, A.M.; Saute, J.A.M.; Longoni, A.; Haas, C.B.; Torrez, V.R.; Brochier, A.W.; Souza, G.N.; Furtado, G.V.; Gheno, T.C.; Russo, A.; et al. Peripheral oxidative stress biomarkers in spinocerebellar ataxia type 3/Machado-Joseph disease. Front. Neurol. 2017, 8, 485. [Google Scholar] [CrossRef] [Green Version]
- Hou, X.; Gong, X.; Zhang, L.; Li, T.; Yuan, H.; Xie, Y.; Peng, Y.; Qiu, R.; Xia, K.; Tang, B.; et al. Identification of a potential exosomal biomarker in spinocerebellar ataxia type 3/Machado-Joseph disease. Epigenomics 2019, 11, 1037–1056. [Google Scholar] [CrossRef] [Green Version]
- Brouillette, A.M.; Öz, G.; Gomez, C.M. Cerebrospinal fluid biomarkers in spinocerebellar ataxia: A pilot study. Did. Markers 2015, 2015, 413098. [Google Scholar] [CrossRef] [Green Version]
- Schulz, J.B.; Borkert, J.; Wolf, S.; Schmitz-Hübsch, T.; Rakowicz, M.; Mariotti, C.; Schöls, L.; Timmann, D.; van de Warrenburg, B.; Dürr, A.; et al. Visualization, quantification and correlation of brain atrophy with clinical symptoms in spinocerebellar ataxia types 1,3 and 6. Neuroimage 2010, 49, 158–168. [Google Scholar] [CrossRef]
- Reetz, K.; Costa, A.S.; Mirzazade, S.; Lehmann, A.; Juzek, A.; Rakowicz, M.; Boguslawska, R.; Schöls, L.; Linnemann, C.; Mariotti, C.; et al. Genotype-specific patterns of atrophy progression are more sensitive than clinical declines in SCA1, SCA3, and SCA6. Brain 2013, 136, 905–917. [Google Scholar] [CrossRef]
- D’Abreu, A.; França, M.C., Jr.; Yasuda, C.L.; Campos, B.A.; Lopes-Cendes, I.; Cendes, F. Neocortical atrophy in Machado-Joseph disease: A longitudinal neuroimaging study. J. Neuroimaging 2012, 22, 285–291. [Google Scholar] [CrossRef] [Green Version]
- Meira, A.T.; Arruda, W.O.; Ono, S.E.; Neto, A.C.; Raskin, S.; Camargo, C.H.F.; Teive, H.A.G. Neuroradiological findings in spinocerebellar ataxias. Tremor Other Hyperkinet. Mov. 2019, 9. [Google Scholar] [CrossRef]
- Olivito, G.; Siciliano, L.; Clausi, S.; Lupo, M.; Romano, S.; Masciullo, M.; Molinari, M.; Cercignani, M.; Bozzali, M.; Leggio, M. Functional changes of mentalizing network in SCA2 patients: Novel insights into understanding of the social cerebellum. Cerebellum 2020, 19, 235–242. [Google Scholar] [CrossRef]
- Adanyeguh, I.M.; Perlbarg, V.; Henry, P.G.; Rinaldi, D.; Petit, E.; Valabregue, R.; Brice, A.; Durr, A.; Mochel, F. Autosomal dominant cerebellar ataxias; Imaging biomarkers with high effect sizes. Neuroimage Clin. 2018, 19, 858–867. [Google Scholar] [CrossRef]
- Doss, S.; Brandt, A.U.; Oberwahrenbrock, T.; Endres, M.; Paul, F.; Rinnenthal, J.L. Metabolic evidence for neurodegeneration in spinocerebellar ataxia type 1. Cerebellum 2014, 13, 199–206. [Google Scholar] [CrossRef]
- Etchebehere, E.C.; Cendes, F.; Lopes-Cendes, I.; Pereira, J.A.; Lima, M.C.; Sansana, C.R.; Silva, C.A.; Camargo, M.F.; Santos, A.O.; Ramos, C.D.; et al. Brain single-photon emission computed tomography and magnetic resonance imaging in Machado-Joseph disease. Arch. Neurol. 2001, 58, 1257–1263. [Google Scholar] [CrossRef]
- Braga-Neto, P.; Pedroso, J.; Gadelha, A.; Laureano, M.R.; de Souza Noto, C.; Garrido, G.J.; Barsottini, O.G. Psychosis in Machado-Joseph disease: Clinical correlates, pathophysiological discussion, and functional brain imaging. Expanding the cerebellar cognitive affective syndrome. Cerebellum 2016, 15, 483–490. [Google Scholar] [CrossRef]
- Mitoma, H.; Buffo, A.; Gelfo, F.; Guell, X.; Fucà, E.; Kakei, S.; Lee, J.; Manto, M.; Petrosini, L.; Shaikh, A.G.; et al. Consensus Paper. Cerebellar reserve: From cerebellar physiology to cerebellar disorders. Cerebellum 2020, 19, 131–153. [Google Scholar] [CrossRef] [Green Version]
- Mitoma, H.; Kakei, S.; Yamaguchi, K.; Manto, M. Physiology of cerebellar reserve: Redundancy and plasticity of a modular machine. Int. J. Mol. Sci. 2021, 22, 4777. [Google Scholar] [CrossRef]
- Mitoma, H.; Manto, M.; Hampe, C.S. Time is cerebellum. Cerebellum 2018, 17, 387–391. [Google Scholar] [CrossRef] [Green Version]
- Mitoma, H.; Manto, M.; Hampe, C.S. Immune-mediated cerebellar ataxias: From bench to bedside. Cerebellum Ataxias 2017, 4, 16. [Google Scholar] [CrossRef] [Green Version]
- Stern, Y. Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol. 2012, 11, 1006–1012. [Google Scholar] [CrossRef] [Green Version]
- Stern, Y. An approach to studying the neural correlates of reserve. Brain Imaging Behav. 2017, 11, 410–416. [Google Scholar] [CrossRef]
- Manto, M.; Kakei, S.; Mitoma, H. The critical need to develop tools assessing cerebellar reserve for the delivery and assessment of non-invasive cerebellar stimulation. Cerebellum Ataxias 2021, 8, 2. [Google Scholar] [CrossRef]
- Khan, N.C.; Pandey, V.; Gajos, K.Z.; Gupta, A.S. Free-living motor activity monitoring in ataxia-telangiectasia. Cerebellum 2021. [Google Scholar] [CrossRef]
- Bolzan, G.; Leotti, V.B.; de Oliveira, C.M.; Ecco, G.; Cappelli, A.H.; Rocha, A.G.; Kersting, N.; Rieck, M.; de Sena, L.S.; Martins, A.C.; et al. Quality of life since pre-ataxic phases of spinocerebellar ataxia type 3/Machado-Joseph disease. Cerebellum 2021. [Google Scholar] [CrossRef]
- Almaguer-Mederos, L.E.; Pérez-Ávila, I.; Aguilera-Rodríguez, R.; Velázquez-Garcés, M.; Almaguer-Gotay, D.; Hechavarría-Pupo, R.; Rodríguez-Estupiñán, A.; Auburger, G. Body mass index is significantly associated with disease severity in spinocerebellar ataxia with type 2 patients. Mov. Disord. 2021, 36, 1372–1380. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manto, M.; Shaikh, A.G.; Mitoma, H. Identification of the Prodromal Symptoms and Pre-Ataxic Stage in Cerebellar Disorders: The Next Challenge. Int. J. Environ. Res. Public Health 2021, 18, 10057. https://doi.org/10.3390/ijerph181910057
Manto M, Shaikh AG, Mitoma H. Identification of the Prodromal Symptoms and Pre-Ataxic Stage in Cerebellar Disorders: The Next Challenge. International Journal of Environmental Research and Public Health. 2021; 18(19):10057. https://doi.org/10.3390/ijerph181910057
Chicago/Turabian StyleManto, Mario, Aasef G. Shaikh, and Hiroshi Mitoma. 2021. "Identification of the Prodromal Symptoms and Pre-Ataxic Stage in Cerebellar Disorders: The Next Challenge" International Journal of Environmental Research and Public Health 18, no. 19: 10057. https://doi.org/10.3390/ijerph181910057
APA StyleManto, M., Shaikh, A. G., & Mitoma, H. (2021). Identification of the Prodromal Symptoms and Pre-Ataxic Stage in Cerebellar Disorders: The Next Challenge. International Journal of Environmental Research and Public Health, 18(19), 10057. https://doi.org/10.3390/ijerph181910057







