Bioconversion of Organic Pollutants in Fish-Canning Wastewater into Volatile Fatty Acids and Polyhydroxyalkanoate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fish-Canning Wastewater Characterization
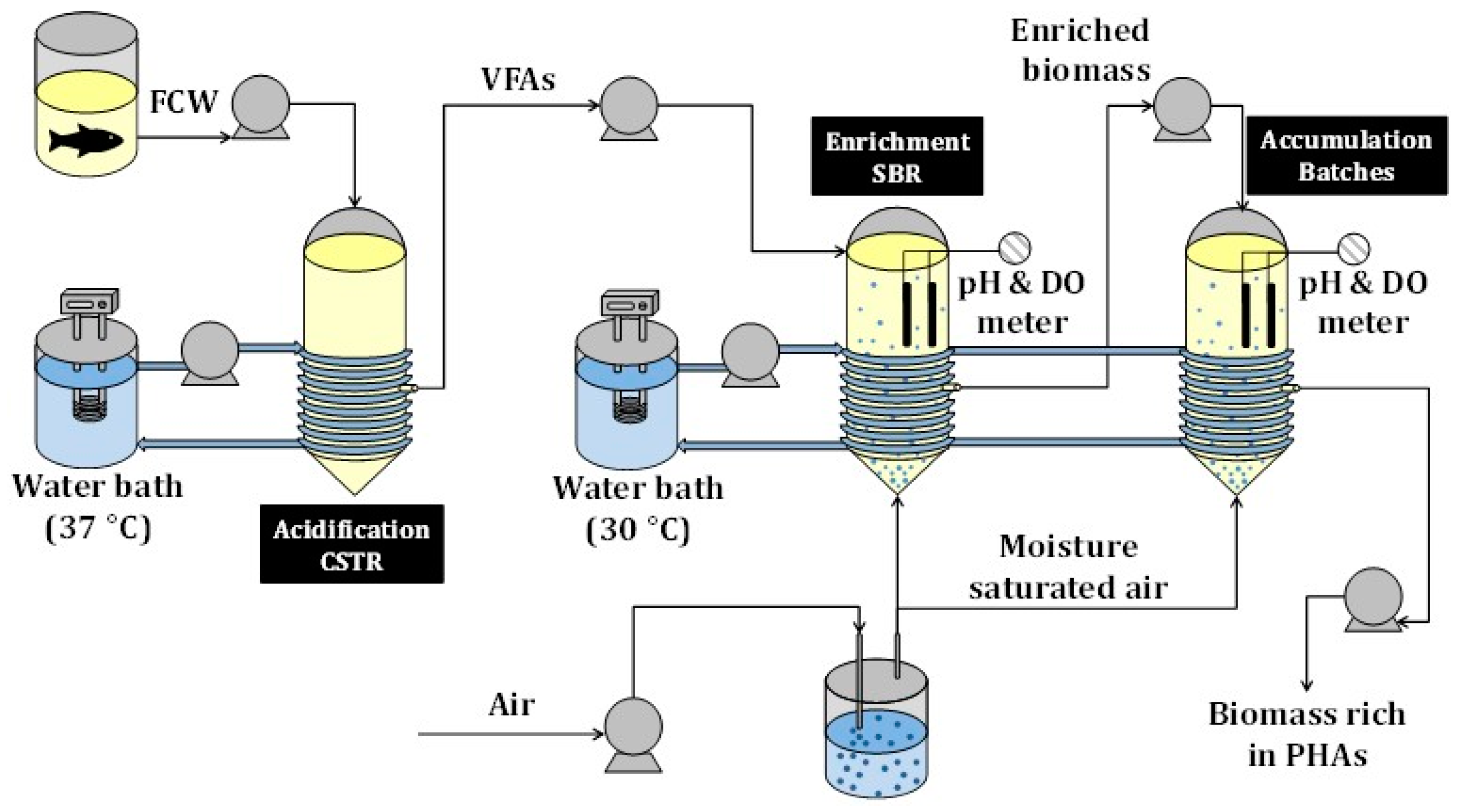
2.2. Experimental Set-Up
2.2.1. Acidification
2.2.2. Enrichment
2.2.3. Accumulation
- Fed-batch assay: The substrate was added in pulses. The complete consumption of the VFAs present in the feeding was noticed by the increase of the DO concentration, which was monitored and measured on-line. Each time the DO concentration rose up, a new pulse of feeding was added. The volume of each pulse was of 40 mL, which corresponded to 28.3 Cmmol VFAs per pulse.
- Batch assay: A volume of 520 mL of substrate was added just once, which means a solely pulse of approximately 250 Cmmol VFA.
2.3. Analytical Methods
2.4. Calculations
reactor per unit of time (g))/(Reactor volume (L) · time (h))
3. Results and Discussion
3.1. Acidification
3.2. Enrichment of a MMC Using the Effluent of the Acidification Reactor Diluted
3.3. Accumulation Performance of a MMC Using the Effluent of the Acidification Reactor without Any Dilution
- Fed-batch accumulation
- Batch accumulation
3.4. Influence of the Feeding Strategy on the PHA Accumulation Stage
4. Future Perspectives
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- European Commission-Executive Agency for Small and Medium-sized Enterprises. Available online: https://ec.europa.eu/easme/ (accessed on 3 June 2021).
- European Commission-Maritime Affairs. Available online: http://ec.europa.eu/maritimeaffairs/policy/blue_growth_en (accessed on 3 June 2021).
- Soto, M.; Méndez, R.; Lema, J.M. Efluentes industriales en la industria de procesado de productos marinos. Ing. Química 1990, 255, 203–209. [Google Scholar]
- Lefebvre, O.; Moletta, R. Treatment of organic pollution in industrial saline wastewater: A literature review. Water Res. 2006, 40, 3671–3682. [Google Scholar] [CrossRef]
- Omil, F. Augas residuais da industria: Implantacion de tecnoloxias limpas no sector conserveiro de productos mariños. Adega Cad. 2003, 11, 23–29. [Google Scholar]
- Feijoo, G.; Soto, M.; Méndez, R.; Lema, J.M. Sodium inhibition in the anaerobic digestion process: Antagonism and adaptation phenomena. Enzym. Microb. Technol. 1995, 17, 180–188. [Google Scholar] [CrossRef]
- Gebauer, R. Mesophilic anaerobic treatment of sludge from saline fish farm effluents with biogas production. Bioresour. Technol. 2004, 93, 155–167. [Google Scholar] [CrossRef]
- Hamoda, M.F.; Al-Attar, I.M.S. Effects of high sodium chloride concentrations on activated sludge treatment. Water Sci. Technol. 1995, 31, 61–72. [Google Scholar] [CrossRef]
- Intrasungkha, N.; Keller, J.; Blackall, L.L. Biological nutrient removal efficiency in treatment of saline wastewater. Water Sci. Technol. 1999, 39, 183–190. [Google Scholar] [CrossRef]
- Palmeiro-Sánchez, T.; Val del Río, A.; Mosquera-Corral, A.; Campos, J.L.; Méndez, R. Comparison of the anaerobic digestion of activated and aerobic granular sludges under brackish conditions. Chem. Eng. J. 2013, 231, 449–454. [Google Scholar] [CrossRef]
- Uygur, A.; Kargı, I. Salt inhibition on biological nutrient removal from saline wastewater in a sequencing batch reactor. Enzym. Microb. Technol. 2004, 34, 313–318. [Google Scholar] [CrossRef]
- Fajardo, C.; Mosquera-Corral, A.; Campos, J.L.; Méndez, R. Post-treatment of fish canning effluents by sequential nitrification and autotrophic denitrification processes. Process Biochem. 2013, 48, 1368–1374. [Google Scholar]
- Chowdhury, P.; Viraraghavan, T.; Srinivasan, A. Biological treatment processes for fish processing wastewater—A review. Bioresour. Technol. 2010, 101, 439–449. [Google Scholar] [CrossRef]
- European Commission-Environment. Circular Economy Strategy. Available online: http://ec.europa.eu/environment/circular-economy/index_en.htm (accessed on 3 June 2021).
- Nikodinovic-Runic, J.; Guzik, M.; Kenny, S.T.; Babu, R.; Werker, A.; O’Connor, K.E. Chapter Four—Carbon-Rich Wastes as Feedstocks for Biodegradable Polymer (Polyhydroxyalkanoate) Production Using Bacteria. In Advances in Applied Microbiology; Sima, S., Geoffrey, M.G., Eds.; Academic Press: Cambridge, MA, USA, 2013; pp. 139–200. [Google Scholar]
- Dionisi, D.; Carucci, G.; Papini, M.P.; Riccardi, C.; Majone, M.; Carrasco, F. Olive oil mill effluents as a feedstock for production of biodegradable polymers. Water Res. 2005, 39, 2076–2084. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, M.G.E.; Eiroa, M.; Torres, C.; Nunes, B.R.; Reis, M.A.M. Strategies for the development of a side stream process for polyhydroxyalkanoate (PHA) production from sugar cane molasses. J. Biotechnol. 2007, 130, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, M.G.E.; Torres, C.A.V.; Reis, M.A.M. Polyhydroxyalkanoate (PHA) production by a mixed microbial culture using sugar molasses: Effect of the influent substrate concentration on culture selection. Water Res. 2010, 44, 3419–3433. [Google Scholar] [CrossRef] [PubMed]
- Bengtsson, S.; Pisco, A.R.; Reis, M.A.M.; Lemos, P.C. Production of polyhydroxyalkanoates from fermented sugar cane molasses by a mixed culture enriched in glycogen accumulating organisms. J. Biotechnol. 2010, 145, 253–263. [Google Scholar] [CrossRef]
- Duque, A.F.; Oliveira, C.S.S.; Carmo, I.T.D.; Gouveia, A.R.; Pardelha, F.; Ramos, A.M.; Reis, M.A.M. Response of a three-stage process for PHA production by mixed microbial cultures to feedstock shift: Impact on polymer composition. New Biotechnol. 2014, 31, 276–288. [Google Scholar] [CrossRef]
- Bengtsson, S.; Werker, A.; Christensson, M.; Welander, T. Production of polyhydroxyalkanoates by activated sludge treating a paper mill wastewater. Bioresour. Technol. 2008, 99, 509–516. [Google Scholar] [CrossRef]
- Jiang, Y.; Marang, L.; Tamis, J.; van Loosdrecht, M.C.M.; Dijkman, H.; Kleerebezem, R. Waste to resource: Converting paper mill wastewater to bioplastic. Water Res. 2012, 46, 5517–5530. [Google Scholar] [CrossRef]
- Morgan-Sagastume, F.; Hjort, M.; Cirne, D.; Gérardin, F.; Lacroix, S.; Gaval, G.; Karabegovic, L.; Alexandersson, T.; Johansson, P.; Karlsson, A.; et al. Integrated production of polyhydroxyalkanoates (PHAs) with municipal wastewater and sludge treatment at pilot scale. Bioresour. Technol. 2015, 181, 78–89. [Google Scholar] [CrossRef]
- Morgan-Sagastume, F.; Karlsson, A.; Johansson, P.; Pratt, S.; Boon, N.; Lant, P.; Werker, A. Production of polyhydroxyalkanoates in open, mixed cultures from a waste sludge stream containing high levels of soluble organics, nitrogen and phosphorus. Water Res. 2010, 44, 5196–5211. [Google Scholar] [CrossRef]
- Liu, H.Y.; Hall, P.V.; Darby, J.L.; Coats, E.R.; Green, P.G.; Thompson, D.E.; Loge, F.J. Production of polyhydroxyalkanoate during treatment of tomato cannery wastewater. Water Env. Res. 2008, 80, 367–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamis, J.; Lužkov, K.; Jiang, Y.; van Loosdrecht, M.C.M.; Kleerebezem, R. Enrichment of Plasticicumulans acidivorans at pilot-scale for PHA production on industrial wastewater. J. Biotechnol. 2014, 192, 161–169. [Google Scholar] [CrossRef]
- Palmeiro-Sánchez, T.; Fra-Vázquez, A.; Rey-Martínez, N.; Campos, J.L.; Mosquera-Corral, A. Transient concentrations of NaCl affect the PHA accumulation in mixed microbial culture. J. Hazard. Mat. 2016, 306, 332–339. [Google Scholar] [CrossRef]
- Palmeiro-Sánchez, T.; Oliveira, C.S.S.; Gouveia, A.R.; Noronha, J.P.; Ramos, A.M.; Mosquera-Corral, A.; Reis, M.A.M. NaCl presence and purification affect the properties of mixed culture PHAs. Eur. Polym. J. 2016, 85, 256–265. [Google Scholar] [CrossRef]
- Johnson, K.; Kleerebezem, R.; van Loosdrecht, M.C.M. Influence of ammonium on the accumulation of polyhydroxybutyrate (PHB) in aerobic open mixed cultures. J. Biotechnol. 2010, 147, 73–79. [Google Scholar] [CrossRef]
- Moralejo-Gárate, H.; Palmeiro-Sánchez, T.; Kleerebezem, R.; Mosquera-Corral, A.; Campos, J.L.; van Loosdrecht, M.C.M. Influence of the cycle length on the production of PHA and polyglucose from glycerol by bacterial enrichments in sequencing batch reactors. Biotechnol. Bioeng. 2013, 110, 3148–3155. [Google Scholar] [CrossRef]
- Buntner, D.; Sánchez, A.; Garrido, J.M. Feasibility of combined UASB and MBR system in dairy wastewater treatment at ambient temperatures. Chem. Eng. J. 2013, 230, 475–481. [Google Scholar] [CrossRef]
- Ripley, L.E.; Boyle, W.C.; Converse, J.C. Improved Alkalimetric Monitoring for Anaerobic Digestion of High-Strength Wastes. J. Water Pollut. Control Fed. 1986, 58, 406–411. [Google Scholar]
- APHA-AWWA-WPCF. Standard Methods for the Examination of Water and Wastewater; American Public Health Association/American Water Works Association/Water Environment Federation: Washington DC, USA, 2005. [Google Scholar]
- Bower, C.E.; Holm-Hansen, T. A Salicylate–Hypochlorite Method for Determining Ammonia in Seawater. Can. J. Fish. Aquat. Sci. 1980, 37, 794–798. [Google Scholar] [CrossRef]
- Smolders, G.J.; van der Meij, J.; van Loosdrecht, M.C.M.; Heijnen, J.J. Stoichiometric model of the aerobic metabolism of the biological phosphorus removal process. Biotechnol. Bioeng. 1994, 44, 837–848. [Google Scholar] [CrossRef]
- Oktem, Y.A.; Ince, O.; Donnelly, T.; Sallis, P.; Ince, B.K. Determination of optimum operating conditions of an acidification reactor treating a chemical synthesis-based pharmaceutical wastewater. Process Biochem. 2006, 41, 2258–2263. [Google Scholar] [CrossRef]
- Beun, J.; Dircks, K.; Van Loosdrecht, M.C.M.; Heijnen, J.J. Poly-beta-hydroxybutyrate metabolism in dynamically fed mixed microbial cultures. Water Res. 2002, 36, 1167–1180. [Google Scholar] [CrossRef]
- Su, G.; Wang, S.; Yuan, Z.; Peng, Y. Enhanced volatile fatty acids production of waste activated sludge under salinity conditions: Performance and mechanisms. J. Biosci. Bioeng. 2016, 121, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.; Jiang, Y.; Kleerebezem, R.; Muyzer, G.; van Loosdrecht, M.C.M. Enrichment of a mixed bacterial culture with a high polyhydroxyalkanoate storage capacity. Biomacromolecules 2009, 10, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Marang, L.; Kleerebezem, R.; Muyzer, G.; van Loosdrecht, M.C.M. Effect of temperature and cycle length on microbial competition in PHB-producing sequencing batch reactor. ISME J. 2011, 5, 896–907. [Google Scholar] [CrossRef] [PubMed]
- Pardelha, F.; Albuquerque, M.G.E.; Reis, M.A.M.; Oliveira, R.; Dias, J.M.L. Dynamic metabolic modelling of volatile fatty acids conversion to polyhydroxyalkanoates by a mixed microbial culture. New Biotechnol. 2014, 31, 335–344. [Google Scholar] [CrossRef]
- Serafim, L.S.; Lemos, P.C.; Oliveira, R.; Reis, M.A.M. Optimization of polyhydroxybutyrate production by mixed cultures submitted to aerobic dynamic feeding conditions. Biotechnol. Bioeng. 2004, 87, 145–160. [Google Scholar] [CrossRef]
- Albuquerque, M.G.E.; Martino, V.; Pollet, E.; Avérous, L.; Reis, M.A.M. Mixed culture polyhydroxyalkanoate (PHA) production from volatile fatty acid (VFA)-rich streams: Effect of substrate composition and feeding regime on PHA productivity, composition and properties. J. Biotechnol. 2011, 151, 66–76. [Google Scholar] [CrossRef]





| Parameter | Wastewater | |
|---|---|---|
| Fats | g/L | 1.2 ± 0.1 |
| Proteins | g/L | 7.3 ± 2.5 |
| Carbohydrates | g/L | 0.15 ± 0.03 |
| VFA | g/L | 11.3 ± 3.3 |
| COD | g/L | 28.4 ± 4.0 |
| BOD5 | g/L | 17.9 ± 0.3 |
| TSS | g/L | 2.2 ± 0.9 |
| VSS | g/L | 1.6 ± 0.7 |
| VSS/TSS | % | 70.4 ± 6.8 |
| NaCl | g/L | 19.8 ± 5.9 |
| Ions | ||
| Na+ | g/L | 8.2 ± 2.8 |
| K+ | g/L | 2.2 ± 0.8 |
| Mg2+ | g/L | n.d. |
| Ca2+ | g/L | n.d. |
| SO42− | g/L | 0.9 ± 0.2 |
| PO43− | g/L | 2.0 ± 0.9 |
| Cl− | g/L | 12.0 ± 3.5 |
| NO2− | g/L | 0.07 ± 0.02 |
| NO3− | g/L | 0.36 ± 0.04 |
| Parameter | CSTR | |
|---|---|---|
| Operational conditions | ||
| Operation days | d | 203 |
| OLR | g COD/(L · d) | 12.3 ± 3.5 |
| HRT | d | 2 |
| SRT | d | 2 |
| Operational parameters at steady state | ||
| pH | 7.5 ± 0.1 | |
| NH4+ | g/L | 4.4 ± 1.6 |
| COD | g/L | 28.3 ± 8.7 |
| TSS | g/L | 2.5 ± 0.7 |
| VSS | g/L | 1.5 ± 0.4 |
| VSS/TSS | % | 62.3 ± 6.9 |
| NaCl | g/L | 28.2 ± 10.1 |
| VFATOTAL | g COD/L | 25.0 ± 4.6 |
| HAc | g COD/L | 10.9 ± 1.4 |
| HPr | g COD/L | 4.1 ± 1.0 |
| HBu | g COD/L | 8.1 ± 3.2 |
| HVa | g COD/L | 1.9 ± 0.5 |
| TA | g CaCO3/L | 11.5 ± 0.9 |
| PA | g CaCO3/L | 3.6 ± 0.4 |
| Ripley ratio | 2.3 ± 0.3 | |
| Salinity and other relevant ions | ||
| Na+ | g/L | 10.9 ± 4.0 |
| K+ | g/L | 1.4 ± 0.5 |
| Mg2+ | g/L | n.d. |
| Ca2+ | g/L | n.d. |
| SO42− | mg/L | 542.4 ± 153.6 |
| PO43− | mg/L | 636.3 ± 245.4 |
| Cl− | g/L | 13.8 ± 2.8 |
| NO2− | mg/L | 49.1 ± 16.8 |
| NO3− | mg/L | 304.1 ± 67.4 |
| Parameter | Enrichment SBR | |
|---|---|---|
| Operational conditions | ||
| Operation time | d | 384 |
| HRT | d | 1 |
| SRT | d | 1 |
| Cycle | h | 12 |
| Operational parameters in steady state at the end of the feast phase | ||
| DOSATURATION | mg/L | 7.2 ± 0.1 |
| T | °C | 30.9 ± 1.0 |
| pH | 8.7 ± 0.4 | |
| NH4+ | mg/L | 256.4 ± 20.3 |
| TS | g/L | 1.34 ± 0.47 |
| VS | g/L | 1.21 ± 0.43 |
| VS/TS | % | 90.3 ± 5.2 |
| Experiment | HB | HV | PHA | HB:HV | Productivity | TS | VS |
|---|---|---|---|---|---|---|---|
| wt% | wt% | wt% | g/g | mg PHA/L h | g/L | g/L | |
| Fed-batch (0 h) | 2.21 | 2.33 | 4.54 | 0.95 | - | 0.72 | 0.56 |
| Fed-batch (27 h) | 7.14 | 1.21 | 8.35 | 5.91 | 10.31 | 3.29 | 2.50 |
| Batch (0 h) | 0.12 | 0.01 | 0.13 | 15.3 | - | 1.71 | 0.87 |
| Batch (2.7 h) | 5.36 | 0.34 | 5.70 | 15.9 | 55.4 | 2.59 | 1.19 |
| Batch (24 h) | 0.09 | 0.00 | 0.09 | - | - | 3.66 | 2.12 |
| −qVFA | qPHA | qX | qCO2 | YPHB | YPHV | YPHA | |
|---|---|---|---|---|---|---|---|
| Cmol/ Cmol X h | Cmol/ Cmol X h | Cmol/ Cmol X h | Cmol/ Cmol X h | Cmol/ Cmol VFA | Cmol/ Cmol VFA | Cmol/ Cmol VFA | |
| Fed-batch | 0.187 | 0.006 | 0.068 | 0.064 | 0.034 | 0.366 | 0.345 |
| Batch | 0.536 | 0.071 | 0.121 | 0.372 | 0.133 | 0.226 | 0.694 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palmeiro-Sánchez, T.; Campos, J.L.; Mosquera-Corral, A. Bioconversion of Organic Pollutants in Fish-Canning Wastewater into Volatile Fatty Acids and Polyhydroxyalkanoate. Int. J. Environ. Res. Public Health 2021, 18, 10176. https://doi.org/10.3390/ijerph181910176
Palmeiro-Sánchez T, Campos JL, Mosquera-Corral A. Bioconversion of Organic Pollutants in Fish-Canning Wastewater into Volatile Fatty Acids and Polyhydroxyalkanoate. International Journal of Environmental Research and Public Health. 2021; 18(19):10176. https://doi.org/10.3390/ijerph181910176
Chicago/Turabian StylePalmeiro-Sánchez, Tania, José Luis Campos, and Anuska Mosquera-Corral. 2021. "Bioconversion of Organic Pollutants in Fish-Canning Wastewater into Volatile Fatty Acids and Polyhydroxyalkanoate" International Journal of Environmental Research and Public Health 18, no. 19: 10176. https://doi.org/10.3390/ijerph181910176
APA StylePalmeiro-Sánchez, T., Campos, J. L., & Mosquera-Corral, A. (2021). Bioconversion of Organic Pollutants in Fish-Canning Wastewater into Volatile Fatty Acids and Polyhydroxyalkanoate. International Journal of Environmental Research and Public Health, 18(19), 10176. https://doi.org/10.3390/ijerph181910176








