Feasibility of Using Bioelectrical Impedance Analysis for Assessing Youth Weight and Health Status: Preliminary Findings
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. General Characteristics
3.2. Baseline Comparisons between BIA and Criterion Measures
3.3. Fasting vs. Non-Fasting State
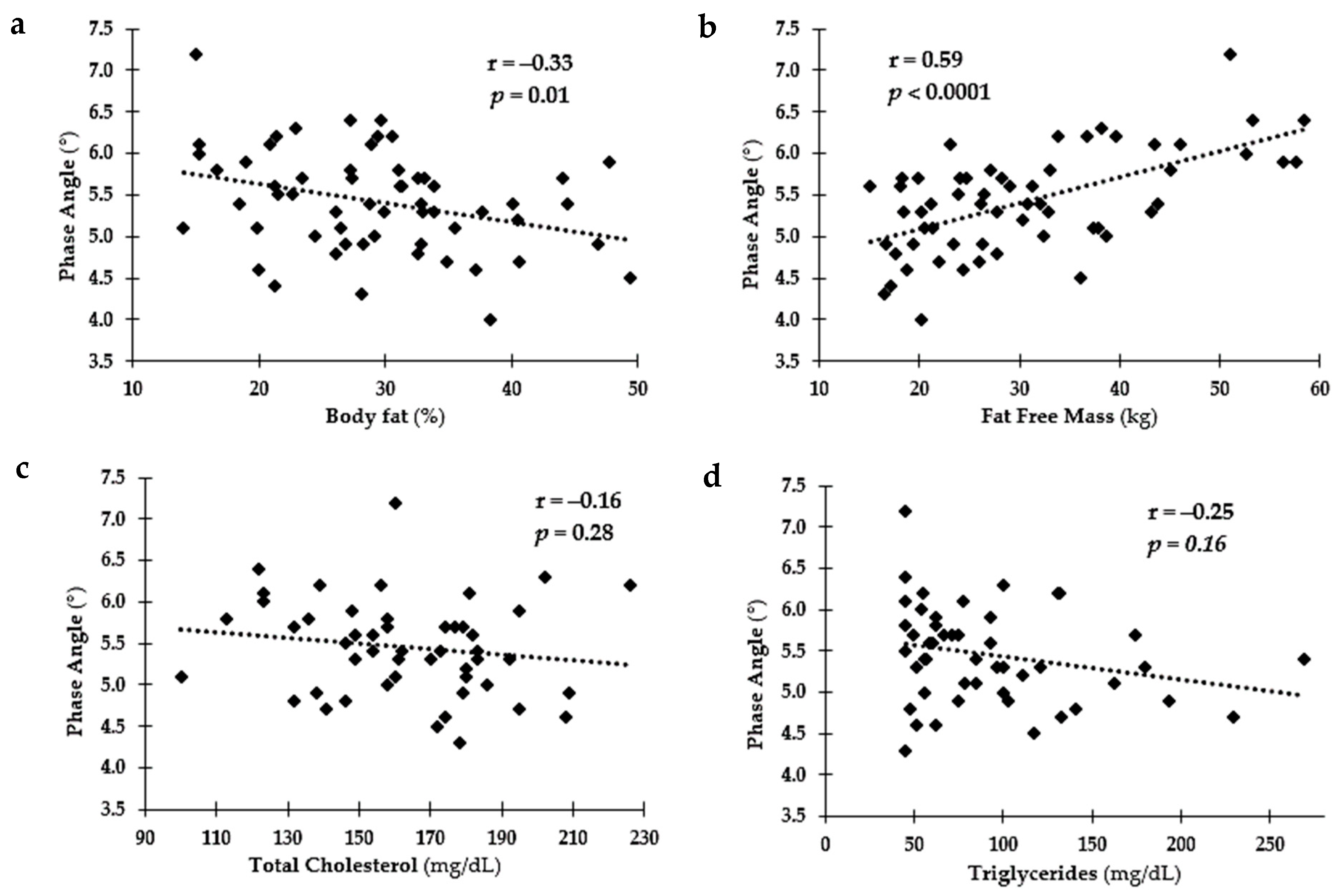
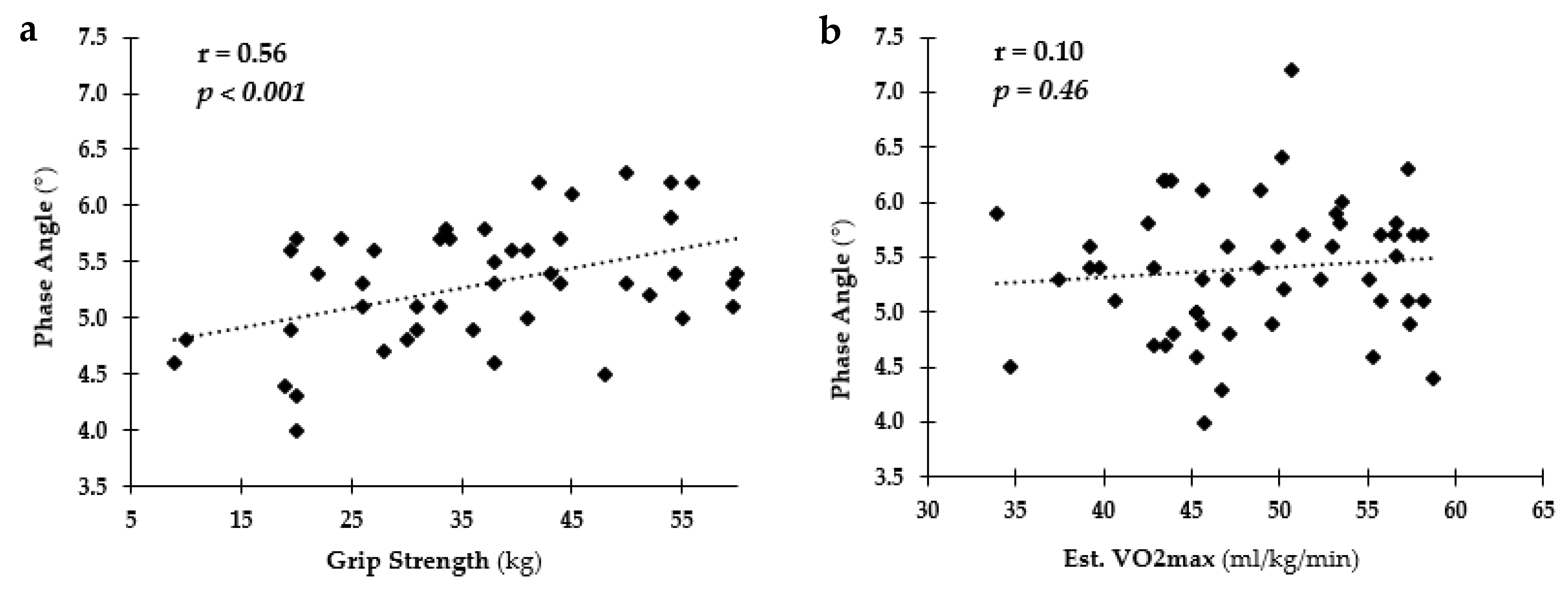
3.4. Relationship among PA and Health and Fitness Measures Overall
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anderson, P.M.; Butcher, K.F.; Schanzenbach, D.W. Understanding recent trends in childhood obesity in the united states. Econ. Hum. Biol. 2019, 34, 16–25. [Google Scholar] [CrossRef]
- Hales, C.M.; Carroll, M.D.; Fryar, C.D.; Ogden, C.L. Prevalence of obesity among adults and youth: United states, 2015–2016. NCHS Data Brief 2017, 1–8. Available online: https://www.ncbi.nlm.nih.gov/pubmed/29155689 (accessed on 25 June 2021).
- Tyson, N.; Frank, M. Childhood and adolescent obesity definitions as related to bmi, evaluation and management options. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 48, 158–164. [Google Scholar] [CrossRef]
- Ogden, C.L.; Carroll, M.D.; Lawman, H.G.; Fryar, C.D.; Kruszon-Moran, D.; Kit, B.K.; Flegal, K.M. Trends in obesity prevalence among children and adolescents in the united states, 1988–1994 through 2013–2014. JAMA 2016, 315, 2292–2299. [Google Scholar] [CrossRef]
- Wells, J.C. Commentary: The paradox of body mass index in obesity assessment: Not a good index of adiposity, but not a bad index of cardio-metabolic risk. Int. J. Epidemiol. 2014, 43, 672–674. [Google Scholar] [CrossRef] [Green Version]
- Jensen, N.S.; Camargo, T.F.; Bergamaschi, D.P. Comparison of methods to measure body fat in 7-to-10-year-old children: A systematic review. Public Health 2016, 133, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Binkovitz, L.A.; Henwood, M.J.; Sparke, P. Pediatric dual-energy x-ray absorptiometry: Technique, interpretation, and clinical applications. Semin. Nucl. Med. 2007, 37, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.T.; Chen, Y.Y.; Wang, C.W.; Chuang, C.L.; Chiang, L.M.; Lai, C.L.; Lu, H.K.; Dwyer, G.B.; Chao, S.P.; Shih, M.K.; et al. Comparison of standing posture bioelectrical impedance analysis with dxa for body composition in a large, healthy chinese population. PLoS ONE 2016, 11, e0160105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalil, S.F.; Mohktar, M.S.; Ibrahim, F. The theory and fundamentals of bioimpedance analysis in clinical status monitoring and diagnosis of diseases. Sensors 2014, 14, 10895–10928. [Google Scholar] [CrossRef] [PubMed]
- Ochoa-Aviles, A.; Rivas-Marino, G.; Verstraeten, R. Tackling Overweight and Obesity in Ecuador: Policies and Strategies for Prevention; Nutrition Exchange: Cuenca, Ecuador, 2017; p. 12. [Google Scholar]
- Dietz, W.H.; Robinson, T.N. Assessment and treatment of childhood obesity. Pediatrics Rev. 1993, 14, 337–343. Available online: http://www.ncbi.nlm.nih.gov/pubmed/8247968 (accessed on 20 January 2012). [CrossRef] [PubMed]
- Saladino, C.F. The efficacy of bioelectrical impedance analysis (bia) in monitoring body composition changes during treatment of restrictive eating disorder patients. J. Eat. Disord. 2014, 2, 34. [Google Scholar] [CrossRef] [PubMed]
- Saunders, M.J.; Blevins, J.E.; Broeder, C.E. Effects of hydration changes on bioelectrical impedance in endurance trained individuals. Med. Sci. Sports Exerc. 1998, 30, 885–892. [Google Scholar] [CrossRef]
- Brodie, D.A.; Eston, R.G.; Coxon, A.Y.; Kreitzman, S.N.; Stockdale, H.R.; Howard, A.N. Effect of changes of water and electrolytes on the validity of conventional methods of measuring fat-free mass. Ann. Nutr. Metab. 1991, 35, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Barreira, T.V.; Tseh, W. The effects of acute water ingestion on body composition analyses via dual-energy x-ray absorptiometry. Clin. Nutr. 2020, 39, 3836–3838. [Google Scholar] [CrossRef] [PubMed]
- Di Vincenzo, O.; Marra, M.; Scalfi, L. Bioelectrical impedance phase angle in sport: A systematic review. J. Int. Soc. Sports Nutr. 2019, 16, 49. [Google Scholar] [CrossRef] [Green Version]
- Amini, M.; Hisdal, J.; Kalvoy, H. Applications of bioimpedance measurement techniques in tissue engineering. J. Electr. Bioimpedance 2018, 9, 142–158. [Google Scholar] [CrossRef] [Green Version]
- Chula de Castro, J.A.; Lima, T.R.; Silva, D.A.S. Body composition estimation in children and adolescents by bioelectrical impedance analysis: A systematic review. J. Bodyw. Mov. Ther. 2018, 22, 134–146. [Google Scholar] [CrossRef]
- Farias, C.L.; Campos, D.J.; Bonfin, C.M.; Vilela, R.M. Phase angle from bia as a prognostic and nutritional status tool for children and adolescents undergoing hematopoietic stem cell transplantation. Clin. Nutr. 2013, 32, 420–425. [Google Scholar] [CrossRef]
- Marino, L.V.; Meyer, R.; Johnson, M.; Newell, C.; Johnstone, C.; Magee, A.; Sykes, K.; Wootton, S.A.; Pappachan, J.V. Bioimpedance spectroscopy measurements of phase angle and height for age are predictive of outcome in children following surgery for congenital heart disease. Clin. Nutr. 2018, 37, 1430–1436. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.H.; Wong, S.W.; Martinez, J. Programmed necrosis and disease:We interrupt your regular programming to bring you necroinflammation. Cell Death Differ. 2019, 26, 25–40. [Google Scholar] [CrossRef] [Green Version]
- Mirwald, R.L.; Baxter-Jones, A.D.; Bailey, D.A.; Beunen, G.P. An assessment of maturity from anthropometric measurements. Med. Sci. Sports Exerc. 2002, 34, 689–694. Available online: https://www.ncbi.nlm.nih.gov/pubmed/11932580 (accessed on 7 February 2019).
- Zubac, D.; Sekulic, D.; Karnincic, H. Reagent strips are reliable and valid measure in defining status of (de)hydration among male junior boxers. Res. Sport Exerc. Health 2014, 3, 25–28. [Google Scholar]
- Ersoy, N.; Ersoy, G.; Kutlu, M. Assessment of hydration status of elite young male soccer players with different methods and new approach method of substitute urine strip. J. Int. Soc. Sports Nutr. 2016, 13, 34. [Google Scholar] [CrossRef] [Green Version]
- McDoniel, S.O. A systematic review on use of a handheld indirect calorimeter to assess energy needs in adults and children. Int. J. Sport Nutr. Exerc. Metab. 2007, 17, 491–500. [Google Scholar] [CrossRef]
- Fields, D.A.; Kearney, J.T.; Copeland, K.C. Medgem hand-held indirect calorimeter is valid for resting energy expenditure measurement in healthy children. Obesity 2006, 14, 1755–1761. Available online: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17062805 (accessed on 5 February 2020). [CrossRef] [Green Version]
- Pluddemann, A.; Thompson, M.; Price, C.P.; Wolstenholme, J.; Heneghan, C. Point-of-care testing for the analysis of lipid panels: Primary care diagnostic technology update. Br. J. Gen. Pract. 2012, 62, e224–e226. [Google Scholar] [CrossRef] [Green Version]
- Parikh, P.; Mochari, H.; Mosca, L. Clinical utility of a fingerstick technology to identify individuals with abnormal blood lipids and high-sensitivity c-reactive protein levels. Am. J. Health Promot. 2009, 23, 279–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, S.H.; Yang, H.I.; Kim, D.I.; Gonzales, T.I.; Brage, S.; Jeon, J.Y. Validation of submaximal step tests and the 6-min walk test for predicting maximal oxygen consumption in young and healthy participants. Int. J. Environ. Res. Public Health 2019, 16, 4858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jankowski, M.; Niedzielska, A.; Brzezinski, M.; Drabik, J. Cardiorespiratory fitness in children: A simple screening test for population studies. Pediatr. Cardiol. 2015, 36, 27–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, J.S.; Hwang, J.S.; Lee, J.A.; Kim, D.H.; Park, K.D.; Jeong, J.S.; Cheon, G.J. Cross-calibration of multi-frequency bioelectrical impedance analysis with eight-point tactile electrodes and dual-energy x-ray absorptiometry for assessment of body composition in healthy children aged 6-18 years. Pediatr. Int. 2009, 51, 263–268. [Google Scholar] [CrossRef]
- Tompuri, T.T.; Lakka, T.A.; Hakulinen, M.; Lindi, V.; Laaksonen, D.E.; Kilpelainen, T.O.; Jaaskelainen, J.; Lakka, H.M.; Laitinen, T. Assessment of body composition by dual-energy x-ray absorptiometry, bioimpedance analysis and anthropometrics in children: The physical activity and nutrition in children study. Clin. Physiol. Funct. Imaging 2015, 35, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Kabiri, L.S.; Hernandez, D.C.; Mitchell, K. Reliability, validity, and diagnostic value of a pediatric bioelectrical impedance analysis scale. Child. Obes. 2015, 11, 650–655. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.C.; Gortmaker, S.L.; Sobol, A.M.; Kuntz, K.M. Estimating the energy gap among us children: A counterfactual approach. Pediatrics 2006, 118, e1721–e1733. Available online: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17142497 (accessed on 13 November 2009). [CrossRef] [PubMed]
- Goran, M.I.; Treuth, M.S. Energy expenditure, physical activity, and obesity in children. Pediatr. Clin. North Am. 2001, 48, 931–953. Available online: https://doi-org.proxy.library.ohio.edu/10.1016/S0031-3955(05)70349-7 (accessed on 4 September 2021). [CrossRef]
- Tinsley, G.M.; Morales, E.; Forsse, J.S.; Grandjean, P.W. Impact of acute dietary manipulations on dxa and bia body composition estimates. Med. Sci. Sports Exerc. 2017, 49, 823–832. [Google Scholar] [CrossRef]
- Selberg, O.; Selberg, D. Norms and correlates of bioimpedance phase angle in healthy human subjects, hospitalized patients, and patients with liver cirrhosis. Eur. J. Appl. Physiol. 2002, 86, 509–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baumgartner, R.N.; Chumlea, W.C.; Roche, A.F. Bioelectric impedance phase angle and body composition. Am. J. Clin. Nutr. 1988, 48, 16–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Sex | Weight Status | Age Group | |||||
|---|---|---|---|---|---|---|---|
| Sample Size | Girls | Boys | HW | OW | OB | Child | Teen |
| 29 | 29 | 37 | 10 | 11 | 39 | 19 | |
| Anthropometrics | |||||||
| Age (years) | 11.1 ± 2.6 | 11.7 ± 3.2 | 11.8 ± 3.0 | 11.1 ± 2.9 | 10.4 ± 2.7 | 9.7 ± 1.7 * | 14.8 ± 1.5 * |
| Weight (kg) | 46.3 ± 18.4 | 48.0 ± 18.2 | 43.1 ± 14.2 * | 48.2 ± 16.2 | 59.8 ± 26.3 * | 41.3 ± 17.7 * | 59.1 ± 12.8 * |
| Height (cm) | 147.3 ± 14.3 | 150.1 ± 19.6 | 150.0 ± 17.7 | 147.0 ± 17.1 | 145.7 ± 15.9 | 140.3 ± 12.6 * | 165.9 ± 11.0 * |
| BMI (%ile) | 65.9 ± 27.9 | 67.0 ± 32.2 | 51.4 ± 27.5 * | 88.2 ± 2.1 * | 97.3 ± 1.4 * | 72.0 ± 28.2 * | 55.1 ± 30.8 * |
| RMR (Kcals/day) | 1042 ± 210 * | 1202 ± 308 * | 1068 ± 257 | 1209 ± 251 | 1231 ± 312 | 1043 ± 232 * | 1267 ± 290 * |
| Blood Analysis | |||||||
| TC (mg/dL) | 165.2 ± 5.0 | 161.1 ± 5.9 | 158.6 ± 4.7 | 169.6 ± 9.9 | 174.5 ± 6.6 | 168.9 ± 22.7 * | 151.5 ± 30.6 * |
| HDL (mg/dL) | 52.0 ± 2.7 | 53.1 ± 3.6 | 54.7 ± 3.0 | 51.0 ± 4.8 | 46.1 ± 2.7 | 55.6 ± 16.0 | 46.5 ± 12.4 |
| TRG (mg/dL) | 97.0 ± 10.2 | 85.0 ± 10.6 | 83.9 ± 8.0 | 98.8 ± 19.2 | 114.1 ± 22.8 | 100.2 ± 57.0 | 73.9 ± 30.3 |
| LDL (mg/dL) | 94.1 ± 5.5 | 90.7 ± 4.7 | 87.3 ± 4.8 | 98.7 ± 6.9 | 105.0 ± 6.5 | 93.2 ± 25.2 | 90.3 ± 26.5 |
| Glucose (mg/dL) | 83.2 ± 1.7 * | 91.0 ± 1.3 * | 85.8 ± 1.5 | 90.0 ± 3.0 | 86.9 ± 3.1 | 87.7 ± 7.6 | 84.9 ± 10.2 |
| Resting Hemodynamics | |||||||
| Systolic BP (mmHg) | 99.7 ± 1.6 * | 106.7 ± 2.0 * | 103.5 ± 1.7 | 102.6 ± 1.7 | 101.4 ± 4.2 | 100.9 ± 9.4 * | 107.3 ± 8.8 * |
| Diastolic BP (mmHg) | 58.1 ± 1.7 | 59.4 ± 1.9 | 57.6 ± 1.3 | 57.9 ± 2.2 | 62.8 ± 5.0 | 57.3 ± 9.8 | 61.2 ± 6.5 |
| HR (bpm) | 78.9 ± 2.1 | 79.4 ± 2.6 | 77.4 ± 2.2 | 79.2 ± 3.4 | 83.9 ± 4.2 | 81.6 ± 11.3 * | 73.1 ± 12.9 * |
| Fitness Measures | |||||||
| Grip Strength (kg) | 39.1 ± 3.2 | 45.7 ± 4.2 | 42.6 ± 3.6 | 44.9 ± 5.9 | 38.6 ± 4.6 | 35.0 ± 14.8 * | 57.8 ± 18.9 * |
| Est.VO2max (mL/kg/min) | 43.6 ± 0.7 * | 54.4 ± 0.6 * | 48.9 ± 1.0 | 49.5 ± 2.1 | 46.7 ± 3.1 | 49.2 ± 6.9 * | 47.5 ± 5.8 * |
| DXA Measures | |||||||
| FM (kg) | 14.9 ± 1.8 | 12.6 ± 1.3 | 10.3 ± 0.6 * | 15.2 ± 1.6 * | 23.9 ± 3.8 * | 13.5 ± 9.4 | 14.2 ± 5.5 |
| FMI (kg/m2) | 6.7 ± 0.7 | 5.6 ± 0.5 | 4.6 ± 0.2 * | 6.9 ± 0.4 * | 10.7 ± 1.3 * | 6.6 ± 3.6 | 5.2 ± 2.1 |
| BF (%) | 31.6 ± 1.4 * | 27.0 ± 1.7 * | 25.1 ± 1.0 * | 33.0 ± 1.7 * | 40.1 ± 2.1 * | 31.6 ± 8.1 * | 24.6 ± 7.8 * |
| VAT (cm2) | 39.6 ± 6.6 | 46.4 ± 3.4 | 30.9 ± 1.7 * | 47.6 ± 4.7 * | 79.3 ± 13.1 * | 43.9 ± 32.2 | 41.2 ± 17.2 |
| FFM (kg) | 28.8 ± 1.8 | 32.6 ± 2.5 | 30.1 ± 1.8 | 30.2 ± 3.6 | 32.9 ± 4.2 | 25.5 ± 8.5 * | 41.3 ± 9.7 * |
| FFMI (kg/m2) | 12.9 ± 0.4 | 13.8 ± 0.4 | 12.9 ± 0.3 * | 13.4 ± 0.6 | 14.8 ± 0.9 * | 12.6 ± 2.1 * | 14.8 ± 2.0 * |
| BIA Measures | |||||||
| BMR (kcals/day) | 1101 ± 38 | 1207 ± 62 | 1150 ± 45 | 1159 ± 92 | 1168 ± 92 | 1024 ± 30 * | 1424 ± 55 * |
| Phase Angle (°) | 5.2 ± 0.1 * | 5.6 ± 0.1 * | 5.4 ± 0.1 | 5.4 ± 0.2 | 5.4 ± 0.2 | 5.2 ± 0.1 * | 5.9 ± 0.1 * |
| FM (kg) | 12.3 ± 2.1 | 9.3 ± 1.3 | 7.0 ± 0.6 * | 11.6 ± 1.5 * | 22.8 ± 4.5 * | 11.0 ± 1.7 | 10.4 ± 1.3 |
| FMI (kg/m2) | 5.5 ± 0.8 | 4.1 ± 0.5 | 3.1 ± 0.3 * | 5.2 ± 0.5 * | 10.1 ± 1.6 * | 5.3 ± 0.7 | 3.9 ± 0.5 |
| BF (%) | 24.0 ± 1.9 | 18.8 ± 1.9 | 16.4 ± 1.1 * | 24.4 ± 2.1 * | 35.9 ± 3.1 * | 23.4 ± 1.8 | 17.4 ± 2.0 |
| VAT (cm2) | 53.0 ± 10.0 | 37.8 ± 6.8 | 26.7 ± 2.7* | 47.4 ± 8.1 * | 106.4 ± 21.9 * | 47.7 ± 8.5 | 40.7 ± 6.2 |
| FFM (kg) | 33.9 ± 1.8 | 38.8 ± 2.9 | 36.1 ± 2.1 | 36.5 ± 4.3 | 37.0 ± 4.3 | 30.3 ± 1.4 * | 48.8 ± 2.6 * |
| FFMI (kg/m2) | 15.3 ± 0.3 | 16.4 ± 0.5 | 15.5 ± 0.3 | 16.3 ± 0.7 | 16.8 ± 0.8 | 15.1 ± 0.3 * | 17.5 ± 0.5 * |
| Body Fat (%) | FMI (kg/m2) | FFMI (kg/m2) | VAT (cm2) | RMR (kcal/day) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | DXA | BIA | DXA | BIA | DXA | BIA | DXA | BIA | MG | BIA | |
| All Youth | 58 | 29.3 ± 1.1 | 21.4 ± 1.4 | 6.1 ± 0.4 | 4.8 ± 0.5 | 13.4 ± 0.3 | 15.9 ± 0.3 | 43.0 ± 3.7 | 45.4 ± 6.1 | 1104 ± 39 * | 1244 ± 41 * |
| Sex | |||||||||||
| Girls | 30 | 31.6 ± 1.4 | 24.0 ± 1.9 | 6.7 ± 0.7 | 5.5 ± 0.8 | 12.9 ± 0.4 | 15.3 ± 0.3 | 39.6 ± 6.7 | 53.0 ± 10.0 | 1070 ± 48 * | 1227 ± 44 * |
| Boys | 28 | 27.0 ± 1.7 | 18.8 ± 1.9 | 5.6 ± 0.5 | 4.1 ± 0.5 | 13.8 ± 0.4 | 16.4 ± 0.5 | 46.4 ± 2.9 * | 37.8 ± 6.8 * | 1148 ± 64 * | 1268 ± 75 * |
| Weight Status | |||||||||||
| HW | 37 | 25.1 ± 1.0 * | 16.4 ± 1.1 * | 4.6 ± 0.2 * | 3.1 ± 0.3 * | 12.9 ± 0.3 * | 15.5 ± 0.3 * | 30.9 ± 1.7 | 26.7 ± 2.7 | 1049 ± 44 * | 1231 ± 48 * |
| OW | 10 | 33.0 ± 1.7 | 24.4 ± 2.1 | 6.9 ± 0.4 | 5.2 ± 0.5 | 13.4 ± 0.6 | 16.3 ± 0.7 | 47.6 ± 4.7 | 47.4 ± 8.1 | 1153 ± 105 | 1248 ± 125 |
| OB | 11 | 40.1 ± 2.1 | 35.9 ± 3.1 | 10.7 ± 1.3 | 10.1 ± 1.6 | 14.8 ± 0.9 | 16.8 ± 0.8 | 79.3 ± 13.1 | 106.4 ± 21.9 | 1231 ± 94 | 1285 ± 101 |
| Age Group | |||||||||||
| Child | 39 | 31.6 ± 1.3 | 23.4 ± 1.8 | 6.6 ± 0.6 | 5.3 ± 0.7 | 12.6 ± 0.3 | 15.1 ± 0.3 | 43.9 ± 5.2 | 47.7 ± 8.5 | 1038 ± 42 * | 1113 ± 34 * |
| Teens | 19 | 24.6 ± 1.8 | 17.4 ± 2.0 | 5.2 ± 0.5 * | 3.9 ± 0.5 * | 14.8 ± 0.5 | 17.5 ± 0.5 | 41.2 ± 4.0 | 40.7 ± 6.2 | 1234 ± 72 * | 1541 ± 62 * |
| Variable | Timepoints | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Baseline | N | 0 min | N | 30 min | N | 60 min | N | 90 min | N | 120 min | |
| Weight (kg) | 58 | 47.1 ± 2.4 | 57 | 46.6 ± 2.3 | 57 | 46.7 ± 2.3 | 57 | 46.7 ± 2.3 | 57 | 46.6 ± 2.3 | 52 | 45.9 ± 2.5 |
| BMR (kcals/day) | 58 | 1155 ± 37 | 57 | 1143 ± 35 | 57 | 1144 ± 35 | 57 | 1144 ± 35 | 57 | 1147 ± 35 | 52 | 1144 ± 38 |
| Phase Angle (°) | 58 | 5.42 ± 0.08 | 57 | 5.43 ± 0.08 | 57 | 5.45 ± 0.08 | 57 | 5.44 ± 0.08 | 57 | 5.41 ± 0.08 | 52 | 5.40 ± 0.08 |
| Fat Mass Analysis | ||||||||||||
| FM (kg) | 58 | 10.8 ± 1.2 | 57 | 11.0 ± 1.3 | 57 | 10.9 ± 1.2 | 57 | 10.6 ± 1.3 | 57 | 12.0 ± 1.8 | 52 | 10.0 ± 1.2 |
| BF (%) | 58 | 21.4 ± 1.4 * | 57 | 22.0 ± 1.4 * | 57 | 21.9 ± 1.4 * | 57 | 21.7 ± 1.4 | 57 | 21.4 ± 1.4 | 52 | 20.6 ± 1.3 |
| VAT (cm2) | 58 | 45.4 ± 6.1 * | 57 | 46.2 ± 6.2 * | 57 | 45.5 ± 6.6 | 57 | 45.2 ± 6.7 | 57 | 45.0 ± 5.9 | 52 | 40.7 ± 5.6 * |
| FMI (kg/m2) | 58 | 4.7 ± 9.5 | 57 | 4.9 ± 1.5 | 57 | 4.8 ± 7.5 | 57 | 4.8 ± 4.5 | 57 | 5.4 ± 1.8 | 54 | 4.2 ± 7.5 |
| Fat-Free Mass Analysis | ||||||||||||
| FFM (kg) | 58 | 36.3 ± 1.7 | 57 | 35.8 ± 1.6 | 57 | 35.8 ± 1.6 | 57 | 35.8 ± 1.6 | 57 | 36.0 ± 1.6 | 52 | 35.8 ± 1.7 |
| FFMI (kg/m2) | 58 | 15.8 ± 6.3 * | 57 | 15.7 ± 5.3 | 57 | 15.7 ± 7.3 | 57 | 15.7 ± 8.3 | 57 | 15.8 ± 3.3 * | 54 | 15.2 ± 0.5 * |
| Body Water Analysis | ||||||||||||
| TBW (L) | 58 | 26.6 ± 1.2 * | 57 | 26.2 ± 1.2 | 57 | 26.2 ± 1.2 | 57 | 26.2 ± 1.2 | 57 | 26.3 ± 1.2 * | 52 | 26.2 ± 1.3 * |
| ECW/TBW | 58 | 0.3 ± 8.0 * | 57 | 0.3 ± 8.0 * | 57 | 0.3 ± 8.0 | 57 | 0.3 ± 8.0 | 57 | 0.3 ± 8.0 * | 52 | 0.3 ± 8.0 * |
| TBW/LBM | 58 | 73.2 ± 4.0 | 57 | 73.2 ± 1.0 | 57 | 73.2 ± 0.0 | 57 | 73.2 ± 1.0 | 57 | 73.2 ± 2.0 | 52 | 73.2 ± 4.0 |
| %TBW | 58 | 57.5 ± 1.0 | 57 | 57.5 ± 1.2 | 57 | 57.2 ± 1.0 | 57 | 57.3 ± 1.0 | 57 | 57.6 ± 1.0 | 52 | 58.1 ± 1.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Howe, C.A.; Corrigan, R.J.; Djalali, M.; McManaway, C.; Grbcich, A.; Aidoo, G.S. Feasibility of Using Bioelectrical Impedance Analysis for Assessing Youth Weight and Health Status: Preliminary Findings. Int. J. Environ. Res. Public Health 2021, 18, 10094. https://doi.org/10.3390/ijerph181910094
Howe CA, Corrigan RJ, Djalali M, McManaway C, Grbcich A, Aidoo GS. Feasibility of Using Bioelectrical Impedance Analysis for Assessing Youth Weight and Health Status: Preliminary Findings. International Journal of Environmental Research and Public Health. 2021; 18(19):10094. https://doi.org/10.3390/ijerph181910094
Chicago/Turabian StyleHowe, Cheryl A., Riley J. Corrigan, Maya Djalali, Chris McManaway, Alexandra Grbcich, and Grace Sam Aidoo. 2021. "Feasibility of Using Bioelectrical Impedance Analysis for Assessing Youth Weight and Health Status: Preliminary Findings" International Journal of Environmental Research and Public Health 18, no. 19: 10094. https://doi.org/10.3390/ijerph181910094
APA StyleHowe, C. A., Corrigan, R. J., Djalali, M., McManaway, C., Grbcich, A., & Aidoo, G. S. (2021). Feasibility of Using Bioelectrical Impedance Analysis for Assessing Youth Weight and Health Status: Preliminary Findings. International Journal of Environmental Research and Public Health, 18(19), 10094. https://doi.org/10.3390/ijerph181910094






