Body Composition Results of Caucasian Young Normal Body Mass Women in the Follicular Proliferative Phase, Measured for the Different Positions of Limbs
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Studied Population
- −
- Females;
- −
- Age 18–30 years;
- −
- Caucasian ethnicity;
- −
- Current Body Mass Index (BMI) of 18.5–25.0 kg/m2;
- −
- Stable body mass declared within the previous 6 months;
- −
- Living in Warsaw (being able to participate in the study conducted in the Dietary Outpatient Clinic of the Institute of Human Nutrition Sciences).
- −
- Irregular menstrual cycle declared;
- −
- Amenorrhea declared;
- −
- Applied hormonal contraception declared;
- −
- Epilepsy;
- −
- Having pacemakers or other simulators implanted;
- −
- Having orthopedic prosthesis or other metal implants;
- −
- Having visually abnormal body; limb; or trunk build (after serious surgical procedures and resections, after limb amputations, with serious scoliosis, etc.);
- −
- Practicing sports professionally;
- −
- Being pregnant or during lactation;
- −
- Any metabolic disorders or other chronic diseases declared;
- −
- Current undergoing body mass reduction declared;
- −
- Current being on any special diet declared.
2.3. Measurements
- −
- −
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lemos, T.; Gallagher, D. Current body composition measurement techniques. Curr. Opin. Endocrinol. Diabetes Obes. 2017, 24, 310–314. [Google Scholar] [CrossRef]
- Duren, D.L.; Sherwood, R.J.; Czerwinski, S.A.; Lee, M.; Choh, A.C.; Siervogel, R.M.; Chumlea, W.C. Body composition methods: Comparisons and interpretation. J. Diabetes Sci. Technol. 2008, 2, 1139–1146. [Google Scholar] [CrossRef]
- Jeon, K.C.; Kim, S.-Y.; Jiang, F.L.; Chung, S.; Ambegaonkar, J.P.; Park, J.-H.; Kim, Y.-J.; Kim, C.-H. Prediction equations of the multifrequency standing and supine bioimpedance for appendicular skeletal muscle mass in Korean older people. Int. J. Environ. Res. Public Health 2020, 17, 5847. [Google Scholar] [CrossRef]
- Ræder, H.; Kværner, A.S.; Henriksen, C.; Florholmen, G.; Henriksen, H.B.; Bøhn, S.K.; Paur, I.; Smeland, S.; Blomhoff, R. Validity of bioelectrical impedance analysis in estimation of fat-free mass in colorectal cancer patients. Clin. Nutr. 2018, 37, 292–300. [Google Scholar] [CrossRef]
- Siddiqui, N.I.; Alam Khan, S.; Shoeb, M.; Bose, S. Anthropometric predictors of bio-impedance analysis (BIA) phase angle in healthy adults. J. Clin. Diagn. Res. 2016, 10, CC01–CC04. [Google Scholar] [CrossRef]
- Wells, J.C.K.; Fewtrell, M.S. Measuring body composition. Arch. Dis. Child. 2006, 91, 612–617. [Google Scholar] [CrossRef] [PubMed]
- Kyle, U.G.; Bosaeus, I.; de Lorenzo, A.D.; Deurenberg, P.; Elia, M.; Gomez, J.M.; Heitmann, B.L.; Kent-Smith, L.; Melchior, J.-C.; Pirlich, M.; et al. Bioelectrical impedance analysis? Part I: Review of principles and methods. Clin. Nutr. 2004, 23, 1226–1243. [Google Scholar] [CrossRef] [PubMed]
- Moissl, U.M.; Wabel, P.; Chamney, P.W.; Bosaeus, I.; Levin, N.W.; Bosy-Westphal, A.; Korth, O.; Müller, M.J.; Ellegård, L.; Malmros, V.; et al. Body fluid volume determination via body composition spectroscopy in health and disease. Physiol. Meas. 2006, 27, 921–933. [Google Scholar] [CrossRef] [PubMed]
- Lyons-Reid, J.; Ward, L.C.; Kenealy, T.; Cutfield, W. Bioelectrical impedance analysis—An easy tool for quantifying body composition in infancy? Nutrients 2020, 12, 920. [Google Scholar] [CrossRef] [PubMed]
- Walter-Kroker, A.; Kroker, A.; Mattiucci-Guehlke, M.; Glaab, T. A practical guide to bioelectrical impedance analysis using the example of chronic obstructive pulmonary disease. Nutr. J. 2011, 10, 35. [Google Scholar] [CrossRef]
- Mitra, S. Extracellular hydration, cardiovascular risk, and the interstitium: A three-dimensional view. Kidney Int. 2014, 85, 510–512. [Google Scholar] [CrossRef] [PubMed]
- Haverkort, E.B.; Reijven, P.L.M.; Binnekade, J.M.; de van der Schueren, M.A.E.; Earthman, C.P.; Gouma, D.J.; de Haan, R.J. Bioelectrical impedance analysis to estimate body composition in surgical and oncological patients: A systematic review. Eur. J. Clin. Nutr. 2015, 69, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Talma, H.; Chinapaw, M.J.M.; Bakker, B.; HiraSing, R.A.; Terwee, C.B.; Altenburg, T.M. Bioelectrical impedance analysis to estimate body composition in children and adolescents: A systematic review and evidence appraisal of validity, responsiveness, reliability and measurement error. Obes. Rev. 2013, 14, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Lyons-Reid, J.; Derraik, J.G.B.; Ward, L.C.; Tint, M.; Kenealy, T.; Cutfield, W.S. Bioelectrical impedance analysis for assessment of body composition in infants and young children—A systematic literature review. Clin. Obes. 2021, 11, e12441. [Google Scholar] [CrossRef]
- Kyle, U.G.; Bosaeus, I.; de Lorenzo, A.D.; Deurenberg, P.; Elia, M.; Gómez, J.M.; Heitmann, B.L.; Kent-Smith, L.; Melchior, J.-C.; Pirlich, M.; et al. Bioelectrical impedance analysis—Part II: Utilization in clinical practice. Clin. Nutr. 2004, 23, 1430–1453. [Google Scholar] [CrossRef]
- Reiss, J.; Iglseder, B.; Kreutzer, M.; Weilbuchner, I.; Treschnitzer, W.; Kässmann, H.; Pirich, C.; Reiter, R. Case finding for sarcopenia in geriatric inpatients: Performance of bioimpedance analysis in comparison to dual X-ray absorptiometry. BMC Geriatr. 2016, 16, 52. [Google Scholar] [CrossRef] [PubMed]
- Bourdon, C.; Bartels, R.H.; Chimwezi, E.; Kool, J.; Chidzalo, K.; Perot, L.; Brals, D.; Bandsma, R.H.; van Hensbroek, M.B.; Voskuijl, W.P. The clinical use of longitudinal bio-electrical impedance vector analysis in assessing stabilization of children with severe acute malnutrition. Clin. Nutr. 2021, 40, 2078–2090. [Google Scholar] [CrossRef] [PubMed]
- Stapel, S.N.; Looijaard, W.G.P.M.; Dekker, I.M.; Girbes, A.R.J.; Weijs, P.J.M.; Oudemans-van Straaten, H.M. Bioelectrical impedance analysis-derived phase angle at admission as a predictor of 90-day mortality in intensive care patients. Eur. J. Clin. Nutr. 2018, 72, 1019–1025. [Google Scholar] [CrossRef] [PubMed]
- Kahraman, A.; Hilsenbeck, J.; Nyga, M.; Ertle, J.; Wree, A.; Plauth, M.; Gerken, G.; Canbay, A.E. Bioelectrical impedance analysis in clinical practice: Implications for hepatitis C therapy BIA and hepatitis C. Virol. J. 2010, 7, 191. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Body Mass Index—BMI. Available online: https://www.euro.who.int/en/health-topics/disease-prevention/nutrition/a-healthy-lifestyle/body-mass-index-bmi (accessed on 26 July 2021).
- Scalfi, L.; Bedogni, G.; Marra, M.; di Biase, G.; Caldara, A.; Severi, S.; Contaldo, F.; Battistini, N. The prediction of total body water from bioelectrical impedance in patients with anorexia nervosa. Br. J. Nutr. 1997, 78, 357–365. [Google Scholar] [CrossRef]
- Salinari, S.; Bertuzzi, A.; Mingrone, G.; Capristo, E.; Scarfone, A.; Greco, A.V.; Heymsfield, S.B. Bioimpedance analysis: A useful technique for assessing appendicular lean soft tissue mass and distribution. J. Appl. Physiol. 2003, 94, 1552–1556. [Google Scholar] [CrossRef]
- Campa, F.; Levi Micheli, M.; Pompignoli, M.; Cannataro, R.; Gulisano, M.; Toselli, S.; Greco, G.; Coratella, G. The Influence of Menstrual Cycle on Bioimpedance Vector Patterns, Performance, and Flexibility in Elite Soccer Players. Int. J. Sports Physiol. Perform. 2021, 17, 1–9. [Google Scholar] [CrossRef]
- Cáceres, D.I.; Sartor-Messagi, M.; Rodríguez, D.A.; Escalada, F.; Gea, J.; Orozco-Levi, M.; Marco, E. Variability in bioelectrical impedance assessment of body composition depending on measurement conditions: Influence of fast and rest. Nutr. Hosp. 2014, 30, 1359–1365. (In Spanish) [Google Scholar] [CrossRef]
- Głąbska, D.; Cackowska, K.; Guzek, D. Comparison of the body composition of Caucasian young normal body mass women, measured in the follicular phase, depending on the carbohydrate diet level. Medicina 2018, 54, 104. [Google Scholar] [CrossRef]
- Hussain, R.; Levin, N.; Zhu, F.; Kappel, F.; Kotanko, P. Body composition and solute kinetics in hemodialysis patients: A mathematical model. IET Commun. 2012, 6, 3301–3308. [Google Scholar] [CrossRef]
- Jensen, B.; Braun, W.; Both, M.; Gallagher, D.; Clark, P.; González, D.L.; Klückmann, K.; Bosy-Westphal, A. Configuration of bioelectrical impedance measurements affects results for phase angle. Med. Eng. Phys. 2020, 84, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Aandstad, A.; Holtberget, K.; Hageberg, R.; Holme, I.; Anderssen, S.A. Validity and reliability of bioelectrical impedance analysis and skinfold thickness in predicting body fat in military personnel. Mil. Med. 2014, 179, 208–217. [Google Scholar] [CrossRef]
- Langer, R.D.; Borges, J.H.; Pascoa, M.A.; Cirolini, V.X.; Guerra-Junior, G.; Gonçalves, E.M. Validity of bioelectrical impedance analysis to estimation fat-free mass in the army cadets. Nutrients 2016, 8, 121. [Google Scholar] [CrossRef] [PubMed]
- Dittmar, M.; Reber, H. New equations for estimating body cell mass from bioimpedance parallel models in healthy older Germans. Am. J. Physiol. Metab. 2001, 281, E1005–E1014. [Google Scholar] [CrossRef]
- Fujii, K.; Ishizaki, A.; Ogawa, A.; Asami, T.; Kwon, H.; Tanaka, A.; Sekiya, N.; Hironaka, S. Validity of using multi-frequency bioelectrical impedance analysis to measure skeletal muscle mass in preschool children. J. Phys. Ther. Sci. 2017, 29, 863–868. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, C.; Evans, J.; Smye, S.; Holland, P. Total body water measurement using bioelectrical impedance analysis, isotope dilution and total body potassium: A scoring system to facilitate intercomparison. Eur. J. Clin. Nutr. 2002, 56, 326–337. [Google Scholar] [CrossRef][Green Version]
- Landis, J.R.; Koch, G.G. The Measurement of Observer Agreement for Categorical Data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Myles, P.; Cui, J.I. Using the Bland–Altman method to measure agreement with repeated measures. Br. J. Anaesth. 2007, 99, 309–311. [Google Scholar] [CrossRef]
- Kondrup, J.; Allison, S.P.; Elia, M.; Vellas, B.; Plauth, M. Educational and clinical practice committee, European Society of Parenteral and Enteral Nutrition (ESPEN). ESPEN guidelines for nutrition screening 2002. Clin. Nutr. 2003, 22, 415–421. [Google Scholar] [CrossRef]
- Schell, B.; Gross, R. The reliability of bioelectrical impedance measurements in the assessment of body composition in healthy adults. Nutr. Rep. Int. 2000, 36, 449–459. [Google Scholar]
- Deurenberg, P.; Deurenberg-Yap, M. Validation of skinfold thickness and hand-held impedance measurements for estimation of body fat percentage among Singaporean Chinese, Malay and Indian subjects. Asia Pac. J. Clin. Nutr. 2002, 11, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Pigłowska, M.; Kostka, T.; Drygas, W.; Jegier, A.; Leszczyńska, J.; Bill-Bielecka, M.; Kwaśniewska, M. Body composition, nutritional status, and endothelial function in physically active men without metabolic syndrome—A 25 year cohort study. Lipids Health Dis. 2016, 15, 84. [Google Scholar] [CrossRef] [PubMed]
- Mareschal, J.; Achamrah, N.; Norman, K.; Genton, L. Clinical value of muscle mass assessment in clinical conditions associated with malnutrition. J. Clin. Med. 2019, 8, 1040. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, S.; Kehayias, J.J. Sarcopenia and the analysis of body composition. Adv. Nutr. 2014, 5, 260–267. [Google Scholar] [CrossRef]
- De-Mateo-Silleras, B.; Camina-Martín, M.A.; de-Frutos-Allas, J.M.; de-la-Cruz-Marcos, S.; Carreño-Enciso, L.; Redondo-del-Río, M.P. Bioimpedance analysis as an indicator of muscle mass and strength in a group of elderly subjects. Exp. Gerontol. 2018, 113, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Fiaccadori, E.; Morabito, S.; Cabassi, A.; Regolisti, G. Body cell mass evaluation in critically ill patients: Killing two birds with one stone. Crit. Care 2014, 18, 139. [Google Scholar] [CrossRef]
- Ugras, S. Evaluating of altered hydration status on effectiveness of body composition analysis using bioelectric impedance analysis. Libyan J. Med. 2020, 15, 1741904. [Google Scholar] [CrossRef]
- Tai, R.; Ohashi, Y.; Mizuiri, S.; Aikawa, A.; Sakai, K. Association between ratio of measured extracellular volume to expected body fluid volume and renal outcomes in patients with chronic kidney disease: A retrospective single-center cohort study. BMC Nephrol. 2014, 15, 1–10. [Google Scholar] [CrossRef]
- Knudsen, N.N.; Kjærulff, T.M.; Ward, L.C.; Sæbye, D.; Holst, C.; Heitmann, B.L. Body water distribution and risk of cardiovascular morbidity and mortality in a healthy population: A prospective cohort study. PLoS ONE 2014, 9, e87466. [Google Scholar] [CrossRef]
- Dehghan, M.; Merchant, A.T. Is bioelectrical impedance accurate for use in large epidemiological studies? Nutr. J. 2008, 7, 26. [Google Scholar] [CrossRef]
- Bongiovanni, T.; Mascherini, G.; Genovesi, F.; Pasta, G.; Iaia, F.M.; Trecroci, A.; Ventimiglia, M.; Alberti, G.; Campa, F. Bioimpedance vector references need to be period-specific for assessing body composition and cellular health in elite soccer players: A brief report. J. Funct. Morphol. Kinesiol. 2020, 5, 73. [Google Scholar] [CrossRef]
- Khalil, S.F.; Mohktar, M.S.; Ibrahim, F. The theory and fundamentals of bioimpedance analysis in clinical status monitoring and diagnosis of diseases. Sensors 2014, 14, 10895–10928. [Google Scholar] [CrossRef]
- Ismail, A.H.; Schlieper, G.; Walter, M.; Floege, J.; Leonhardt, S. Knee-to-knee bioimpedance measurements to monitor changes in extracellular fluid in haemodynamic-unstable patients during dialysis. J. Electr. Bioimpedance 2019, 10, 55–62. [Google Scholar] [CrossRef]
- Ismail, A.H.; Gross, T.; Schlieper, G.; Walter, M.; Eitner, F.; Floege, J.; Leonhardt, S. Monitoring transcellular fluid shifts during episodes of intradialytic hypotension using bioimpedance spectroscopy. Clin. Kidney J. 2021, 14, 149–155. [Google Scholar] [CrossRef]
- Campa, F.; Toselli, S.; Mazzilli, M.; Gobbo, L.A.; Coratella, G. Assessment of body composition in athletes: A narrative review of available methods with special reference to quantitative and qualitative bioimpedance analysis. Nutrients 2021, 13, 1620. [Google Scholar] [CrossRef]
- Barrea, L.; Muscogiuri, G.; Macchia, P.E.; di Somma, C.; Falco, A.; Savanelli, M.C.; Colao, A.; Savastano, S. Mediterranean diet and phase angle in a sample of adult population: Results of a pilot study. Nutrients 2017, 9, 151. [Google Scholar] [CrossRef] [PubMed]
- Brantlov, S.; Jødal, L.; Lange, A.; Rittig, S.; Ward, L.C. Standardisation of bioelectrical impedance analysis for the estimation of body composition in healthy paediatric populations: A systematic review. J. Med. Eng. Technol. 2017, 41, 460–479. [Google Scholar] [CrossRef] [PubMed]



| Body Composition | Position with Arms Abducted at Least 30° and Legs Abducted at Approximately 45°; n = 100 | Position with Arms and Legs Separated to Not Touch the Body; n = 100 | p ** | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Median | Minimum | Maximum | Mean ± SD | Median | Minimum | Maximum | ||
| Fat mass (kg) | 16.4 ± 4.0 | 16.3 | 5.5 | 26.9 | 16.0 ± 4.2 | 16.2 | 5.3 | 26.9 | 0.5545 |
| Fat mass (%) | 27.5 ± 4.5 | 27.2 | 13.1 | 37.5 | 26.9 ± 4.8 | 26.8 | 11.8 | 37.5 | 0.3739 |
| Fat-free mass (kg) | 42.6 ± 3.5 | 42.2 | 33.6 | 52.6 | 42.9 ± 3.5 | 42.6 | 33.6 | 52.9 | 0.5028 |
| Fat-free mass (%) | 72.0 ± 6.5 | 72.6 * | 24.9 | 86.9 | 72.6 ± 5.5 | 73.1 * | 51.5 | 88.2 | 0.6927 |
| Body cell mass (kg) | 19.2 ± 3.0 | 19.2 * | 9.2 | 29.8 | 20.8 ± 4.4 | 20.2 * | 12.6 | 35.9 | 0.0165 |
| Body cell mass (%) | 45.1 ± 6.0 | 45.0 | 25.7 | 61.6 | 48.4 ± 8.7 | 47.8 * | 28.5 | 75.3 | 0.0075 |
| Total body water (kg) | 31.1 ± 2.6 | 30.9 | 24.6 | 38.5 | 31.4 ± 2.6 | 31.2 * | 24.6 | 38.7 | 0.4495 |
| Total body water (%) | 53.1 ± 3.3 | 53.1 | 44.8 | 63.6 | 53.5 ± 3.5 | 53.5 | 44.7 | 64.5 | 0.4475 |
| Extracellular water (kg) | 14.6 ± 1.5 | 14.3 | 11.6 | 18.7 | 14.2 ± 1.6 | 14.0 | 10.9 | 19.4 | 0.0982 |
| Extracellular water (%) | 46.8 ± 3.6 | 46.6 * | 38.3 | 64.2 | 45.2 ± 4.2 | 45.3 | 35.9 | 57.2 | 0.0049 |
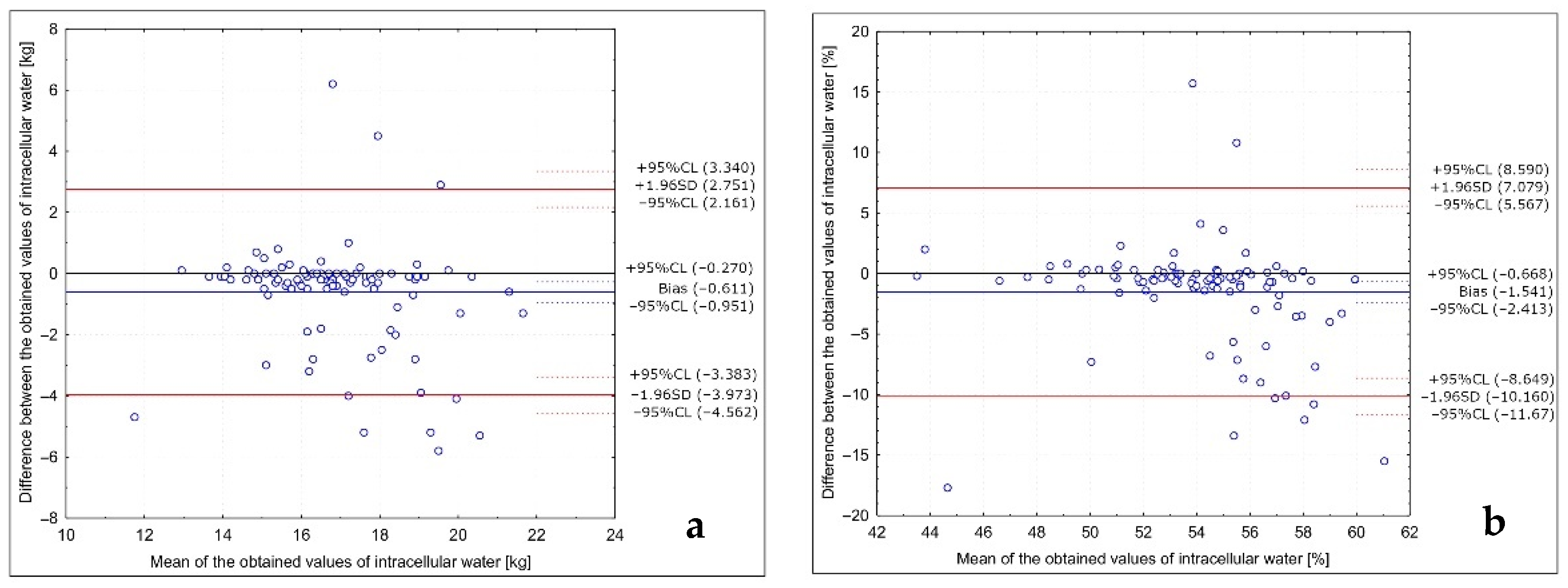
| Intracellular water (kg) | 16.6 ± 1.8 | 16.6 * | 9.4 | 21.0 | 17.2 ± 2.1 | 16.9 * | 12.9 | 23.2 | 0.0759 |
| Intracellular water (%) | 53.2 ± 3.6 | 53.4 * | 35.8 | 61.7 | 54.7 ± 4.3 | 54.6 | 42.8 | 68.8 | 0.0115 |
| Muscle mass (kg) | 24.0 ± 3.5 | 23.9 * | 10.8 | 35.8 | 26.1 ± 4.8 | 25.4 * | 17.1 | 42.7 | 0.0025 |
| Muscle mass (%) | 41.0 ± 6.1 | 40.8 * | 27.3 | 67.2 | 44.7 ± 8.6 | 43.8 * | 28.9 | 76.5 | 0.0011 |
| Body Composition | p | R |
|---|---|---|
| Fat mass (kg) | <0.0001 | 0.9335 * |
| Fat mass (%) | <0.0001 | 0.9122 * |
| Fat-free mass (kg) | <0.0001 | 0.9059 * |
| Fat-free mass (%) | <0.0001 | 0.8613 ** |
| Body cell mass (kg) | <0.0001 | 0.6103 ** |
| Body cell mass (%) | <0.0001 | 0.6974 ** |
| Total body water (kg) | <0.0001 | 0.9052 ** |
| Total body water (%) | <0.0001 | 0.9119 * |
| Extracellular water (kg) | <0.0001 | 0.8645 * |
| Extracellular water (%) | <0.0001 | 0.5050 ** |
| Intracellular water (kg) | <0.0001 | 0.6677 ** |
| Intracellular water (%) | <0.0001 | 0.5620 ** |
| Muscle mass (kg) | <0.0001 | 0.6374 ** |
| Muscle mass (%) | <0.0001 | 0.7152 ** |
| Position with Arms Abducted at Least 30° and Legs Abducted at Approximately 45° | ||||||
|---|---|---|---|---|---|---|
| Quartile | 1st Quartile | 2nd Quartile | 3rd Quartile | 4th Quartile | ||
| Position with arms and legs separated to not touch the body | Body cell mass (kg) | 1st quartile | 20% | 1% | 1% | 3% |
| 2nd quartile | 2% | 16% | 7% | 0% | ||
| 3rd quartile | 1% | 1% | 12% | 11% | ||
| 4th quartile | 2% | 7% | 5% | 11% | ||
| Body cell mass (%) | 1st quartile | 17% | 6% | 0% | 2% | |
| 2nd quartile | 3% | 15% | 7% | 0% | ||
| 3rd quartile | 2% | 0% | 13% | 10% | ||
| 4th quartile | 3% | 4% | 5% | 13% | ||
| Position with Arms Abducted at Least 30° and Legs Abducted at Approximately 45° | ||||||
|---|---|---|---|---|---|---|
| Quartile | 1st Quartile | 2nd Quartile | 3rd Quartile | 4th Quartile | ||
| Position with arms and legs separated to not touch the body | Extracellular water (kg) | 1st quartile | 16% | 5% | 3% | 1% |
| 2nd quartile | 6% | 17% | 1% | 1% | ||
| 3rd quartile | 1% | 3% | 17% | 4% | ||
| 4th quartile | 2% | 0% | 4% | 19% | ||
| Extracellular water (%) | 1st quartile | 11% | 3% | 8% | 3% | |
| 2nd quartile | 11% | 13% | 0% | 1% | ||
| 3rd quartile | 1% | 8% | 12% | 4% | ||
| 4th quartile | 2% | 1% | 5% | 17% | ||
| Position with Arms Abducted at Least 30° and Legs Abducted at Approximately 45° | ||||||
|---|---|---|---|---|---|---|
| Quartile | 1st Quartile | 2nd Quartile | 3rd Quartile | 4th Quartile | ||
| Position with arms and legs separated to not touch the body | Intracellular water (kg) | 1st quartile | 20% | 3% | 0% | 2% |
| 2nd quartile | 0% | 19% | 5% | 1% | ||
| 3rd quartile | 3% | 2% | 13% | 7% | ||
| 4th quartile | 2% | 1% | 7% | 15% | ||
| Intracellular water (%) | 1st quartile | 18% | 3% | 1% | 3% | |
| 2nd quartile | 4% | 14% | 6% | 1% | ||
| 3rd quartile | 0% | 0% | 15% | 10% | ||
| 4th quartile | 3% | 8% | 3% | 11% | ||
| Position with Arms Abducted at Least 30° and Legs Abducted at Approximately 45° | ||||||
|---|---|---|---|---|---|---|
| Quartile | 1st Quartile | 2nd Quartile | 3rd Quartile | 4th Quartile | ||
| Position with arms and legs separated to not touch the body | Muscle mass (kg) | 1st quartile | 19% | 2% | 1% | 3% |
| 2nd quartile | 2% | 15% | 8% | 0% | ||
| 3rd quartile | 0% | 3% | 11% | 11% | ||
| 4th quartile | 4% | 5% | 5% | 11% | ||
| Muscle mass (%) | 1st quartile | 15% | 10% | 0% | 0% | |
| 2nd quartile | 1% | 13% | 10% | 1% | ||
| 3rd quartile | 3% | 1% | 10% | 11% | ||
| 4th quartile | 6% | 1% | 5% | 13% | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Głąbska, D.; Wojciechowska, A.; Cackowska, K.; Guzek, D. Body Composition Results of Caucasian Young Normal Body Mass Women in the Follicular Proliferative Phase, Measured for the Different Positions of Limbs. Int. J. Environ. Res. Public Health 2021, 18, 10214. https://doi.org/10.3390/ijerph181910214
Głąbska D, Wojciechowska A, Cackowska K, Guzek D. Body Composition Results of Caucasian Young Normal Body Mass Women in the Follicular Proliferative Phase, Measured for the Different Positions of Limbs. International Journal of Environmental Research and Public Health. 2021; 18(19):10214. https://doi.org/10.3390/ijerph181910214
Chicago/Turabian StyleGłąbska, Dominika, Agata Wojciechowska, Karolina Cackowska, and Dominika Guzek. 2021. "Body Composition Results of Caucasian Young Normal Body Mass Women in the Follicular Proliferative Phase, Measured for the Different Positions of Limbs" International Journal of Environmental Research and Public Health 18, no. 19: 10214. https://doi.org/10.3390/ijerph181910214
APA StyleGłąbska, D., Wojciechowska, A., Cackowska, K., & Guzek, D. (2021). Body Composition Results of Caucasian Young Normal Body Mass Women in the Follicular Proliferative Phase, Measured for the Different Positions of Limbs. International Journal of Environmental Research and Public Health, 18(19), 10214. https://doi.org/10.3390/ijerph181910214








