An Unrecognized Hazard in E-Cigarette Vapor: Preliminary Quantification of Methylglyoxal Formation from Propylene Glycol in E-Cigarettes
Abstract
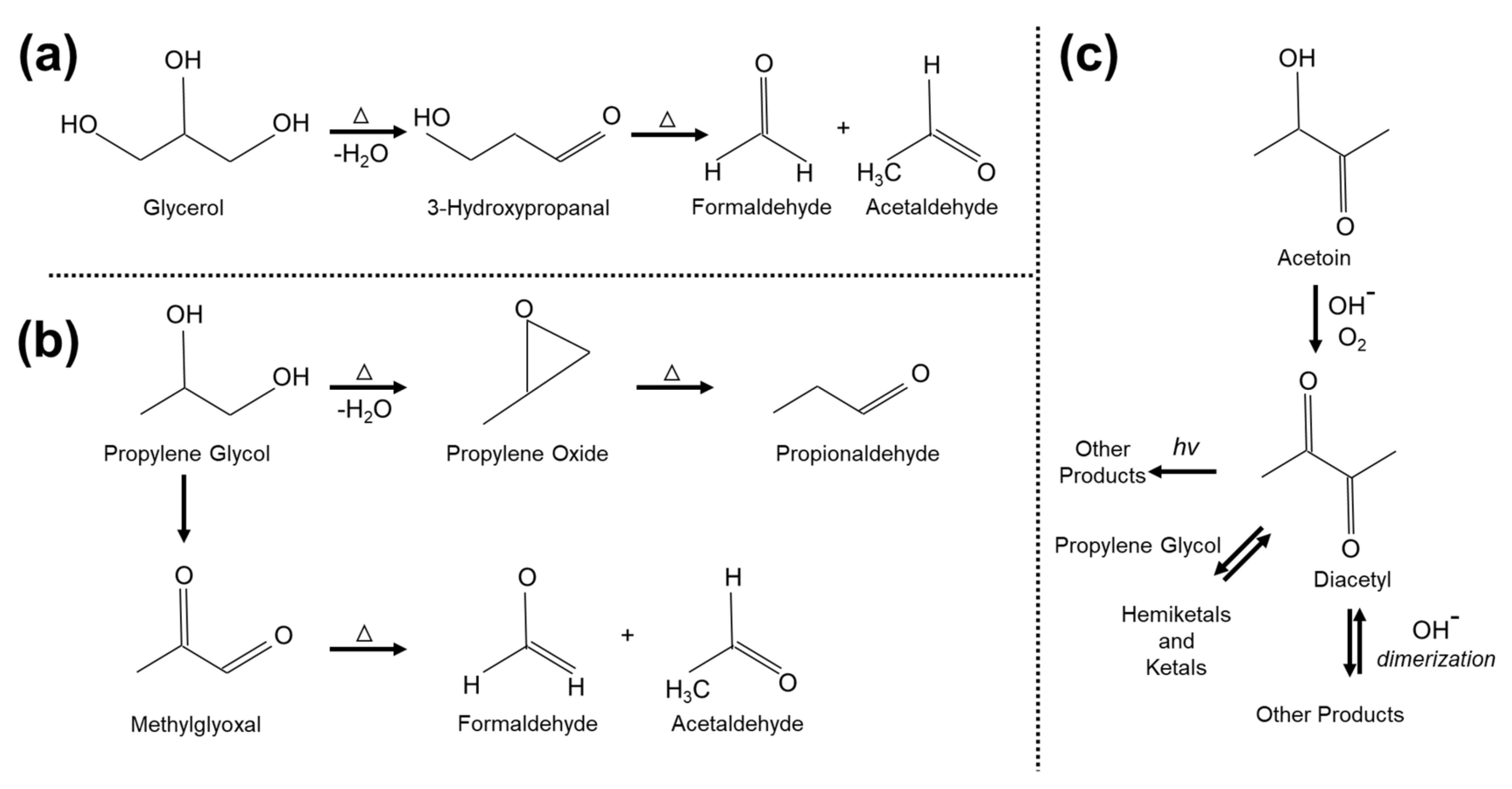
:1. Introduction
2. Methodology
3. Results
4. Discussion
4.1. Public Health Concerns
4.2. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- CDCMMWR. QuickStats: Percentage of Adults Who Ever Used an E-cigarette and Percentage Who Currently Use E-cigarettes, by Age Group—National Health Interview Survey, United States, 2016. MMWR Morb. Mortal. Wkly. Rep. 2017, 66. [Google Scholar] [CrossRef] [Green Version]
- US Food and Drug Administration. Youth Tobacco Use: Results from the National Youth Tobacco Survey; FDA: Silver Spring, MD, USA, 2019. [Google Scholar]
- Siegel, M.B.; Tanwar, K.L.; Wood, K.S. Electronic Cigarettes as a Smoking-Cessation Tool: Results from an Online Survey. Am. J. Prev. Med. 2011, 40, 472–475. [Google Scholar] [CrossRef]
- JUUL Market Share in 2019. Available online: https://blog.technavio.com/blog/juul-market-share-dominating-e-cigarettes-market (accessed on 5 January 2021).
- Burstyn, I. Peering through the mist: Systematic review of what the chemistry of contaminants in electronic cigarettes tells us about health risks. BMC Public Health 2014, 14, 18. [Google Scholar] [CrossRef] [Green Version]
- Qu, Y.; Szulejko, J.E.; Kim, K.-H.; Jo, S.-H. The effect of varying battery voltage output on the emission rate of carbonyls released from e-cigarette smoke. Microchem. J. 2019, 145, 47–54. [Google Scholar] [CrossRef]
- Schneller, L.M.; Vanderbush, T.S.; O′connor, R.J. Can Established Vapers Distinguish Different PG:VG Ratios? A Pilot Study. Tob. Regul. Sci. 2018, 4, 73–78. [Google Scholar] [CrossRef]
- Talih, S.; Salman, R.; El-Hage, R.; Karam, E.; Karaoghlanian, N.; El-Hellani, A.; Saliba, N.; Shihadeh, A. Characteristics and toxicant emissions of JUUL electronic cigarettes. Tob. Control 2019, 28, 678–680. [Google Scholar] [CrossRef] [PubMed]
- Jensen, R.P.; Luo, W.; Pankow, J.F.; Strongin, R.M.; Peyton, D.H. Hidden Formaldehyde in E-Cigarette Aerosols. N. Engl. J. Med. 2015, 372, 392–394. [Google Scholar] [CrossRef] [Green Version]
- Khlystov, A.; Samburova, V. Flavoring Compounds Dominate Toxic Aldehyde Production during E-Cigarette Vaping. Environ. Sci. Technol. 2016, 50, 13080–13085. [Google Scholar] [CrossRef]
- Klager, S.; Vallarino, J.; MacNaughton, P.; Christiani, D.C.; Lu, Q.; Allen, J.G. Flavoring Chemicals and Aldehydes in E-Cigarette Emissions. Environ. Sci. Technol. 2017, 51, 10806–10813. [Google Scholar] [CrossRef] [PubMed]
- Ogunwale, M.A.; Li, M.; Ramakrishnam Raju, M.V.; Chen, Y.; Nantz, M.H.; Conklin, D.J.; Fu, X.-A. Aldehyde Detection in Electronic Cigarette Aerosols. ACS Omega 2017, 2, 1207–1214. [Google Scholar] [CrossRef]
- Vas, C.A.; Porter, A.; McAdam, K. Acetoin is a precursor to diacetyl in e-cigarette liquids. Food Chem. Toxicol. 2019, 133, 110727. [Google Scholar] [CrossRef] [PubMed]
- Allaman, I.; Bélanger, M.; Magistretti, P.J. Methylglyoxal, the dark side of glycolysis. Front. Neurosci. 2015, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bekki, K.; Uchiyama, S.; Ohta, K.; Inaba, Y.; Nakagome, H.; Kunugita, N. Carbonyl Compounds Generated from Electronic Cigarettes. Int. J. Environ. Res. Public Health 2014, 11, 11192–11200. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, S.; Ohta, K.; Inaba, Y.; Kunugita, N. Determination of Carbonyl Compounds Generated from the E-cigarette Using Coupled Silica Cartridges Impregnated with Hydroquinone and 2,4-Dinitrophenylhydrazine, Followed by High-Performance Liquid Chromatography. Anal. Sci. 2013, 29, 1219–1222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohta, K.; Uchiyama, S.; Inaba, Y.; Nakagome, H.; Kunugita, N. Determination of carbonyl compounds generated from the electronic cigarette using coupled silica cartridges impregnated with hydroquinone and 2,4-dinitrophenylhydrazine. Bunseki Kagaku 2011, 60, 791–797. [Google Scholar] [CrossRef] [Green Version]
- Uchiyama, S.; Senoo, Y.; Hayashida, H.; Inaba, Y.; Nakagome, H.; Kunugita, N. Determination of Chemical Compounds Generated from Second-generation E-cigarettes Using a Sorbent Cartridge Followed by a Two-step Elution Method. Anal. Sci. 2016, 32, 549–555. [Google Scholar] [CrossRef] [Green Version]
- American Chemical Society E-cigarettes Can Damage DNA. Available online: https://www.sciencedaily.com/releases/2018/08/180820085208.htm (accessed on 23 December 2019).
- Hubbs, A.F.; Battelli, L.A.; Goldsmith, W.T.; Porter, D.W.; Frazer, D.; Friend, S.; Schwegler-Berry, D.; Mercer, R.R.; Reynolds, J.S.; Grote, A.; et al. Necrosis of nasal and airway epithelium in rats inhaling vapors of artificial butter flavoring. Toxicol. Appl. Pharmacol. 2002, 185, 128–135. [Google Scholar] [CrossRef]
- Morgan, D.L.; Flake, G.P.; Kirby, P.J.; Palmer, S.M. Respiratory Toxicity of Diacetyl in C57Bl/6 Mice. Toxicol. Sci. 2008, 103, 169–180. [Google Scholar] [CrossRef] [Green Version]
- Van Rooy, F.G.B.G.J.; Rooyackers, J.M.; Prokop, M.; Houba, R.; Smit, L.A.M.; Heederik, D.J.J. Bronchiolitis Obliterans Syndrome in Chemical Workers Producing Diacetyl for Food Flavorings. Am. J. Respir. Crit. Care Med. 2007, 176, 498–504. [Google Scholar] [CrossRef]
- Muttray, A.; Gosepath, J.; Brieger, J.; Faldum, A.; Pribisz, A.; Mayer-Popken, O.; Jung, D.; Rossbach, B.; Mann, W.; Letzel, S. No acute effects of an exposure to 50 ppm acetaldehyde on the upper airways. Int. Arch. Occup. Environ. Health 2009, 82, 481–488. [Google Scholar] [CrossRef]
- Silverman, L.; Schulte, H.F.; First, M.W. Further studies on sensory response to certain industrial solvent vapors. J. Ind. Hyg. Toxicol. 1946, 28, 262–266. [Google Scholar] [PubMed]
- Sim, V.M.; Pattle, R.E. Effect of possible smog irritants on human subjects. J. Am. Med. Assoc. 1957, 165, 1908–1913. [Google Scholar] [CrossRef] [PubMed]
- US Department of Health and Human Services. Registry of Toxic Effects of Chemical Substances (RTECS, Online Database); National Toxicology Information Program, National Library of Medicine: Bethesda, MD, USA, 1993. [Google Scholar]
- US Department of Health and Human Services. Hazardous Substances Data Bank (HSDB, Online Database); National Toxicology Information Program, National Library of Medicine: Bethesda, MD, USA, 1993. [Google Scholar]
- Hiraki, B.; Misra, M.; Cook, D. HPHC Analysis of Seven Flavors of a Temperature-Regulated Nicotine Salt Pod System; JUUL Labs, Inc.: San Francisco, CA, USA, 2019. [Google Scholar]
- Allen Joseph, G.; Flanigan Skye, S.; Mallory, L.; Jose, V.; Piers, M.; Stewart James, H.; Christiani David, C. Flavoring Chemicals in E-Cigarettes: Diacetyl, 2,3-Pentanedione, and Acetoin in a Sample of 51 Products, Including Fruit-, Candy-, and Cocktail-Flavored E-Cigarettes. Environ. Health Perspect. 2016, 124, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Munch, J.W.; Munch, D.J.; Winslow, S.D.; Wendelken, S.C.; Pepich, B.V. Determination of carbonyl compounds in drinking water by pentafluorobenzylhydroxylamine derivatization and capillary gas chromatography with electron capture detection. US EPA Method 556 1998, 1, 1–37. [Google Scholar]
- Bao, M.; Pantani, F.; Griffini, O.; Burrini, D.; Santianni, D.; Barbieri, K. Determination of carbonyl compounds in water by derivatization–solid-phase microextraction and gas chromatographic analysis. J. Chromatogr. A 1998, 809, 75–87. [Google Scholar] [CrossRef]
- Bao, M.; Joza, P.J.; Masters, A.; Rickert, W.S. Analysis of Selected Carbonyl Compounds in Tobacco Samples by Using Pentafluorobenzylhydroxylamine Derivatization and Gas Chromatography-Mass Spectrometry. Beitr. Tab. Int. Contrib. Tob. Res. 2014, 26, 86–97. [Google Scholar] [CrossRef] [Green Version]
- Health Canada. Determination of “tar”, Nicotine and Carbon Monoxide in Mainstream Tobacco Smoke; Health Canada: Ottawa, ON, Canada, 1999. [Google Scholar]
- Baylor, K.; Morison, G.; Taylor, D.R. Laboratory Data Review for the Non-Chemist; United States Environmental Protection Agency Region 9: San Francisco, CA, USA, 2014. [Google Scholar]
- Henningfield, J.E.; Zaatari, G.S. Electronic nicotine delivery systems: Emerging science foundation for policy. Tob. Control 2010, 19, 89–90. [Google Scholar] [CrossRef] [Green Version]
- Laugesen, M. Second safety report on the Ruyan® e-cigarette. Cell 2008, 27, 4375. [Google Scholar]
- Tinghino, B.; Enea, D. E-cigarette: Primi dati e possibili prospettive. Tabaccologia 2009, 2–3, 36–39. [Google Scholar]
- Laugesen, M. Safety Report on the Ruyan E-Cigarette Cartridge and Inhaled Aerosol; Health New Zealand Ltd.: Christchurch, New Zealand, 2008. [Google Scholar]
- Cobb, N.K.; Byron, M.J.; Abrams, D.B.; Shields, P.G. Novel Nicotine Delivery Systems and Public Health: The Rise of the “E-Cigarette”; American Public Health Association: Washington, DC, USA, 2010. [Google Scholar]
- Kreiss, K.; Gomaa, A.; Kullman, G.; Fedan, K.; Simoes, E.J.; Enright, P.L. Clinical Bronchiolitis Obliterans in Workers at a Microwave-Popcorn Plant. N. Engl. J. Med. 2002, 347, 330–338. [Google Scholar] [CrossRef] [Green Version]
- Hubbs, A.F.; Goldsmith, W.T.; Kashon, M.L.; Frazer, D.; Mercer, R.R.; Battelli, L.A.; Kullman, G.J.; Schwegler-Berry, D.; Friend, S.; Castranova, V. Respiratory toxicologic pathology of inhaled diacetyl in Sprague-Dawley rats. Toxicol. Pathol. 2008, 36, 330–344. [Google Scholar] [CrossRef] [Green Version]
- Anderson, S.E.; Franko, J.; Wells, J.R.; Lukomska, E.; Meade, B.J. Evaluation of the hypersensitivity potential of alternative butter flavorings. Food Chem. Toxicol. 2013, 62, 373–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hubbs, A.F.; Cumpston, A.M.; Goldsmith, W.T.; Battelli, L.A.; Kashon, M.L.; Jackson, M.C.; Frazer, D.G.; Fedan, J.S.; Goravanahally, M.P.; Castranova, V. Respiratory and olfactory cytotoxicity of inhaled 2, 3-pentanedione in Sprague-Dawley rats. Am. J. Pathol. 2012, 181, 829–844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Margham, J.; McAdam, K.; Forster, M.; Liu, C.; Wright, C.; Mariner, D.; Proctor, C. Chemical composition of aerosol from an e-cigarette: A quantitative comparison with cigarette smoke. Chem. Res. Toxicol. 2016, 29, 1662–1678. [Google Scholar] [CrossRef] [PubMed]
- Farsalinos, K.E.; Kistler, K.A.; Gillman, G.; Voudris, V. Evaluation of Electronic Cigarette Liquids and Aerosol for the Presence of Selected Inhalation Toxins. Nicotine Tob. Res. 2015, 17, 168–174. [Google Scholar] [CrossRef]
- Tierney, P.A.; Karpinski, C.D.; Brown, J.E.; Luo, W.; Pankow, J.F. Flavour chemicals in electronic cigarette fluids. Tob. Control 2016, 25, e10–e15. [Google Scholar] [CrossRef] [Green Version]
- Varlet, V.; Farsalinos, K.; Augsburger, M.; Thomas, A.; Etter, J.-F. Toxicity assessment of refill liquids for electronic cigarettes. Int. J. Environ. Res. Public Health 2015, 12, 4796–4815. [Google Scholar] [CrossRef] [Green Version]
- U.S. Department of Health & Human Services. Criteria for A Recommended Standard: Occupational Exposure to Diacetyl and 2,3-Pentanedione; U.S. Department of Health & Human Services: Washington, DC, USA, 2016. [Google Scholar] [CrossRef] [Green Version]
- Egilman, D.S.; Schilling, J.H.; Menendez, L. A Proposal for a Safe Exposure Level for Diacetyl. Int. J. Occup. Environ. Health 2011, 17, 122–134. [Google Scholar] [CrossRef]
- Saliba, N.A.; El Hellani, A.; Honein, E.; Salman, R.; Talih, S.; Zeaiter, J.; Shihadeh, A. Surface chemistry of electronic cigarette electrical heating coils: Effects of metal type on propylene glycol thermal decomposition. J. Anal. Appl. Pyrolysis 2018, 134, 520–525. [Google Scholar] [CrossRef]
- Hubbs, A.F.; Kreiss, K.; Cummings, K.J.; Fluharty, K.L.; O’Connell, R.; Cole, A.; Dodd, T.M.; Clingerman, S.M.; Flesher, J.R.; Lee, R.; et al. Flavorings-Related Lung Disease: A Brief Review and New Mechanistic Data. Toxicol. Pathol. 2019, 47, 1012–1026. [Google Scholar] [CrossRef]
- Nigro, C.; Raciti, G.A.; Leone, A.; Fleming, T.H.; Longo, M.; Prevenzano, I.; Fiory, F.; Mirra, P.; D’Esposito, V.; Ulianich, L. Methylglyoxal impairs endothelial insulin sensitivity both in vitro and in vivo. Diabetologia 2014, 57, 1485–1494. [Google Scholar] [CrossRef]
- Nigro, C.; Leone, A.; Raciti, G.; Longo, M.; Mirra, P.; Formisano, P.; Beguinot, F.; Miele, C. Methylglyoxal-glyoxalase 1 balance: The root of vascular damage. Int. J. Mol. Sci. 2017, 18, 188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, S.-J.; Xu, Y.-M.; Lau, A.T.Y. Electronic cigarette: A recent update of its toxic effects on humans. J. Cell. Physiol. 2018, 233, 4466–4478. [Google Scholar] [CrossRef] [PubMed]
- Shimatani, Y.; Nodera, H.; Osaki, Y.; Banzrai, C.; Takayasu, K.; Endo, S.; Shibuta, Y.; Kaji, R. Upregulation of axonal HCN current by methylglyoxal: Potential association with diabetic polyneuropathy. Clin. Neurophysiol. 2015, 126, 2226–2232. [Google Scholar] [CrossRef] [PubMed]
- Thornalley, P.J.; Waris, S.; Fleming, T.; Santarius, T.; Larkin, S.J.; Winklhofer-Roob, B.M.; Stratton, M.R.; Rabbani, N. Imidazopurinones are markers of physiological genomic damage linked to DNA instability and glyoxalase 1-associated tumour multidrug resistance. Nucleic Acids Res. 2010, 38, 5432–5442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eberhardt, M.J.; Filipovic, M.R.; Leffler, A.; de la Roche, J.; Kistner, K.; Fischer, M.J.; Fleming, T.; Zimmermann, K.; Ivanovic-Burmazovic, I.; Nawroth, P.P. Methylglyoxal activates nociceptors through transient receptor potential channel A1 (TRPA1) a possible mechanism of metabolic neuropathies. J. Biol. Chem. 2012, 287, 28291–28306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goniewicz, M.L.; Knysak, J.; Gawron, M.; Kosmider, L.; Sobczak, A.; Kurek, J.; Prokopowicz, A.; Jablonska-Czapla, M.; Rosik-Dulewska, C.; Havel, C.; et al. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob. Control 2014, 23, 133–139. [Google Scholar] [CrossRef] [Green Version]
- Lorenti Garcia, C.; Mechilli, M.; Proietti De Santis, L.; Schinoppi, A.; Katarzyna, K.; Palitti, F. Relationship between DNA lesions, DNA repair and chromosomal damage induced by acetaldehyde. Mutat. Res. 2009, 662, 3–9. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. Toxicological Review of Propionaldehyde (CAS No. 123-38-6); U.S. Environmental Protection Agency: Washington, DC, USA, 2008; p. 78. [Google Scholar]
- CORESTA, E.; Force, C.T. Routine analytical machine for e-cigarette aerosol generation and collection–definitions and standard conditions. Paris CORESTA 2015, 1, 1–6. Available online: https://www.coresta.org/sites/default/files/technical_documents/main/CRM_81.pdf (accessed on 5 January 2021).
- Rao, P.; Liu, J.; Springer, M.L. JUUL and Combusted Cigarettes Comparably Impair Endothelial Function. Tob. Regul. Sci. 2020, 6, 30–37. [Google Scholar] [CrossRef]
- Omaiye, E.E.; McWhirter, K.J.; Luo, W.; Pankow, J.F.; Talbot, P. Toxicity of JUUL Fluids and Aerosols Correlates Strongly with Nicotine and Some Flavor Chemical Concentrations. bioRxiv 2018, 490607. [Google Scholar] [CrossRef] [Green Version]


| Tested JUUL Pods | Ave. Sampling Flow (Start-End) (LPM) | Sampling Time (min) | Air Sampled per Puff (cm3) | Total Air Sampled (L) |
|---|---|---|---|---|
| Classic tobacco (5%) | 0.88 (0.80–0.96) | 240 | 29.3 | 7.036 |
| Crème (3%) | 0.41 (0.32–0.51) | 245 | 13.8 | 3.377 |
| Crème (5%) | 0.52 (0.41–0.64) | 240 | 17.5 | 4.188 |
| Cucumber (3%) | 0.53 (0.52–0.54) | 244 | 17.7 | 4.315 |
| Cucumber (5%) | 0.62 (0.50–0.74) | 240 | 20.6 | 4.952 |
| Fruit (3%) | 0.44 (0.43–0.45) | 251 | 14.8 | 3.715 |
| Fruit (5%) | 0.92 (0.92–0.93) | 245 | 30.8 | 7.546 |
| Mango (5%) | 0.98 (0.96–1.00) | 243 | 32.6 | 7.914 |
| Menthol (5%) | 0.78 (0.77–0.80) | 245 | 26.1 | 6.403 |
| Mint (3%) | 0.61 (0.58–0.63) | 245 | 20.2 | 4.945 |
| Mint (5%) | 0.52 (0.51–0.53) | 245 | 17.3 | 4.230 |
| Virginia Tobacco (3%) | 0.53 (0.50–0.55) | 240 | 17.6 | 4.212 |
| Virginia Tobacco (5%) A | 0.77 (0.73–0.80) | 240 | 25.6 | 6.140 |
| Virginia Tobacco (5%) B | 0.85 (0.84–0.86) | 240 | 28.3 | 6.800 |
| Carbonyls/Di-Carbonyls | LOD (µg/mL) * | LOQ (µg/mL) * | Ave. Recovery Rates (Range) ** |
|---|---|---|---|
| Acetaldehyde | 0.005 | 0.017 | 121 (113–130) |
| Propionaldehyde | 0.002 | 0.008 | 150 (102–176) † |
| Isobutyraldehyde | 0.001 | 0.003 | 142 (102–168) † |
| Acetoin | 0.003 | 0.011 | 167 (120–201) † |
| 2,3-Butanedione (Diacetyl) | 0.001 | 0.003 | 105 (101–108) |
| 2,3-Pentanedione | 0.002 | 0.006 | 92 (89–96) |
| 2,3-Hexanedione | 0.002 | 0.006 | 141 (84–220) † |
| 2,3-Heptanedione | 0.002 | 0.008 | 88 (69–120) |
| Methylglyoxal | 0.001 | 0.003 | 149 (107–175) † |
| Sample | Acetaldehyde | Propionaldehyde | Methylglyoxal | Diacetyl |
|---|---|---|---|---|
| E-cigarette Flavor (Nicotine Strength) | [µg/mL] | [µg/mL] | [µg/mL] | [µg/mL] |
| Blank | <0.017 but ≥0.005 | <0.008 but ≥0.002 | <0.001 | <0.001 |
| Classic tobacco (5%) | 0.031 | 0.011 | 0.284 | 0.005 |
| Crème (3%) | 0.213 | 0.019 | 1.45 | 0.003 |
| Crème (5%) | 0.030 | 0.035 | 0.443 | <0.003 but ≥0.001 |
| Cucumber (3%) | 0.159 | 0.041 | 3.05 | 0.007 |
| Cucumber (5%) | 0.158 | 0.054 | 3.00 | 0.013 |
| Fruit (3%) | 0.320 | 0.022 | 1.82 | 0.004 |
| Fruit (5%) | 0.334 | 0.028 | 5.79 | 0.016 |
| Mango (5%) | 0.167 | 0.022 | 0.978 | 0.008 |
| Menthol (5%) | 0.041 | 0.035 | 0.597 | 0.005 |
| Mint (3%) | 0.063 | 0.025 | 1.73 | 0.009 |
| Mint (5%) | 0.075 | 0.015 | 0.841 | 0.005 |
| Virginia Tobacco (3%) | 0.118 | 0.023 | 0.940 | <0.003 but ≥0.001 |
| Virginia Tobacco (5%) A | 0.034 | 0.009 | 0.230 | <0.003 but ≥0.001 |
| Virginia Tobacco (5%) B | 0.065 | 0.009 | 0.316 | <0.003 but ≥0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azimi, P.; Keshavarz, Z.; Lahaie Luna, M.; Cedeno Laurent, J.G.; Vallarino, J.; Christiani, D.C.; Allen, J.G. An Unrecognized Hazard in E-Cigarette Vapor: Preliminary Quantification of Methylglyoxal Formation from Propylene Glycol in E-Cigarettes. Int. J. Environ. Res. Public Health 2021, 18, 385. https://doi.org/10.3390/ijerph18020385
Azimi P, Keshavarz Z, Lahaie Luna M, Cedeno Laurent JG, Vallarino J, Christiani DC, Allen JG. An Unrecognized Hazard in E-Cigarette Vapor: Preliminary Quantification of Methylglyoxal Formation from Propylene Glycol in E-Cigarettes. International Journal of Environmental Research and Public Health. 2021; 18(2):385. https://doi.org/10.3390/ijerph18020385
Chicago/Turabian StyleAzimi, Parham, Zahra Keshavarz, Marianne Lahaie Luna, Jose Guillermo Cedeno Laurent, Jose Vallarino, David C. Christiani, and Joseph G. Allen. 2021. "An Unrecognized Hazard in E-Cigarette Vapor: Preliminary Quantification of Methylglyoxal Formation from Propylene Glycol in E-Cigarettes" International Journal of Environmental Research and Public Health 18, no. 2: 385. https://doi.org/10.3390/ijerph18020385
APA StyleAzimi, P., Keshavarz, Z., Lahaie Luna, M., Cedeno Laurent, J. G., Vallarino, J., Christiani, D. C., & Allen, J. G. (2021). An Unrecognized Hazard in E-Cigarette Vapor: Preliminary Quantification of Methylglyoxal Formation from Propylene Glycol in E-Cigarettes. International Journal of Environmental Research and Public Health, 18(2), 385. https://doi.org/10.3390/ijerph18020385







