Does Last Year’s Cost Predict the Present Cost? An Application of Machine Leaning for the Japanese Area-Basis Public Health Insurance Database
Abstract
1. Introduction
2. Materials and Methods
2.1. Setting
2.2. Database
2.3. Statistical Modeling
2.3.1. Generalized Linear Model and Zero-Inflated Model
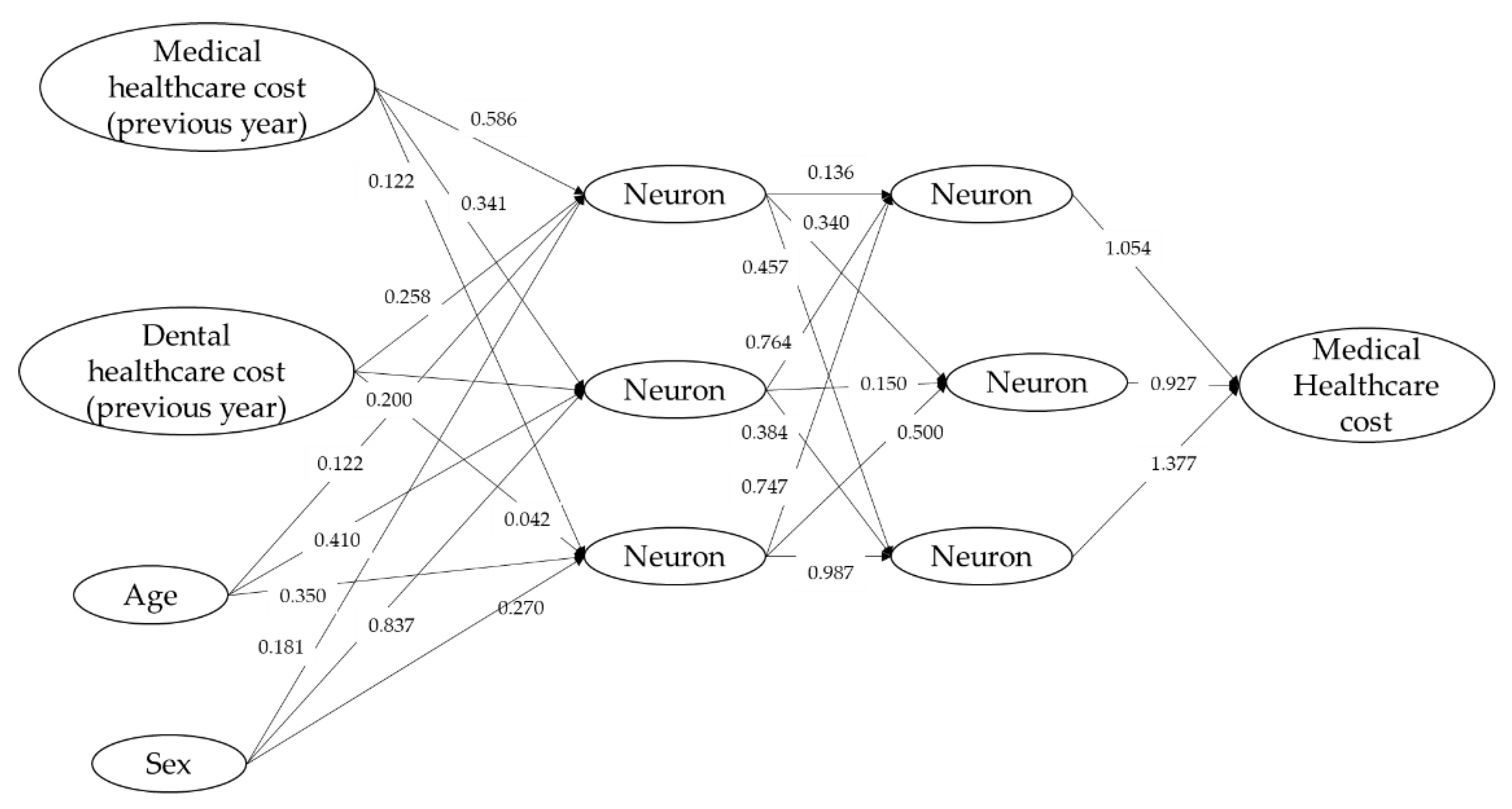
2.3.2. Neural Network Model
2.3.3. Support Vector Machine Regression
2.3.4. Generalized Boosted Regression Models
2.4. Ethics
3. Results
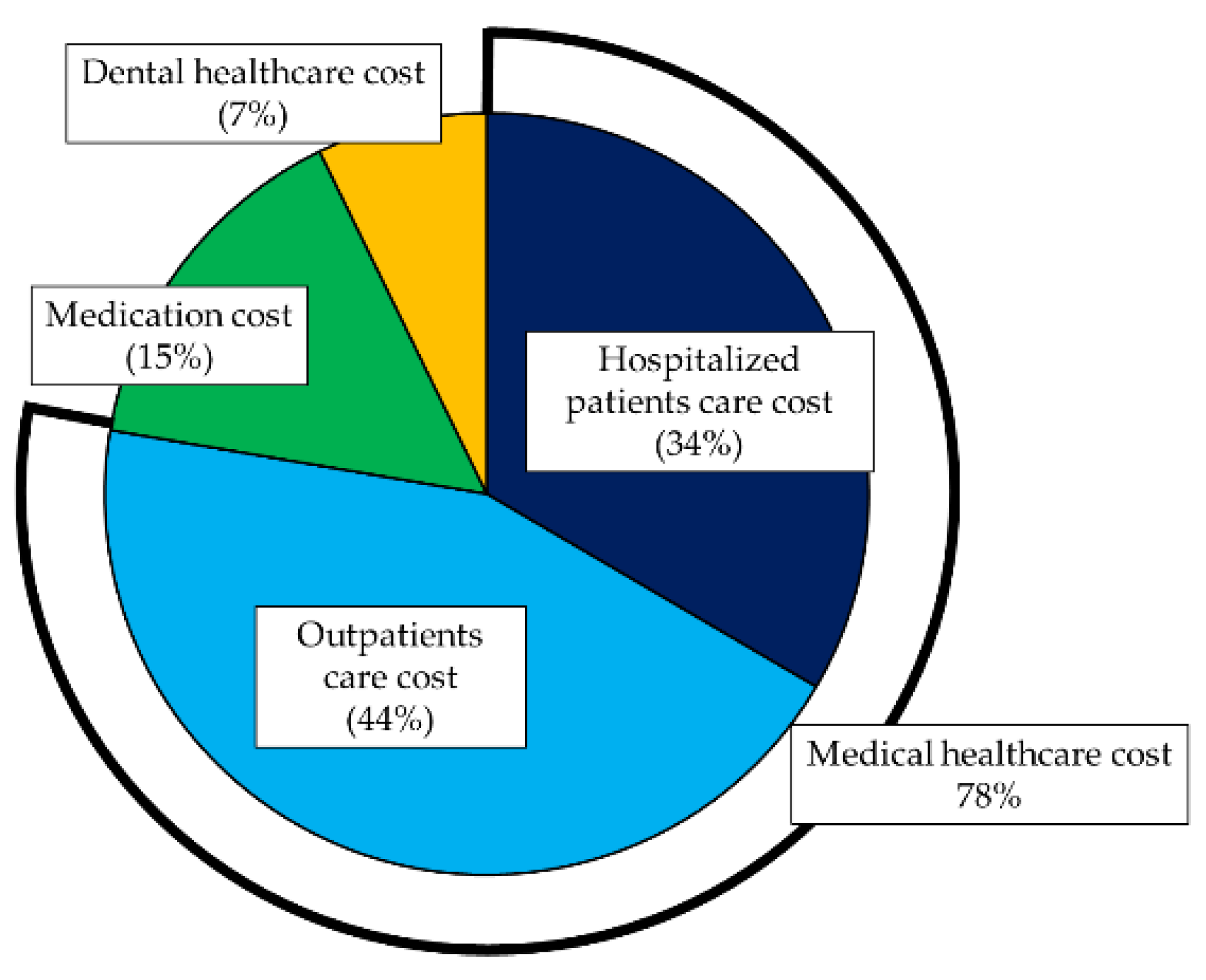
3.1. Descriptive Statistics of the Healthcare Cost
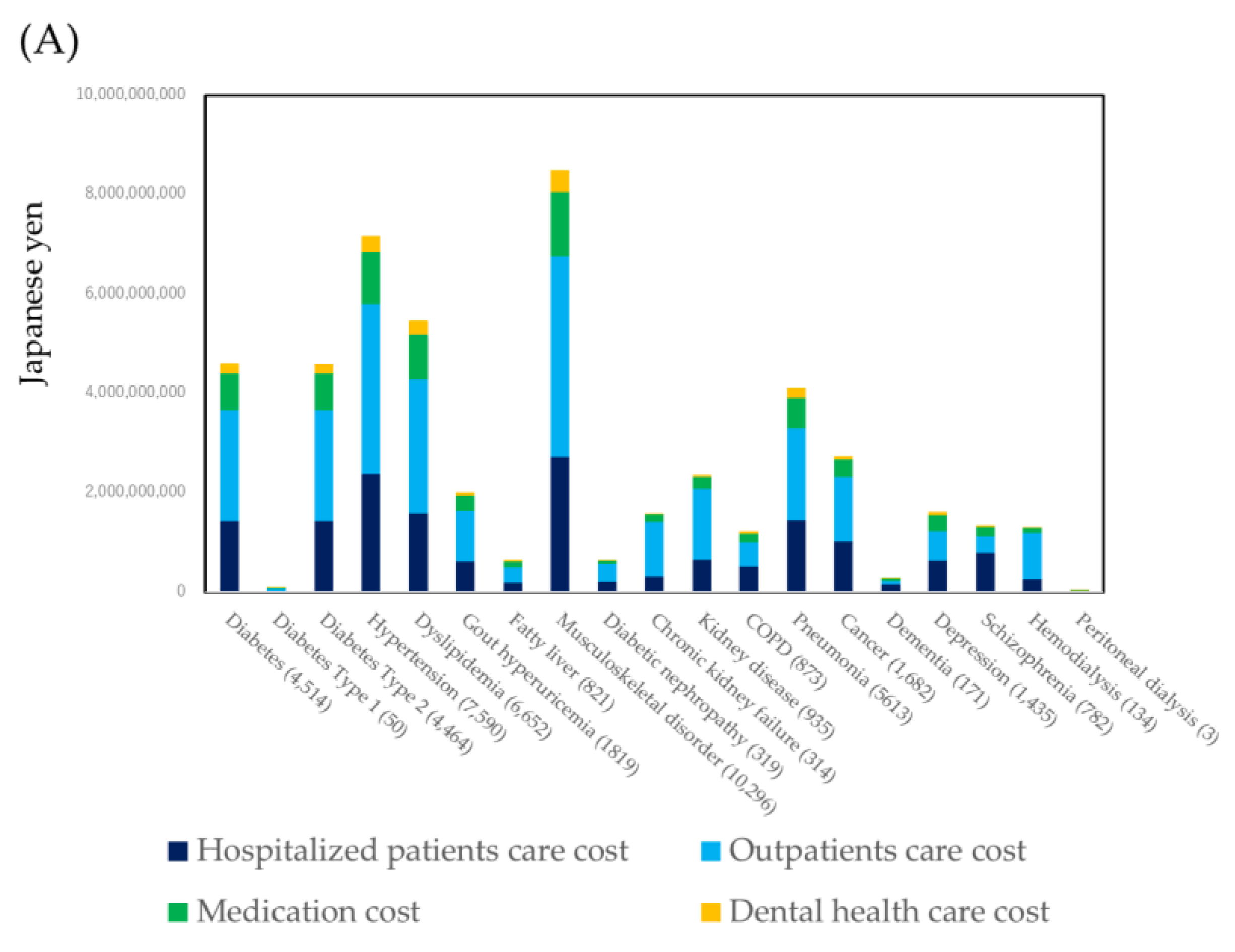
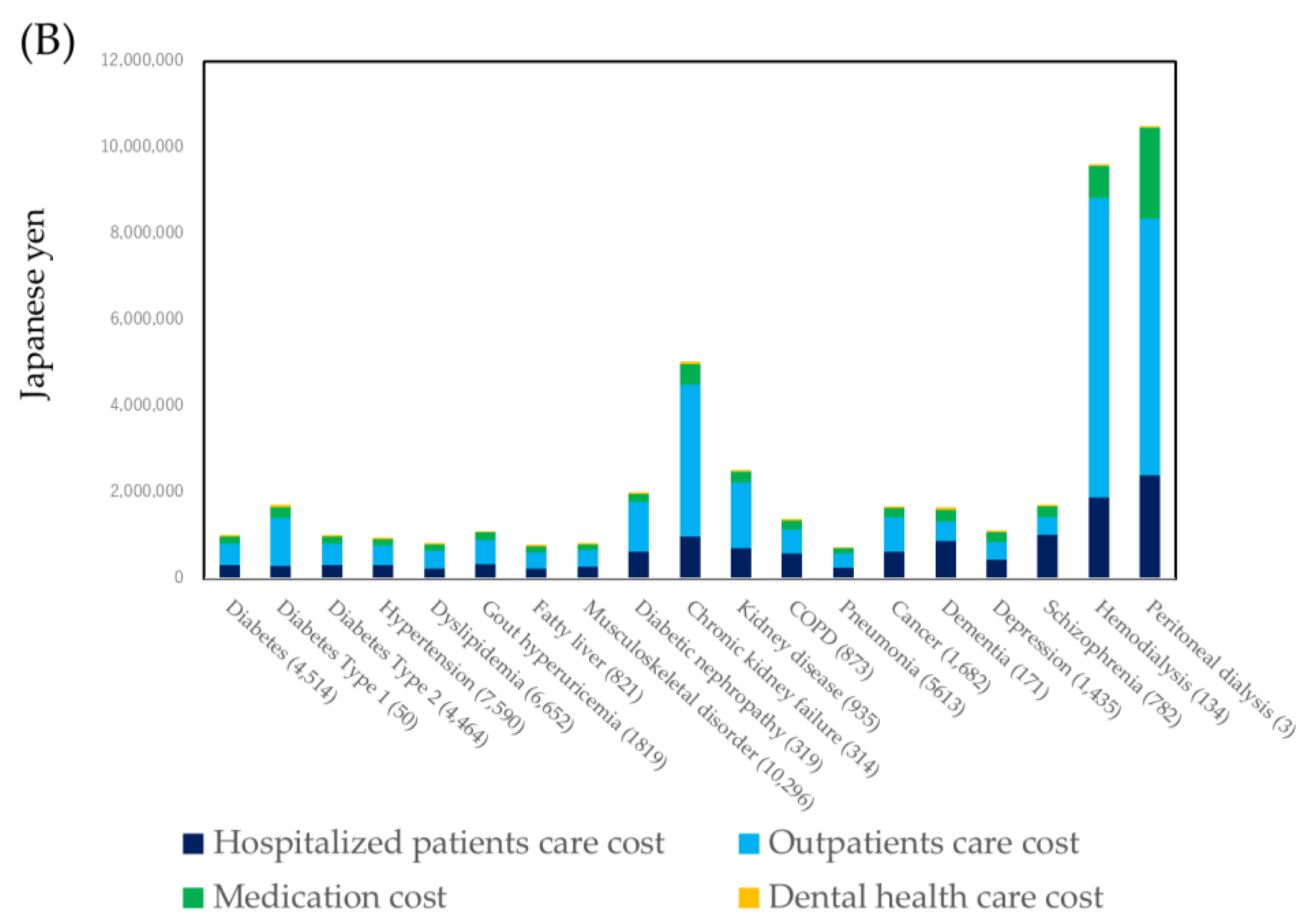
3.2. Healthcare Cost by Specific Diseases
3.3. Prediction of Medical Healthcare Cost
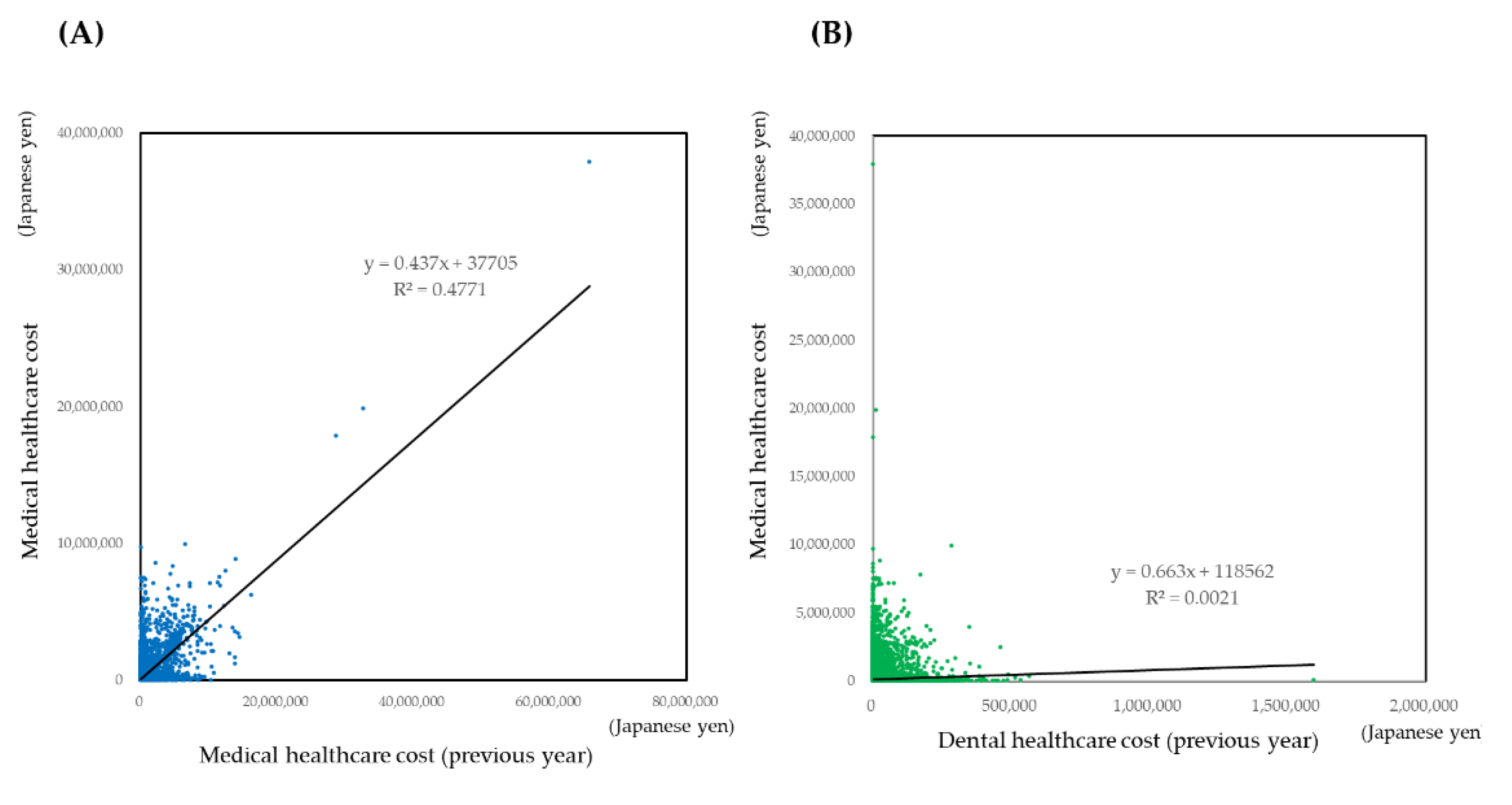
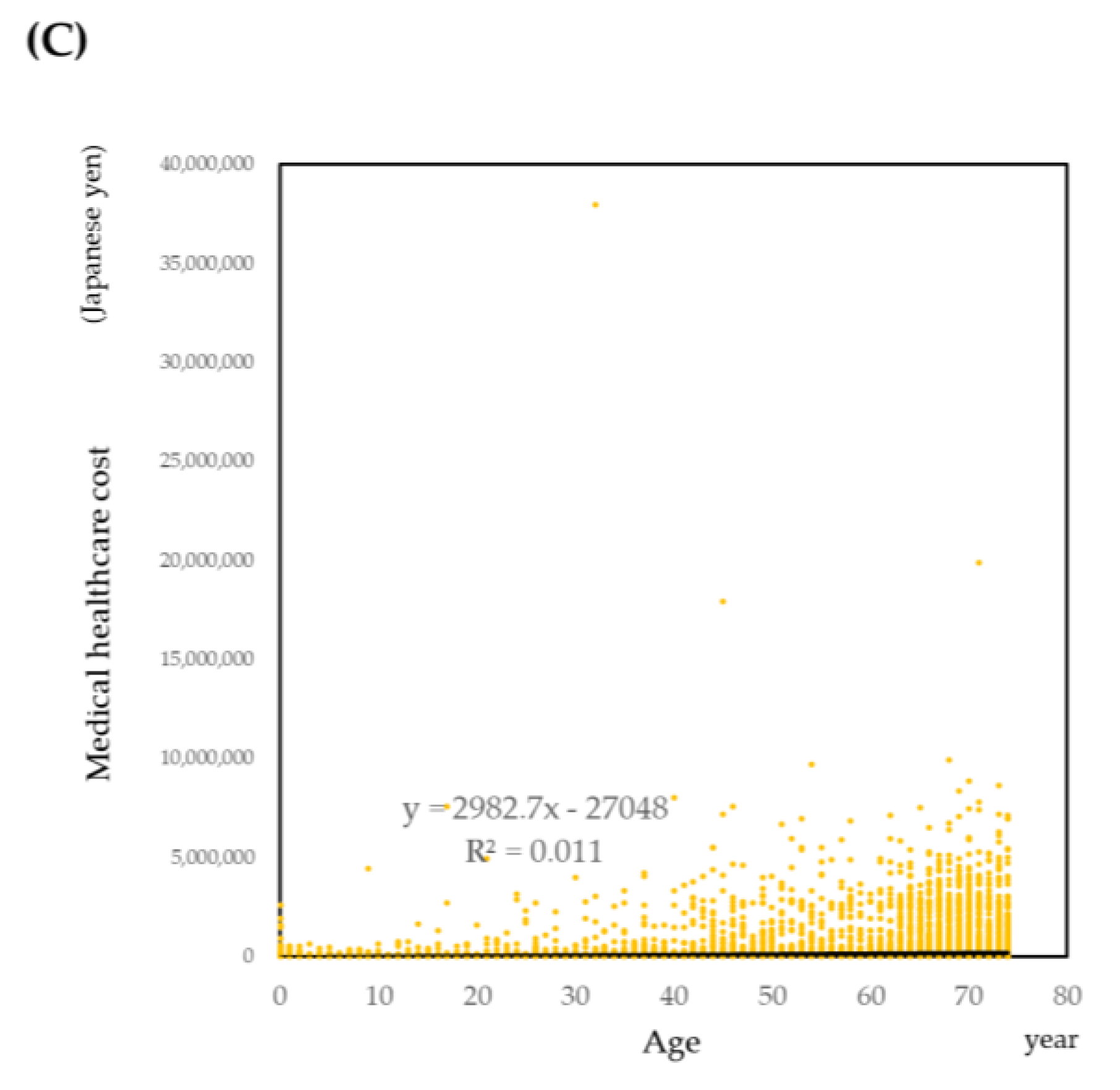
3.3.1. Descriptive Analysis
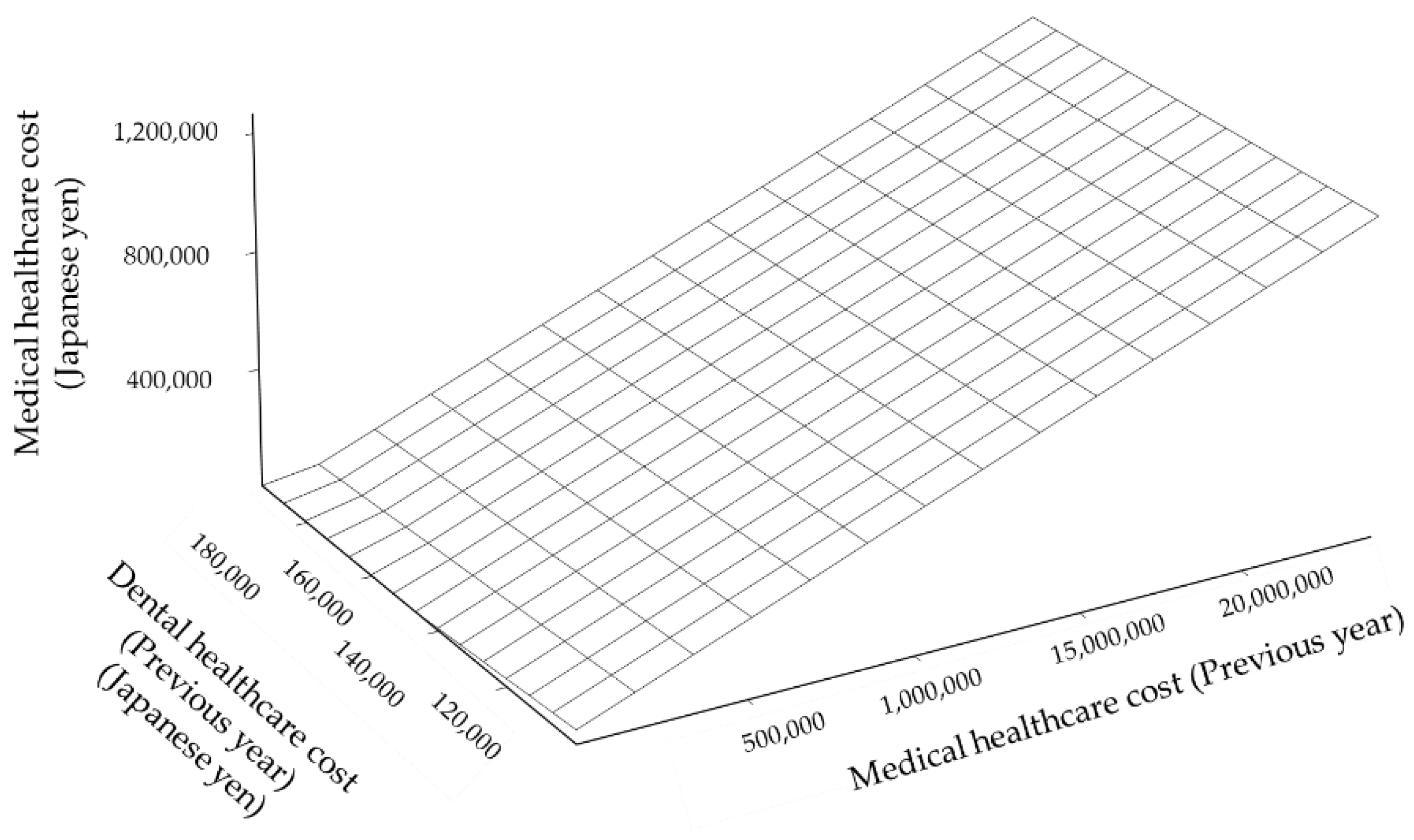
3.3.2. Prediction of Medical Healthcare Cost by Regression Models
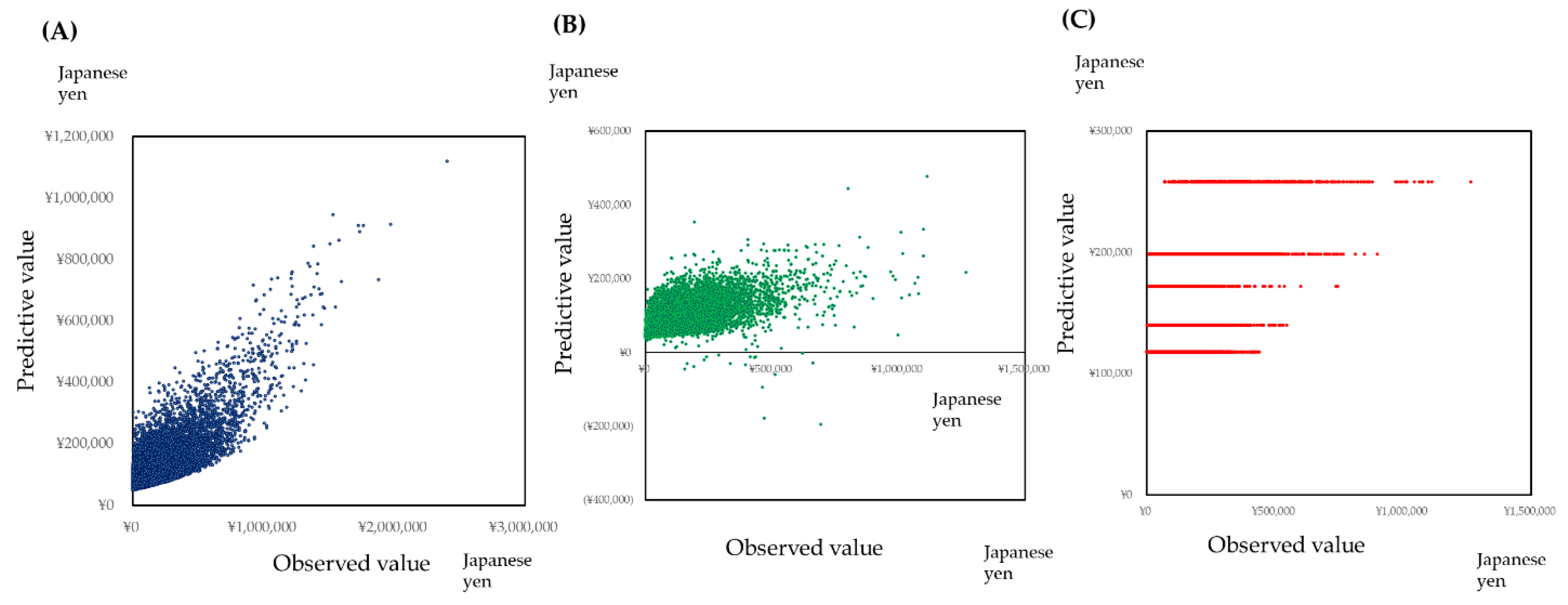
3.3.3. Prediction of Medical Healthcare Cost by the Neural Network Model, Support Vector Machine Regression, and Generalized Boosted Regression Modeling (GBM)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Estimates of National Medical Care Expenditure, FY 2017. Available online: https://www.mhlw.go.jp/english/database/db-hss/enmce_2017.html (accessed on 14 October 2020).
- Romandini, M.; Baima, G.; Antonoglou, G.; Bueno, J.; Figuero, E.; Sanz, M. Periodontitis, Edentulism, and Risk of Mortality: A Systematic Review with Meta-analyses. J. Dent. Res. 2020. [Google Scholar] [CrossRef]
- Gobin, R.; Tian, D.; Liu, Q.; Wang, J. Periodontal Diseases and the Risk of Metabolic Syndrome: An Updated Systematic Review and Meta-Analysis. Front. Endocrinol. 2020, 11, 336. [Google Scholar] [CrossRef] [PubMed]
- Orlandi, M.; Graziani, F.; D’Aiuto, F. Periodontal therapy and cardiovascular risk. Periodontology 2000 2020, 83, 107–124. [Google Scholar] [CrossRef] [PubMed]
- Jepsen, S.; Suvan, J.; Deschner, J. The association of periodontal diseases with metabolic syndrome and obesity. Periodontology 2000 2020, 83, 125–153. [Google Scholar] [CrossRef] [PubMed]
- Genco, R.J.; Sanz, M. Clinical and public health implications of periodontal and systemic diseases: An overview. Periodontology 2000 2020, 83, 7–13. [Google Scholar] [CrossRef]
- Sanz, M.; Castillo, M.D.A.; Jepsen, S.; Juanatey, G.J.R.; D’Aiuto, F.; Bouchard, P.; Chapple, I.; Dietrich, T.; Gotsman, I.; Graziani, F.; et al. Periodontitis and cardiovascular diseases: Consensus report. J. Clin. Periodontol. 2020, 47, 268–288. [Google Scholar] [CrossRef]
- Bourgeois, D.; Inquimbert, C.; Ottolenghi, L.; Carrouel, F. Periodontal Pathogens as Risk Factors of Cardiovascular Diseases, Diabetes, Rheumatoid Arthritis, Cancer, and Chronic Obstructive Pulmonary Disease-Is There Cause for Consideration? Microorganisms 2019, 7, 424. [Google Scholar] [CrossRef]
- Peres, M.A.; Macpherson, L.M.D.; Weyant, R.J.; Daly, B.; Venturelli, R.; Mathur, M.R.; Listl, S.; Celeste, R.K.; Herreño, G.C.C.; Kearns, C.; et al. Oral diseases: A global public health challenge. Lancet 2019, 394, 249–260. [Google Scholar] [CrossRef]
- Sanz, M.; Ceriello, A.; Buysschaert, M.; Chapple, I.; Demmer, R.T.; Graziani, F.; Herrera, D.; Jepsen, S.; Lione, L.; Madianos, P.; et al. Scientific evidence on the links between periodontal diseases and diabetes: Consensus report and guidelines of the joint workshop on periodontal diseases and diabetes by the International Diabetes Federation and the European Federation of Periodontology. J. Clin. Periodontol. 2018, 45, 138–149. [Google Scholar] [CrossRef]
- Cardoso, E.M.; Reis, C.; Céspedes, M.M.C. Chronic periodontitis, inflammatory cytokines, and interrelationship with other chronic diseases. Postgrad. Med. 2018, 130, 98–104. [Google Scholar] [CrossRef]
- Nomura, Y.; Kakuta, E.; Okada, A.; Otsuka, R.; Shimada, M.; Tomizawa, Y.; Taguchi, C.; Arikawa, K.; Daikoku, H.; Sato, T.; et al. Impact of the Serum Level of Albumin and Self-Assessed Chewing Ability on Mortality, QOL, and ADLs for Community-Dwelling Older Adults at the Age of 85: A 15 Year Follow up Study. Nutrients 2020, 12, 3315. [Google Scholar] [CrossRef]
- Nomura, Y.; Kakuta, E.; Okada, A.; Otsuka, R.; Shimada, M.; Tomizawa, Y.; Taguchi, C.; Arikawa, K.; Daikoku, H.; Sato, T.; et al. Effects of self-assessed chewing ability, tooth loss and serum albumin on mortality in 80-year-old individuals: A 20-year follow-up study. BMC Oral Health 2020, 20, 122. [Google Scholar] [CrossRef] [PubMed]
- Kakuta, E.; Nomura, Y.; Naono, Y.; Koresawa, K.; Shimizu, K.; Hanada, N. Correlation between health-care costs and salivary tests. Int. Dent. J. 2013, 63, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Nomura, Y.; Sato, T.; Kamoshida, Y.; Suzuki, S.; Okada, A.; Otsuka, R.; Kakuta, E.; Hanada, N. Prediction of Health Care Costs by Dental Health Care Costs and Periodontal Status. Appl. Sci. 2020, 10, 3140. [Google Scholar] [CrossRef]
- Ebina City Official Website. Available online: https://www.city.ebina.kanagawa.jp/shisei/denshi/toukei/jinko/1009539.html (accessed on 14 October 2020).
- Nomura, Y.; Okada, A.; Kakuta, E.; Otsuka, R.; Saito, H.; Maekawa, H.; Daikoku, H.; Hanada, N.; Sato, T. Workforce and Contents of Home Dental Care in Japanese Insurance System. Int. J. Dent. 2020, 2020, 7316796. [Google Scholar] [CrossRef]
- Otsuka, R.; Nomura, Y.; Okada, A.; Uematsu, H.; Nakano, M.; Hikiji, K.; Hanada, N.; Momoi, Y. Properties of manual toothbrush that influence on plaque removal of interproximal surface in vitro. J. Dent. Sci. 2020, 15, 14–21. [Google Scholar] [CrossRef]
- Morid, M.A.; Sheng, O.R.L.; Kawamoto, K.; Ault, T.; Dorius, J.; Abdelrahman, S. Healthcare cost prediction: Leveraging fine-grain temporal patterns. J. Biomed. Inform. 2019, 91, 103113. [Google Scholar] [CrossRef]
- Morid, M.A.; Sheng, O.R.L.; Kawamoto, K.; Abdelrahman, S. Learning hidden patterns from patient multivariate time series data using convolutional neural networks: A case study of healthcare cost prediction. J. Biomed. Inform. 2020, 111, 103565. [Google Scholar] [CrossRef]
- Mazumdar, M.; Lin, J.-Y.J.; Zhang, W.; Li, L.; Liu, M.; Dharmarajan, K.V.; Sanderson, M.; Isola, L.; Hu, L. Comparison of statistical and machine learning models for healthcare cost data: A simulation study motivated by Oncology Care Model (OCM) data. BMC Health Serv. Res. 2020, 20, 350. [Google Scholar] [CrossRef]
- Orueta, J.F.; Alvarez, G.A.; Aurrekoetxea, J.J.; Goñi, G.M. FINGER (Forming and Identifying New Groups of Expected Risks): Developing and validating a new predictive model to identify patients with high healthcare cost and at risk of admission. BMJ Open 2018, 8, e019830. [Google Scholar] [CrossRef]
- Jones, A.M.; Lomas, J.; Rice, N. Healthcare Cost Regressions: Going Beyond the Mean to Estimate the Full Distribution. Health Econ. 2015, 24, 1192–1212. [Google Scholar] [CrossRef] [PubMed]
- Otani, K.; Baden, W.W. Healthcare cost and predictive factors: High- and low-utilization model development. Health Mark. Q. 2009, 26, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Topics of Ministry Health, Labor and Welfare. Available online: https://www.mhlw.go.jp/topics/2012/02/dl/tp120205-1-13.pdf (accessed on 14 October 2020).
- Nguyen, A.T.M.; Akhter, R.; Garde, S.; Scott, C.; Twigg, S.M.; Colagiuri, S.; Ajwani, S.; Eberhard, J. The association of periodontal disease with the complications of diabetes mellitus. A systematic review. Diabetes Res. Clin. Pract. 2020, 165, 108244. [Google Scholar] [CrossRef] [PubMed]
- Iwashima, Y.; Kokubo, Y.; Ono, T.; Yoshimuta, Y.; Kida, M.; Kosaka, T.; Maeda, Y.; Kawano, Y.; Miyamoto, Y. Additive interaction of oral health disorders on risk of hypertension in a Japanese urban population: The Suita Study. Am. J. Hypertens. 2014, 27, 710–719. [Google Scholar] [CrossRef]
- Nimma, V.; Talla, H.; Poosa, M.; Gopaladas, M.; Meesala, D.; Jayanth, L. Influence of Hypertension on pH of Saliva and Flow Rate in Elder Adults Correlating with Oral Health Status. J. Clin. Diagn. Res. 2016, 10, ZC34–ZC36. [Google Scholar] [CrossRef]
- Sarmento, D.J.D.S.; Caliento, R.; Maciel, R.F.; Braz-Silva, P.H.; Pestana, J.O.M.D.A.; Lockhart, P.B.; Gallottini, M. Poor oral health status and short-term outcome of kidney transplantation. Spec. Care Dent. 2020, 40, 549–554. [Google Scholar] [CrossRef]
- Wallace, K.; Shafique, S.; Piamjariyakul, U. The relationship between oral health and hemodialysis treatment among adults with chronic kidney disease: A systematic review. Nephrol. Nurs. J. 2019, 46, 375–394. [Google Scholar]
- Nomura, Y.; Takei, N.; Ishii, T.; Takada, K.; Amitani, Y.; Koganezawa, H.; Fukuhara, S.; Asai, K.; Uozumi, R.; Bessho, K. Factors that affect oral care outcomes for institutionalized elderly. Int. J. Dent. 2018, 2018, 2478408. [Google Scholar] [CrossRef]
- Scannapieco, F.A.; Shay, K. Oral health disparities in older adults: Oral bacteria, inflammation, and aspiration pneumonia. Dent. Clin. North Am. 2014, 58, 771–782. [Google Scholar] [CrossRef]
- Son, M.; Jo, S.; Lee, J.S.; Lee, D.H. Association between oral health and incidence of pneumonia: A population-based cohort study from Korea. Sci. Rep. 2020, 10, 9576. [Google Scholar] [CrossRef]
- Schwendicke, F.; Stolpe, M.; Müller, F. Professional oral health care for preventing nursing home-acquired pneumonia: A cost-effectiveness and value of information analysis. J. Clin. Periodontol. 2017, 44, 1236–1244. [Google Scholar] [CrossRef] [PubMed]
- Haghighi, A.; Shafipour, V.; Nesami, B.M.; Baradari, G.A.; Charati, Y.J. The impact of oral care on oral health status and prevention of ventilator-associated pneumonia in critically ill patients. Aust. Crit. Care 2017, 30, 69–73. [Google Scholar] [CrossRef] [PubMed]
| Independent Variable | Generalized Linear Model | Zero-Inflated Model | ||
| Zero-Inflation Model (Binomial, Link: Log-log) | ||||
| Estimate | p-Value | |||
| (Intercept) | 0.736 | <0.001 | ||
| Age | −0.0002 | 0.737 | ||
| Sex (Women/Men) | −0.140 | <0.001 | ||
| Medical healthcare cost of the previous year | −0.259 | <0.001 | ||
| Dental healthcare cost of the previous year | −0.265 | <0.001 | ||
| Count model (Poisson, link: Log) | ||||
| Estimate | p-value | Estimate | p-value | |
| (Intercept) | 0.374 | <0.001 | 1.390 | <0.001 |
| Age | 0.004 | <0.001 | 0.004 | <0.001 |
| Sex (Women/Men) | 0.031 | <0.001 | −0.023 | <0.001 |
| Medical healthcare cost of the previous year | 0.114 | <0.001 | 0.058 | <0.001 |
| Dental healthcare cost of the previous year | 0.077 | <0.001 | −0.003 | 0.462 |
| AIC | 178,481 | 130,725 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nomura, Y.; Ishii, Y.; Chiba, Y.; Suzuki, S.; Suzuki, A.; Suzuki, S.; Morita, K.; Tanabe, J.; Yamakawa, K.; Ishiwata, Y.; et al. Does Last Year’s Cost Predict the Present Cost? An Application of Machine Leaning for the Japanese Area-Basis Public Health Insurance Database. Int. J. Environ. Res. Public Health 2021, 18, 565. https://doi.org/10.3390/ijerph18020565
Nomura Y, Ishii Y, Chiba Y, Suzuki S, Suzuki A, Suzuki S, Morita K, Tanabe J, Yamakawa K, Ishiwata Y, et al. Does Last Year’s Cost Predict the Present Cost? An Application of Machine Leaning for the Japanese Area-Basis Public Health Insurance Database. International Journal of Environmental Research and Public Health. 2021; 18(2):565. https://doi.org/10.3390/ijerph18020565
Chicago/Turabian StyleNomura, Yoshiaki, Yoshimasa Ishii, Yota Chiba, Shunsuke Suzuki, Akira Suzuki, Senichi Suzuki, Kenji Morita, Joji Tanabe, Koji Yamakawa, Yasuo Ishiwata, and et al. 2021. "Does Last Year’s Cost Predict the Present Cost? An Application of Machine Leaning for the Japanese Area-Basis Public Health Insurance Database" International Journal of Environmental Research and Public Health 18, no. 2: 565. https://doi.org/10.3390/ijerph18020565
APA StyleNomura, Y., Ishii, Y., Chiba, Y., Suzuki, S., Suzuki, A., Suzuki, S., Morita, K., Tanabe, J., Yamakawa, K., Ishiwata, Y., Ishikawa, M., Sogabe, K., Kakuta, E., Okada, A., Otsuka, R., & Hanada, N. (2021). Does Last Year’s Cost Predict the Present Cost? An Application of Machine Leaning for the Japanese Area-Basis Public Health Insurance Database. International Journal of Environmental Research and Public Health, 18(2), 565. https://doi.org/10.3390/ijerph18020565






