1. Introduction
Pesticides are recognized as reagents for protecting crops against harmful pests and diseases in humans. The beneficial outcome of pesticides makes it become an important tool to maintain and improve the living standard of the global population. An average of 2 million tons of pesticides was used each year globally to confront weeds, insects and pests [
1]. The conventional classification of pesticides based on the target species includes herbicides, insecticide, rodenticide, fungicide and so forth [
2]. Herbicides and insecticides are the most common type of pesticide used, dominating 47.5% and the latter 29.5% of the total pesticide consumption [
1]. The primary pesticide consuming countries including China, the USA, Argentina, India, Japan, Canada, Brazil, France, Italy and Thailand [
2].
The pest control revolution has begun in the 1970s with the development of pesticides based on toxic heavy metals such as copper, lead, mercury and arsenic. This was followed by the discovery of dichlorodiphenyl trichloroethane (DDT) during World War II [
1]. The use of DDT increased enormously due to its effectiveness against almost all pest species at low dosage. Because of the great use, adverse impact on the environment and mankind had become apparent as soon as DDT became popular. After DDT has been banned for agricultural and domestic use, a wide variety of synthetic pesticides has been produced, such as organophosphate and pyrethroid which are still toxic to the environment [
3]. The continuous and excessive use of a wide range of pesticide eventually harm the non-target species and causes the pesticide residues to appear in many unexpected sites [
4]. Under constant chemical pressure, pesticides had led to the development of resistant strains in which the pests and insects get immune to the pesticide [
3].
The application of pesticides give rise to a range of benefits, including increased the quality and quantity of food and reduced insect-borne disease but raised the issues on the potential detrimental effects to the environment, including water resources. The associated environmental impacts are mainly due to the persistent and ubiquitous characteristics of various pesticides that posed havoc to the biodiversity [
2]. The dissolution of pesticides depends on the nature of the compound, pesticide application techniques and climatic factors. The pesticides that are not readily degrading will either get accumulated in soils or mobilized from one site to another in the form of degraded products, with unknown toxicity to human health [
2].
The occurrence of pesticides in the water body is derived by the runoff from the agricultural field and industrial wastewater. Despite the soil matrix that serves as a storage compartment of pesticide due to the high affinity of agrochemicals with soil, surface water resources like streams, estuaries and lakes, as well as the groundwater are susceptible to pesticide contamination because of the close interconnection of soil with water bodies. The low concentration of pesticides built up in water can get magnified through the food chain and enter aquatic organisms that are hazardous for human consumption [
2]. Importantly, chronic exposure to pesticides through water ingestion can mimic the human body’s hormones that reduce body immunity, interrupt hormone balance, trigger reproductive-related issues, posing carcinogenic effects and reduce intelligence particularly towards the children under the body development stage [
5].
This study reviewed the type of pesticide found in the water bodies, the sources of pesticide contamination, the fate and occurrence of pesticides in soil and water, the toxicity impacts on human health and the available treatment method of the pesticide-contaminated water. A recent study of pesticide-contaminated surface water that occurred in Tanjung Karang located at Kuala Selangor, Malaysia was also reviewed in this article.
3. Sources and Fate of Pesticides in Water
The detectable concentration of pesticide in surface waters and groundwater found in agricultural and urban land use areas [
12]. Pesticide moves into water bodies via point source and nonpoint source. Point source that originates from a fixed site including chemical runoff during improper storage, loading, disposal as well as the misapplication of pesticides to water bodies. A direct movement of pesticides into groundwater is a common type of point source pollution, in which the pesticides enter the water wells result from pesticide spills and improper disposal of pesticides. Urban use of insecticide is considered as a point source pesticide in surface waters. The non-point source is the movement of pesticides from large areas across the watersheds and eventually reached the water bodies over the time. Non-point sources of pesticide originate from the agricultural field initiate by the runoff and erosion events, leading to the gradual leaching of pesticides into the ground and surface water [
13].
Contamination of pesticides in water is caused by the persistent chemicals of pesticides released from agricultural activities, urban use and pesticide production factories. Farmers is the key users of pesticide that apply an enormous amount of pesticide to protect and increase crop yields. Besides, the wood treatment industry uses an enormous amount of insecticide to treat the raw material. Depending on the pesticide’s characteristics, chemical compounds from the pesticide applied to the preserved material tend to be released into the environment, becoming one of the sources of pesticide contamination in surface waters. Despite the great use of pesticides in the agricultural sectors, the urban use of pesticides mainly in-house gardening for pest control is an important source of pesticide contamination in water. The insecticide is detected more profoundly in urban settings than other types of pesticides such as herbicide and fungicide [
10]. Since the green revolution, increase consumption of pesticides led to the active production of pesticide formulations, which increase the pesticide manufacturer around the world [
6]. Inevitably pesticide leaching processes throughout the pesticide manufacturing processes as well as in the dumping site and wastewater effluents contribute to point-source pesticide contamination. To summarize, pesticides in surface waters sourced by the run-off event, atmospheric deposition event, wastewater discharge and spills event, while pesticide in groundwater sourced by the pesticide-treated field, waste disposal site and pesticide manufacturing sites.
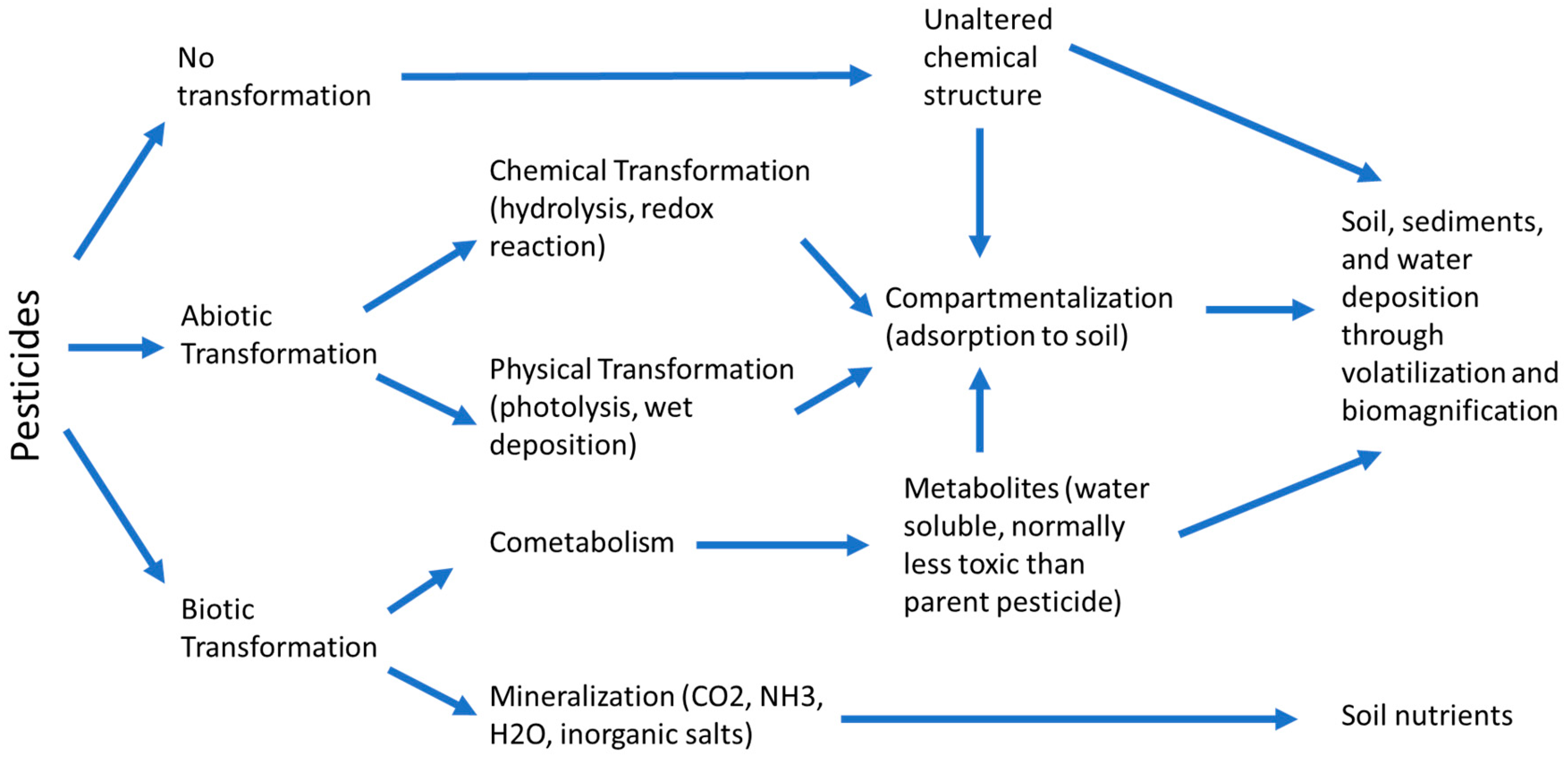
The study on the fate and transport of pesticides is important for knowing their circulation in the biosphere. Pesticides meet a variety of fates after being applied on Earth and
Figure 1 showed the general picture of their fates in the environment. The pesticide that is not taken up by plants will either be retained in the soil or subjected to degradation into other chemical forms. Soluble pesticides will be carried away by water molecules especially during precipitation events by percolating downward into the soil layers and eventually reaching the groundwater. Otherwise, those insoluble chemicals tightly bound to soil particles accumulate in the topsoil layer, which has a high possibility subjected to runoff and erosion to surface waters, contaminating lakes, stream and river with pesticides. Pesticides are most susceptible to runoff immediately after the application on the soil surface between 0.25 to 0.85 cm from the soil surface [
13]. Pesticide contamination in water also contributed by the volatilized pesticides in the atmosphere, in which they redeposited in the rain during the rainfall event and then enter the surface water bodies and soil. However, this pathway is relatively insignificant. In general, pesticides enter the hydrological system mainly via surface loss and leaching through soil layers, whereby the degree of pesticide contamination in water is affected by the properties of pesticide, characteristics of soil, site conditions, as well as the application and management practices of pesticide [
14].
The potential for surface loss and leaching into groundwater is determined by the characteristics of pesticides such as the half-life, solubility and adsorption capacity of the pesticides. Since most pesticides are organic compounds, they typically undergo degradation through microbial, photochemical or chemical reactions. Microbial degradation including the mineralization process in which pesticide breaks down into carbon dioxide and co-metabolization where microbial reaction transforms pesticide into other chemical forms. Photochemical degradation of pesticides is called photolysis in which the pesticides decomposed in the presence of ultraviolet (UV) light. Chemical degradation of pesticide occurs via redox reaction and hydrolysis with air, water and other compounds exist in soil compartments. Pesticides with a low biodegradation rate have a long half-life and tend to persist in the environment that potentially contaminate the water sources. Besides, pesticide degradation processes produce metabolites, inorganic end-product and transformants which can have either lower or higher toxicity than the parent pesticide. Moreover, the mobility of the pesticide is governed by the adsorption capacity and solubility of the pesticide. Pesticides that are strongly adsorbed to soil are less likely to infiltrate downward the soil profile but can easily be carried by eroded soil particles via surface runoff and eventually reached surface water [
14]. For pesticide having low degradation rate, weak adsorption capacity to soil particles and high solubility that is greater than 30 mg/L can potentially leach and dissolve in water. Among the pesticide used, atrazine which is normally used as an herbicide is recognized as a highly potential leach compound into the groundwater due to its high persistency. Cyanazine has a short half-life, therefore lower leaching potential. Methyl parathion is another low leaching potential pesticide because of its high adsorption capacity to soil particles and lower persistency. The 2,4-D is a water-soluble pesticide able to rapidly break down by biological action and therefore is less likely to accumulate in soil and has less persistency [
15].
4. Occurrence of Pesticide and Health Effect
The occurrence of pesticides in specific environmental compartments, such as in soils and streambed sediment, groundwater and surface water is a widespread issue [
16]. Distribution of a range of pesticides in streams and groundwater largely depends on the land-use settings and characteristics of the hydrologic system with consideration of the past and present use of pesticides. Pesticide detected most frequently in streams and groundwater were those in most use and with the compound characteristics of high mobility and persistence in the hydrologic system.
Based on the National Water-Quality Assessment (NAWQA), pesticides are found more often in surface waters than groundwater, being 25 pesticides detected more than 10% of the time in surface waters and 2% of the time in groundwater of various land-use setting in agricultural, urban and mixed land use [
12]. This proves that the occurrence of pesticides in surface waters is prevalent because of the direct and rapid overland mobilization of pesticides via surface runoff. Groundwater that is less vulnerable to pesticide contamination can be explained by the slow water infiltration rate through the soil into the aquifer. However, the extended travel time enables the pesticides to undergo transformation, dispersion and sorption that make contamination of groundwater more difficult to recover once it is contaminated. Of the 25 pesticides, 11 of them are herbicides that are widely applied in the agricultural field, 7 are herbicides used extensively in urban settings and 6 are insecticides applied in both agricultural and urban settings [
12]. In undeveloped areas, detected pesticide in surface water and shallow groundwater is least often. In mixed land-use settings, the frequency of pesticide occurrence detected at stream draining watershed is similar to agricultural or urban settings because of the contribution of pesticides from multiple sources. Similarly, the detection frequency in shallow groundwater is prevalent over the major aquifers. According to the investigation, the pesticides that occurred most frequently in the streams and groundwater are the five agricultural herbicides—atrazine with its degradate, deethylatrazine, metolachlor, cyanazine, alachlor and acetochlor, the five non-agricultural herbicides—simazine, prometon, tebuthiuron, 2,4-D and diuron, as well as the 3 most extensive use insecticide—diazinon, chlorpyrifos and carbaryl [
12]. For comparison, the insecticide was found more frequently in the urban stream than urban groundwater and also found in a higher concentration in comparison to agricultural settings.
Historical use of pesticides with their degradates and residues such as organochlorine is mostly found in soil, sediment and cell tissue of biota [
12]. Review of a largely agricultural country of China, the history used of organochlorine in agricultural activities led to the different level of pesticides contamination in the groundwater, which is mainly driven by the extreme hydrogeological condition. The leaching of pesticides as well as their metabolites downward from soil surface had contaminated the shallow basins in China [
17].
Despite the great importance of pesticides in maintaining good quality and protecting the crops or raw materials, they pose a high degree of concern in human health because of the tendency of pesticide to bioaccumulate in the human cell membrane which interrupts the body functioning system. Humans are exposed to pesticides in water mainly through dermal contact and ingestion [
18,
19]. Pesticide exposure has been proven to result in immunosuppression, hormone disruption, reduce intelligence, reproductive distortion and cancer. Impacts of pesticide exposure to humans can be categorized into acute health problems and chronic health problems. Chronic health problems encompass neurological effects such as onset Parkinson’s disease, reduce the attention span, memory disturbances, reproductive problems, disrupt infant development, birth defect and cancer. Acute health effects depend on the pesticide toxicity and the most common effects are reduced vision, headaches, salivation, diarrhea, nausea, vomiting, wheezing, coma and even death. Moderate pesticide poisoning leads to mimic intrinsic asthma, bronchitis and gastroenteritis [
18].
In Malaysia, there is limited data that documented the effects of pesticides on human health. The study on utilizing biological markers to associate the effects of pesticide exposure to human health would be useful to evaluate the health risk [
19]. A reviewed study by Samsuddin et al. documented that populations that are chronically exposed to low dose mix-pesticide are more likely to have cardiovascular diseases [
20]. Another article revealed that the endosulfan led to the overexpression of stromelysins, a protein from the metalloproteinase family, which in turn degenerated the proteins involved in atherosclerosis progression [
21]. Besides, few works have reviewed whether pesticide exposure could reduce semen quality, lower the sperm count and change the sperm’s morphology [
21,
22]. Literature also reported that farmers had a high chance of inducing prostate cancer and allergic or non-allergic asthma due to the frequent exposure to the chlorinated pesticide [
23,
24]. In animal studies, the genotoxicity effect of exposing organophosphate to orang-asli children was studied, which is observed through the changes in comet tail length [
25,
26]. Other animal study also found that rats exposed to organophosphate resulted in testosterone and hormone disruption in the testis [
27].
As a measure to protect public health, guideline levels for pesticides in drinking water have been implemented by national governments. There are several guideline values, where few of them are issued by World Health Organization (WHO), the United States, Australia, the European Union and Japan. The guideline values may differ based on the socio-economical, dietary, geographical condition and industrial conditions [
28].
Table 3 showed the guideline value for a certain number of pesticides in drinking water issued by WHO aimed for a water quality that is suitable for long-term consumption. These guideline values were made available for the use of regulatory authorities.
5. Pesticide-Contaminated Water Treatment Method—Advanced Oxidation Processes
The conventional method of pesticide treatment processes encompasses coagulation-flocculation, adsorption, filtration and sedimentation, which rely on the phase transfer of pollutants. Those methods are often incurred with a relatively high operational cost and may cause secondary pollution such as sludge formation [
29]. Besides, indiscriminate use and the presence of a wide range of pesticide formulation available around the globe make the compound of pesticide in water harder to be removed. Therefore, alternative treatment processes are required to seek a long-term and feasible method to treat pesticide-contaminated water.
Advanced oxidation processes (AOPs) are recognized as clean technologies for the treatment of water containing recalcitrant and bio-refractory pollutants such as pesticides. It has been adopted as recent water purification technology because of the thermodynamic viability and broad spectrum of applicability [
29,
30]. The main concept of AOPs in the water treatment process is based on the in-situ generation of highly reactive hydroxyl radicals that indiscriminately oxidize a wide range of recalcitrant organic pollutant for complete mineralization of organic contaminants to carbon dioxide, water and mineral salts and capable of transforming the compound of pesticide into more biodegradable species [
31,
32]. Hydroxyl radicals can be produced from different pathways using a combination of oxidants, catalysts and ultraviolet irradiation and this makes the classification of AOPs based on the source of generation of hydroxyl (OH) radicals [
33].
Table 4 showed some of the combinations of AOPs.
The integration of several AOPs into a sequence of complimentary water treatment processes is a common method to yields a more biodegradable effluent which can be further treated by the conventional biological process to reduce the reagent consumption and thus more economical in comparison to AOPs alone [
32]. Based on previous studies, AOPs are recommended as pre-treatment steps to convert the pesticide to a more biodegradable intermediates, then followed by a biological treatment process to convert them into biomass, carbon dioxide, hydrochloric acid, biogas and water. This is mainly due to the inefficient removal of bulk chemical oxygen demand (COD) by AOPs to a level below the regulation standard for some recalcitrant pesticide compounds [
29,
34,
35]. An article by Quiroz et al. also documented the extensive use of AOPs to treat pesticide-contaminated water [
33].
In the field of AOPs, Fenton reactions have been widely studied for the remediation of pesticide-contaminated water because of the faster rate of pollutant removal and its ability to completely mineralized a wide variety of organic compounds [
32,
36]. In the Fenton process, Fenton’s reagent, which is prepared by adding iron salts as a catalyst in the hydrogen peroxide solution to generate strong hydroxyl radicals at an acidic condition. The general mechanisms of the Fenton process are shown in
Table 5. It is worth noting that the depletion rate of Fe
2+ is comparatively higher than the regeneration rate of Fe
2+ from Fe
3+ as illustrated in
Table 5 Reaction 2. Because of this, the addition of Fe
2+ or FeSO
4 in the medium presence with hydrogen peroxide is necessary for continuous Fenton reaction to take place, which is not economical. Moreover, adding more Fe
2+ result in more iron sludge produced that need to be handle in a proper way to avoid secondary pollution [
37]. The drawbacks can be overcome by including the ultraviolet irradiation in the Fenton system, which is commonly known as the photo-Fenton process. The presence of UV visible irradiation with Fenton reagent enhance the process efficiency because of the capability to produce an additional source of hydroxyl radicals through photolysis of hydrogen peroxide and the photoreduction of Fe
3+ to Fe
2+ as illustrated in
Table 5 Reaction 3, which subsequently increase the hydroxyl radicals yields and reduce the amount of Fe
2+ required in Fenton reaction [
38]. The optimum condition of the Fenton system has been studied which focusing on the pH, Fe
2+ dose and the H
2O
2 dose [
31]. From the study, the pH of the system needs to be kept at 3, because this is the optimum condition for the decomposition of hydrogen peroxide to produce hydrogen radicals and prevent the scavenging of hydroxyl radicals via dissociation and auto-decomposition of hydrogen peroxide as illustrated in
Table 5 Reaction 5. Also, a lower pH value that is below or equal to 3 inhibits the occurrence of iron precipitation which enhances the UV radiation transmission into water. The hydrogen peroxide dosage depends on the COD of water, where the optimum COD: H
2O
2 ratio is 1:2.2 and 1:4.4 for photo-Fenton and Fenton treatment, respectively. For the Fe
2+ dosage, the optimum H
2O
2:Fe
2+ ratio is 50:1 and 100:1 for photo-Fenton and Fenton treatment, respectively [
31]. As can be seen, photo-Fenton treatment reduced the consumption of H
2O
2 and Fe
2+ to half in comparison to the Fenton process, which proved that the photo-Fenton process is more economical, more efficient in removing pollutants and produce less sludge. Another literature study by Oller et al. reported that the photo-Fenton reaction successfully eliminated six targeted water-soluble pesticides that are ineffectively removed by the TiO
2 catalytic process [
39]. However, the major drawbacks of the photo-Fenton process are the periodic addition of hydrogen peroxide that increase the operational cost and the use of UV visible light sources [
38].
Moreover, heterogeneous photocatalysis is another appealing option to restore water contaminated with pesticide, that involves the use of solid photocatalyst to form a colloidal suspension under sunlight radiation to remove toxic substance in water [
33]. The heterogeneous photocatalytic degradation has been proved to be able to remove a wide range of pesticides and the common pesticides being tested are triazine, thiocarbamide, phosphorated and chlorinated pesticide [
33]. The solid catalyst act as an active site for adsorption of reactants and desorption of products. TiO
2 is the most common semiconductor used as a photocatalyst because of the cost-effectiveness, high stability, non-toxicity and unique photocatalytic efficiency [
40].
Table 6 summarized the reaction mechanism involves in the heterogeneous photocatalysis reaction. Because TiO
2 has a bandgap energy of 3.2 V, the adsorption of UV light that is equal or greater than the bandgap energy of the semiconductor is required to activate or induce the charge separation. After excitation, the electron will move from valence band to conduction band, forming electron-hole pairs that act as electron donors or acceptors for the molecules that are in contact with the semiconductor [
41]. On the surface of the semiconductor, the generated electron-hole pairs undergo redox reactions in the aqueous solution to produce hydroxyl radicals [
40]. The reactive radicals will then attack the pollutants in the solution. This process gradually decomposes the contaminant, avoiding the residue and sludge production, thereby reducing the probability of secondary pollution. Besides, the catalyst remains unchanged during operation, therefore no consumable chemicals are required. Moreover, the contaminant is strongly adsorbed to the surface of the solid catalyst which make the photocatalytic process capable of removing contaminants effectively even at a very low concentration of contaminants in solution and this saves the water production cost [
41]. However, this process relies on the UV absorption for activation and only 5% of solar irradiation falls within the UV range, resulting in lower production efficiency of reactive radicals. The study to improve the performance of TiO
2 has been studied extensively, which is through the doping of TiO
2 with foreign ions to narrow the TiO
2 bandgap energy, thereby facilitates the generation of reactive radicals under visible light [
38].