Process Simulation and Environmental Aspects of Dimethyl Ether Production from Digestate-Derived Syngas
Abstract
:1. Introduction
CO2 + 3 H2= CH3OH + H2O
CO + H2O = CO2 + H2
2CH3OH = CH3OCH3 + H2O
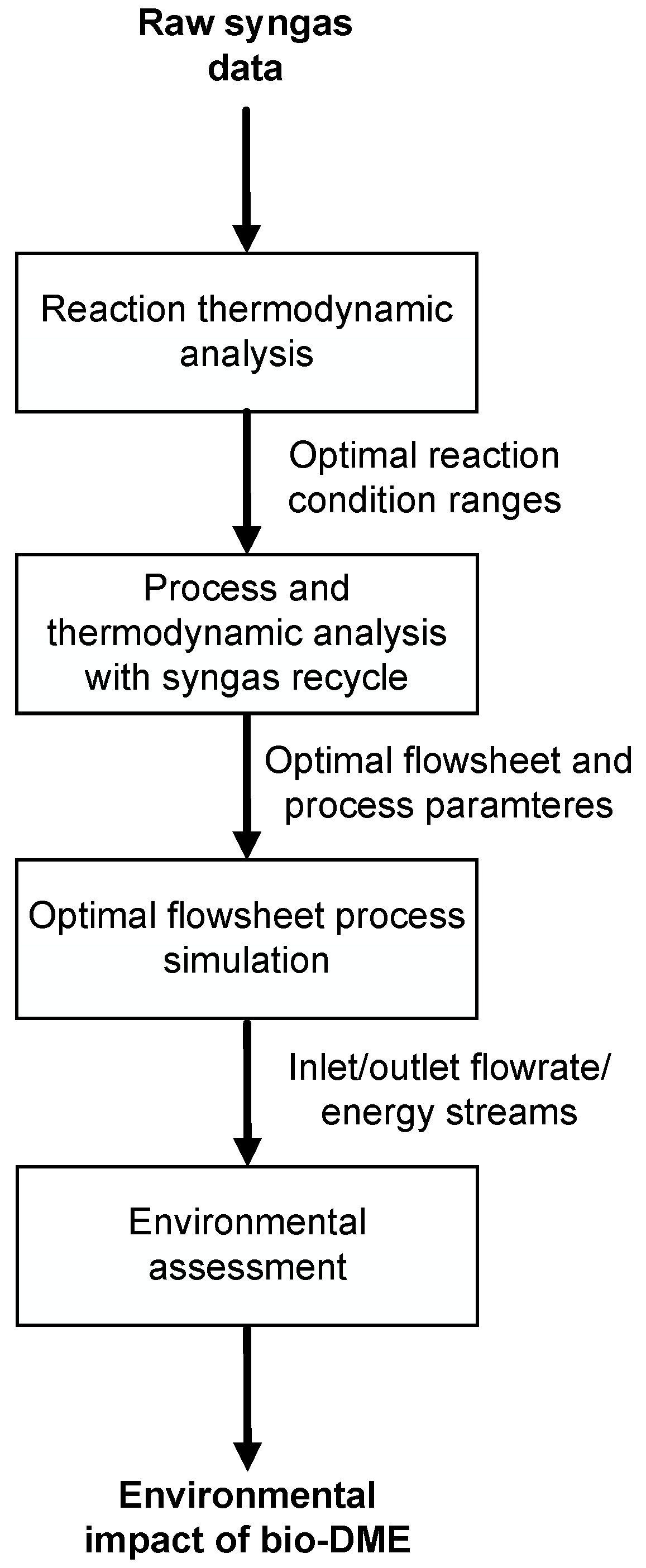
2. Materials and Methods
- (1)
- Thermodynamic analysis to know how the syngas composition, temperature and pressure had an impact on thermodynamic equilibrium;
- (2)
- Thermodynamic and process analysis to individuate the best combination of process parameters (detailed list is shown in Table 1) maximizing the total yield to DME;
- (3)
- Process simulation and evaluation of main process streams and utility consumptions;
- (4)
- Environmental impact analysis of bio-DME.
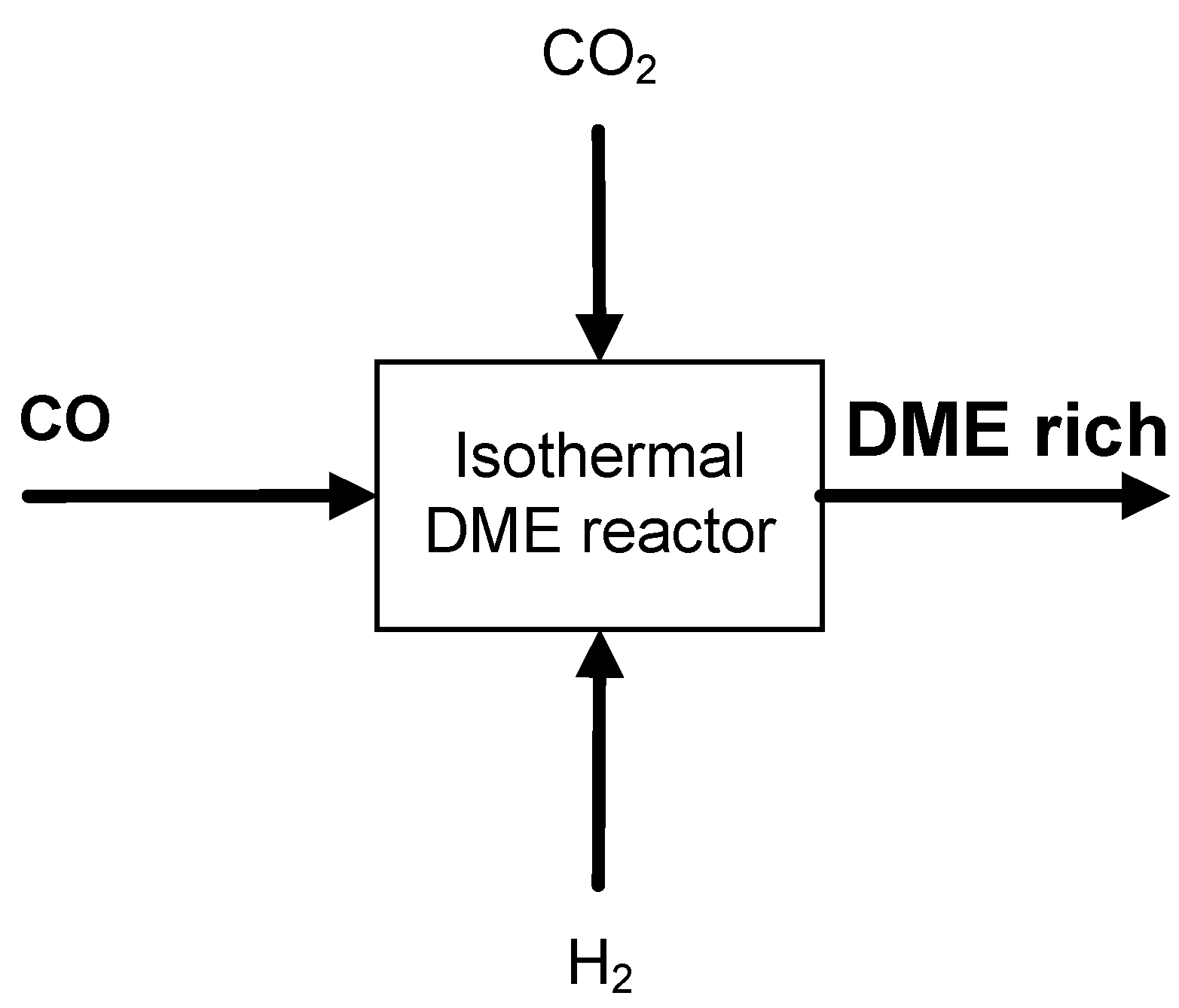
2.1. Thermodynamic Analysis of Direct DME Synthesis from CO2-Rich Syngas
2.2. Thermodynamic and Process Analysis of Direct DME Synthesis from CO2-Rich Syngas
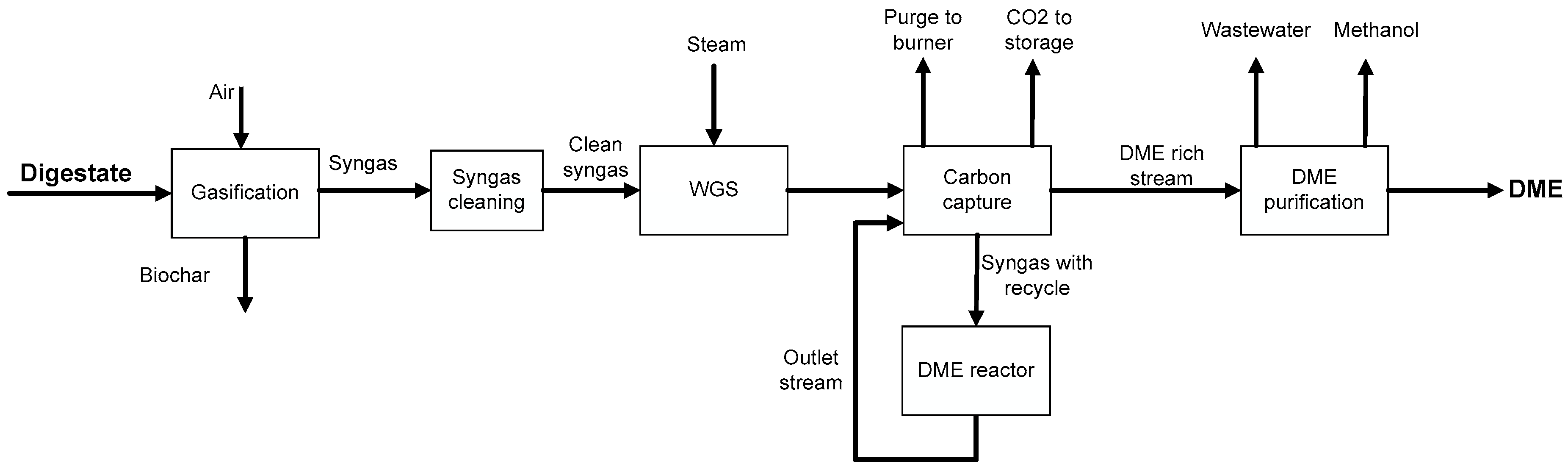
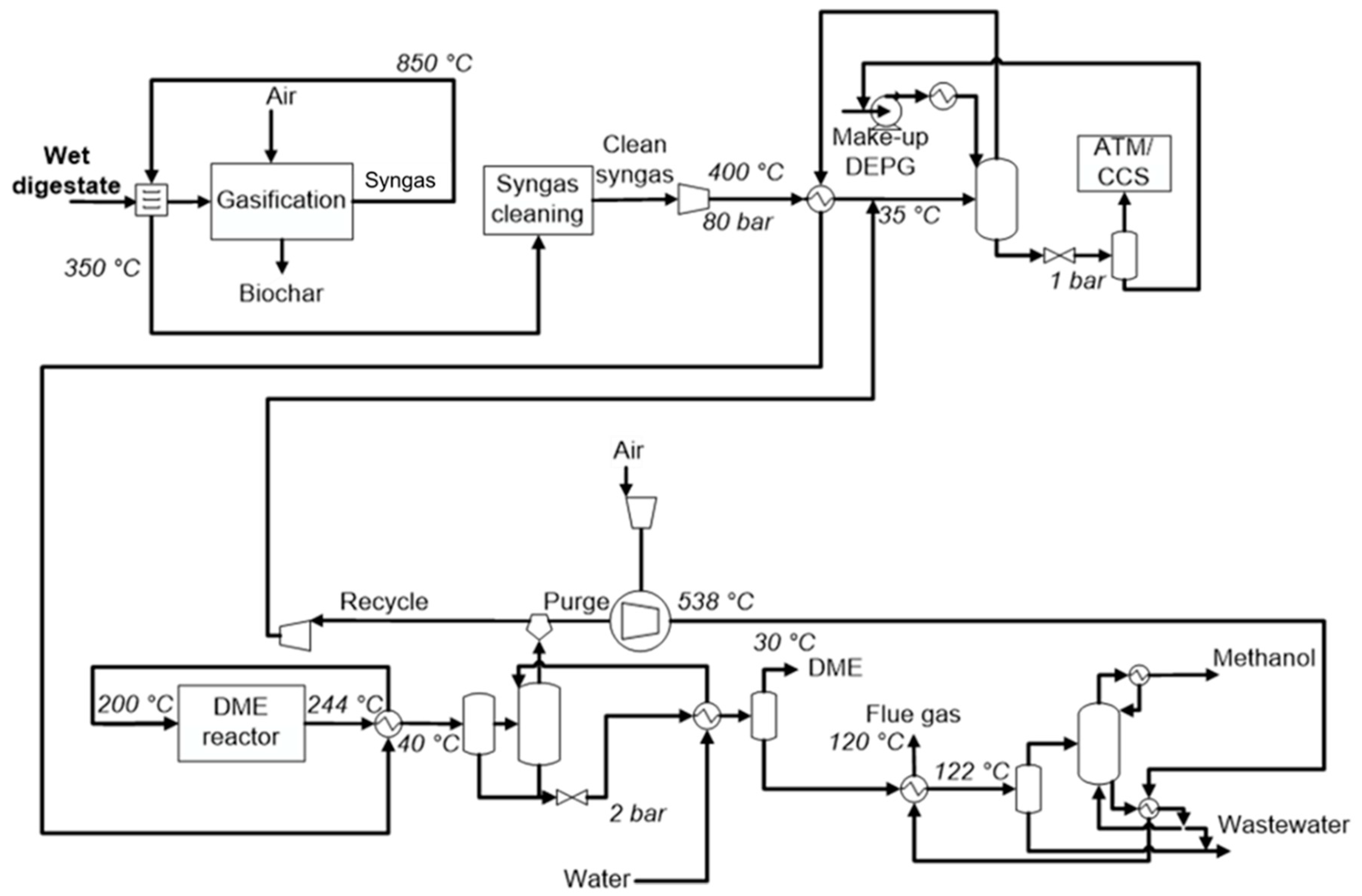
2.3. Process Simulation of the Optimal Flowsheet
2.4. Environmental Assessment
3. Results and Discussion
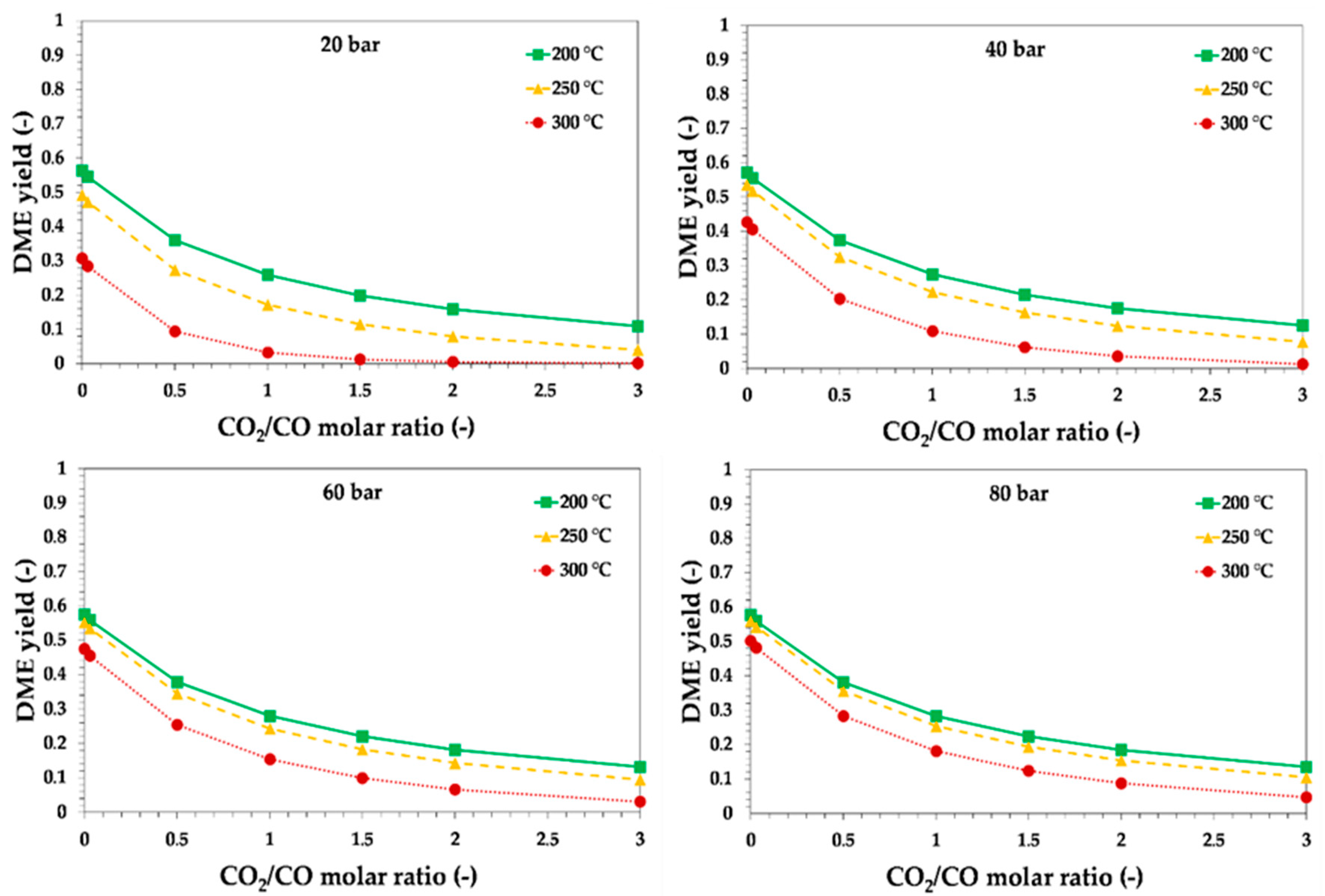
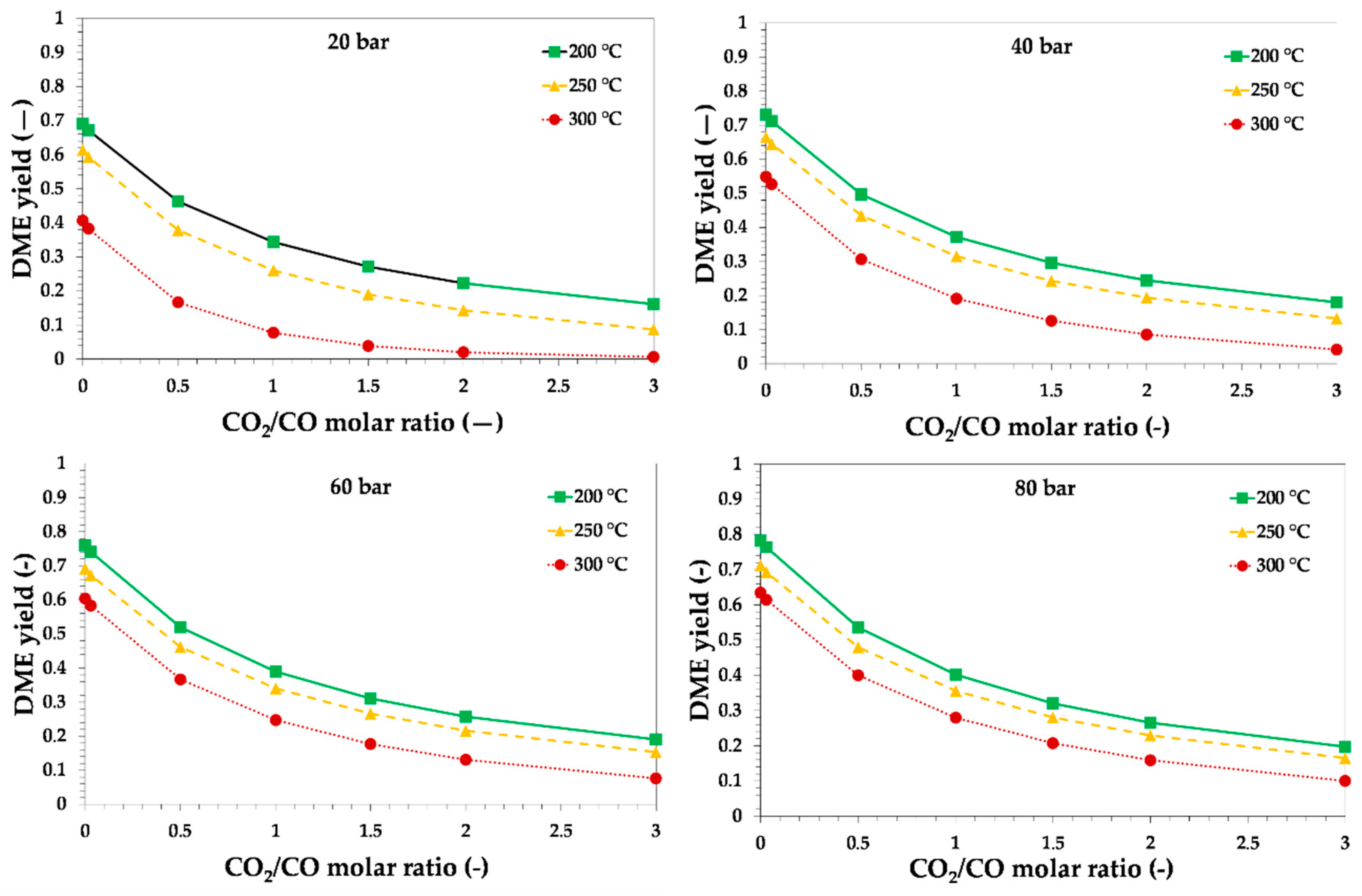
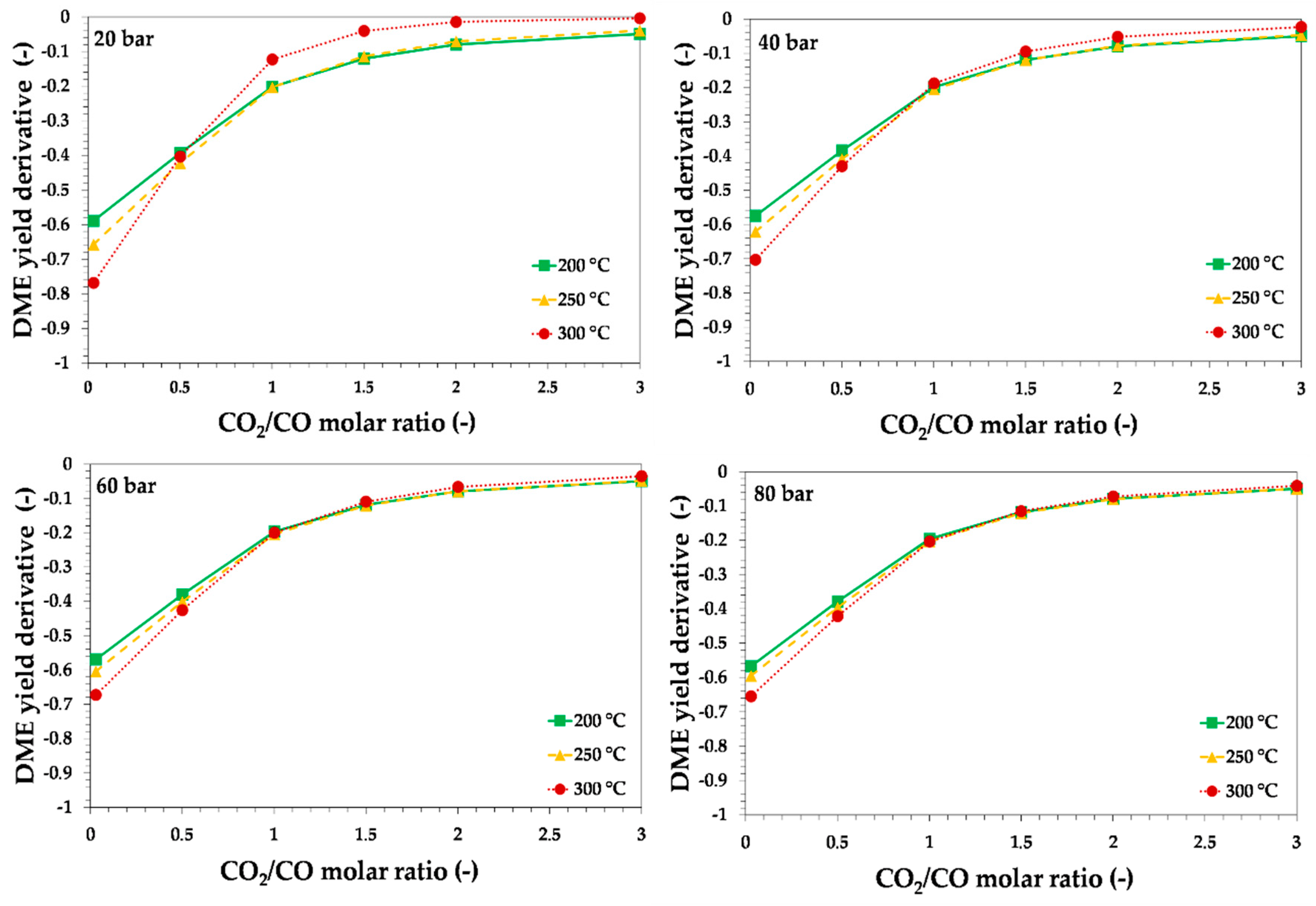
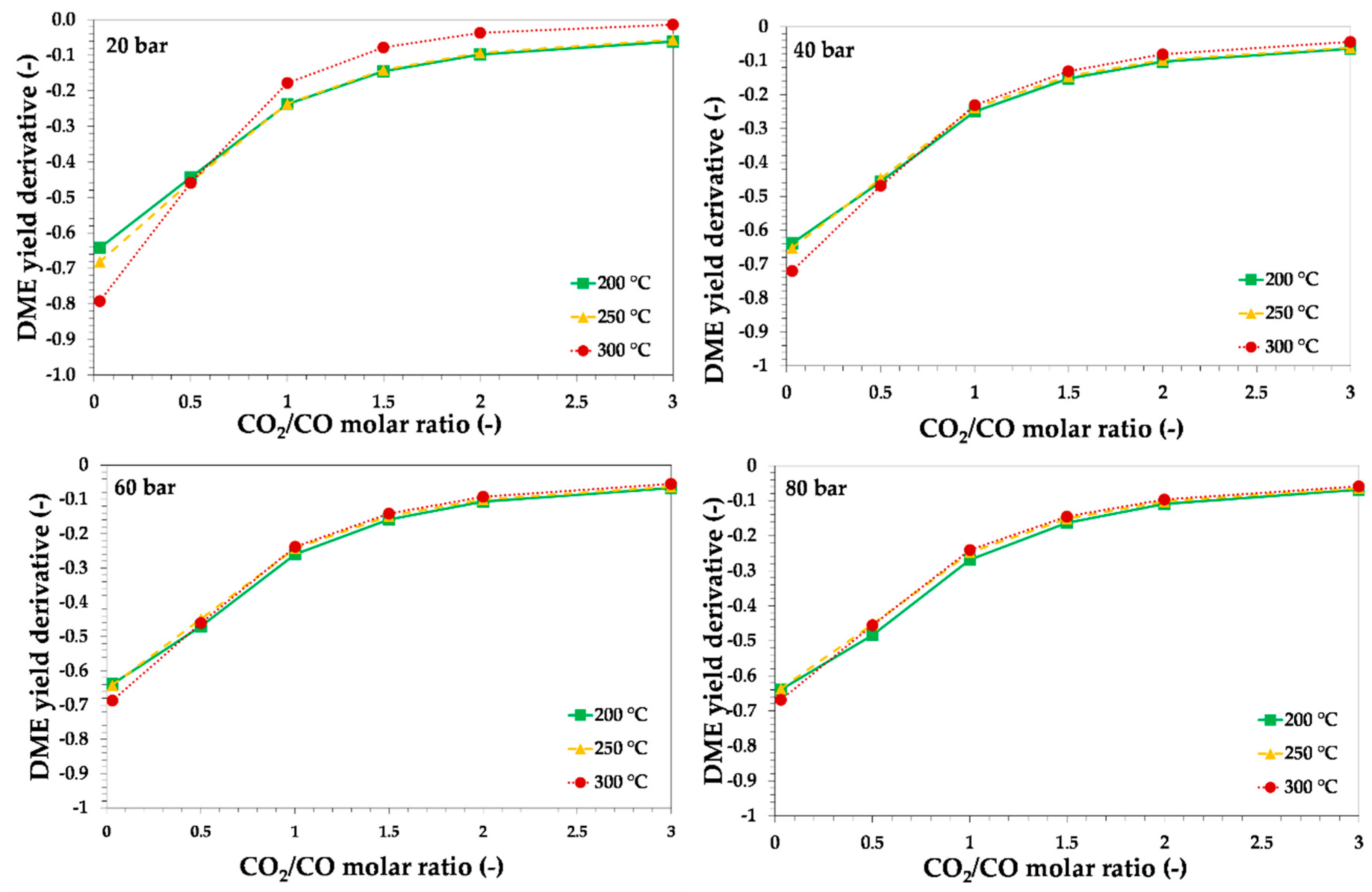
3.1. Thermodynamic Assessment of Direct DME Synthesis from CO2-Rich Syngas
3.2. Thermodynamic and Process Analysis of Direct DME Synthesis from CO2-Rich Syngas
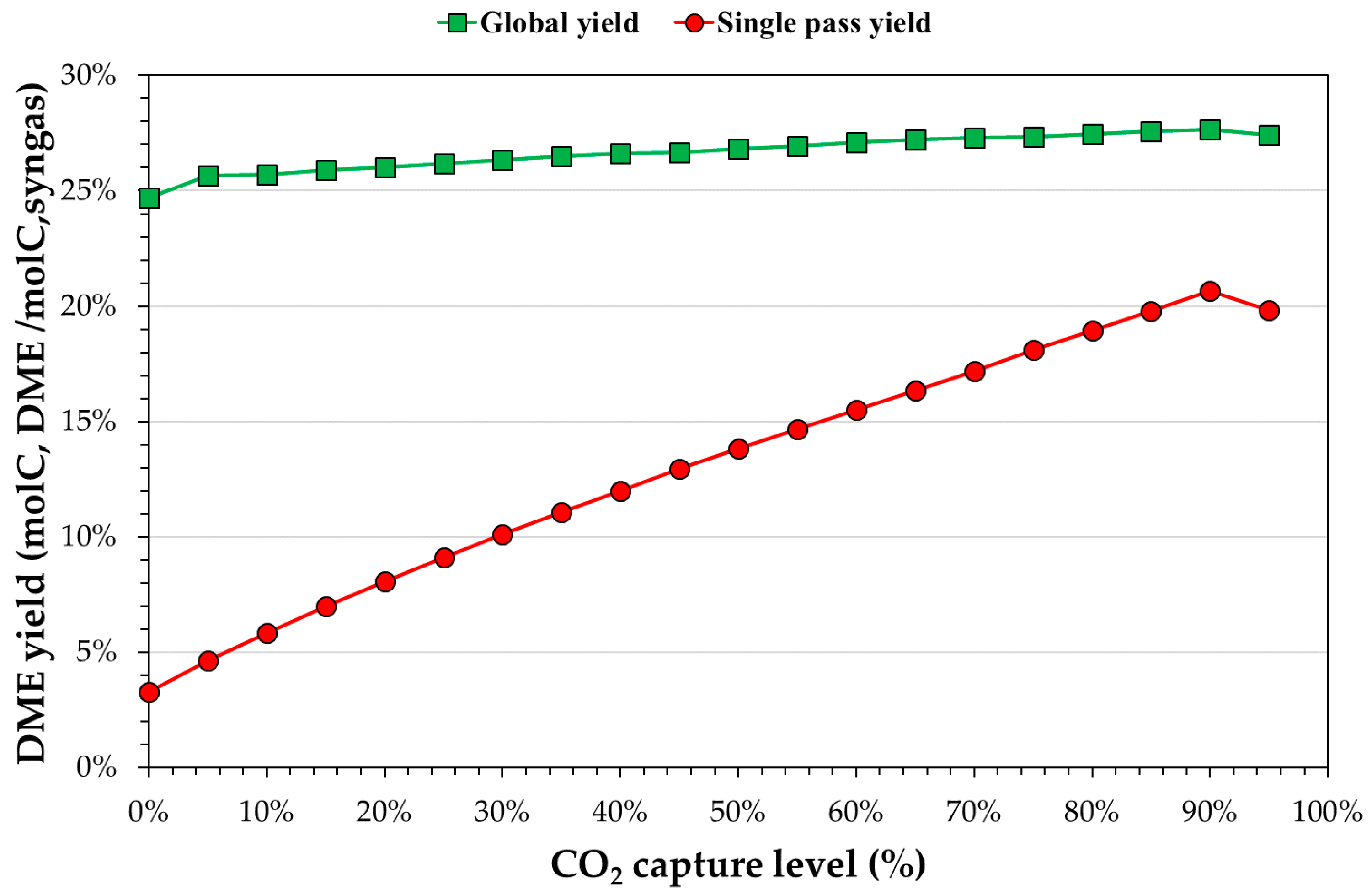
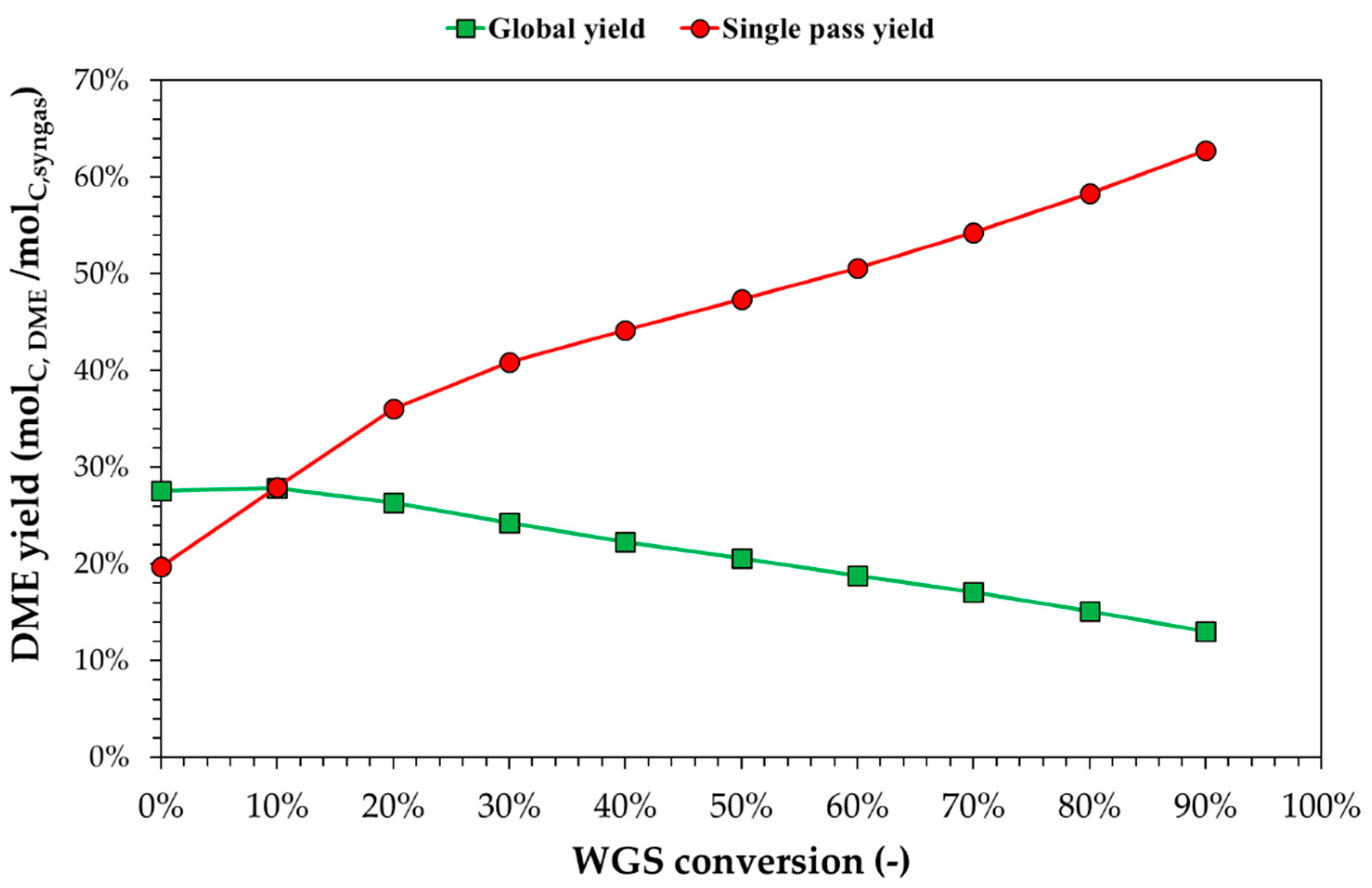
3.3. Process Simulation of DME Synthesis from Digestate-Derived Syngas
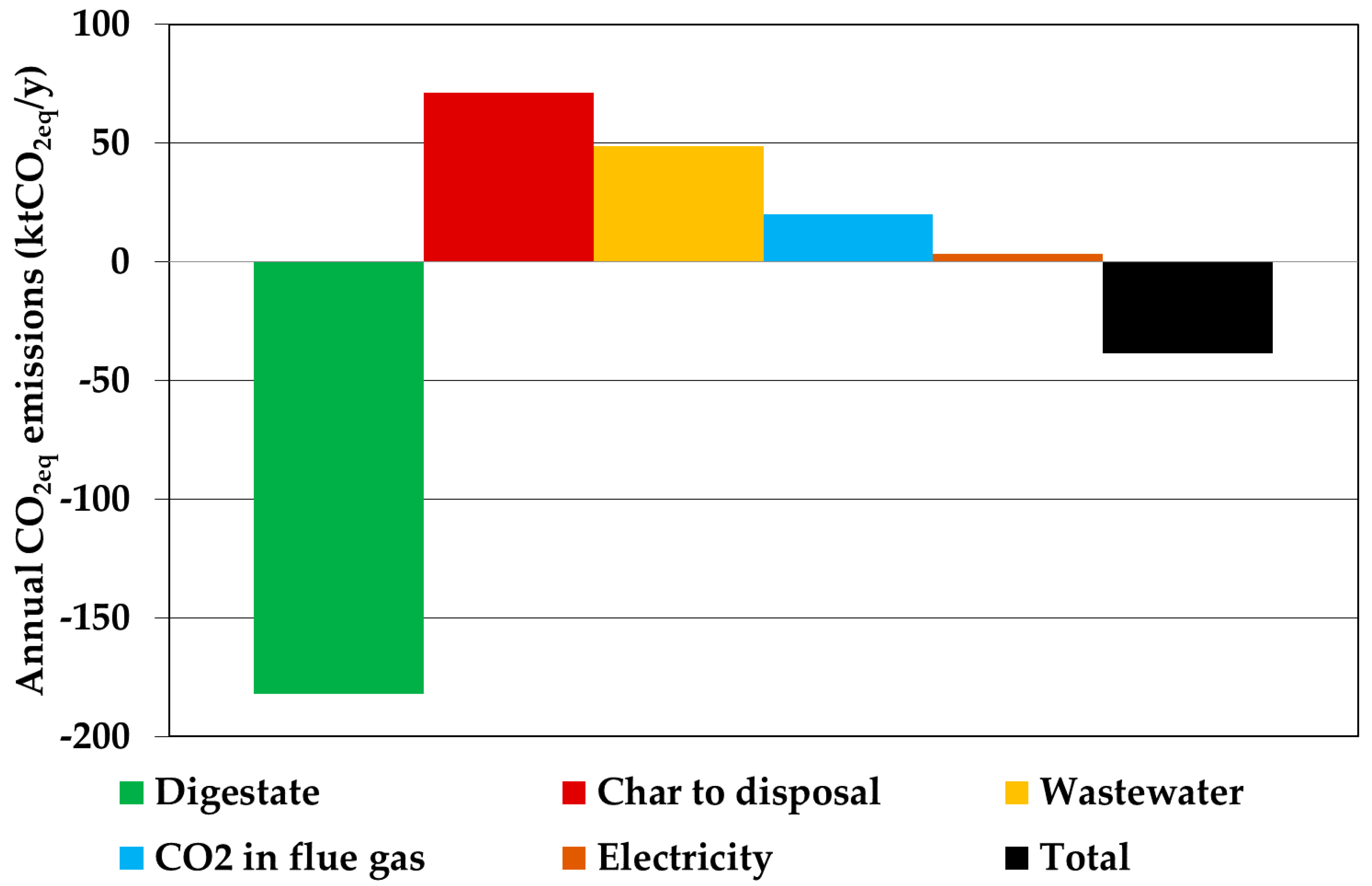
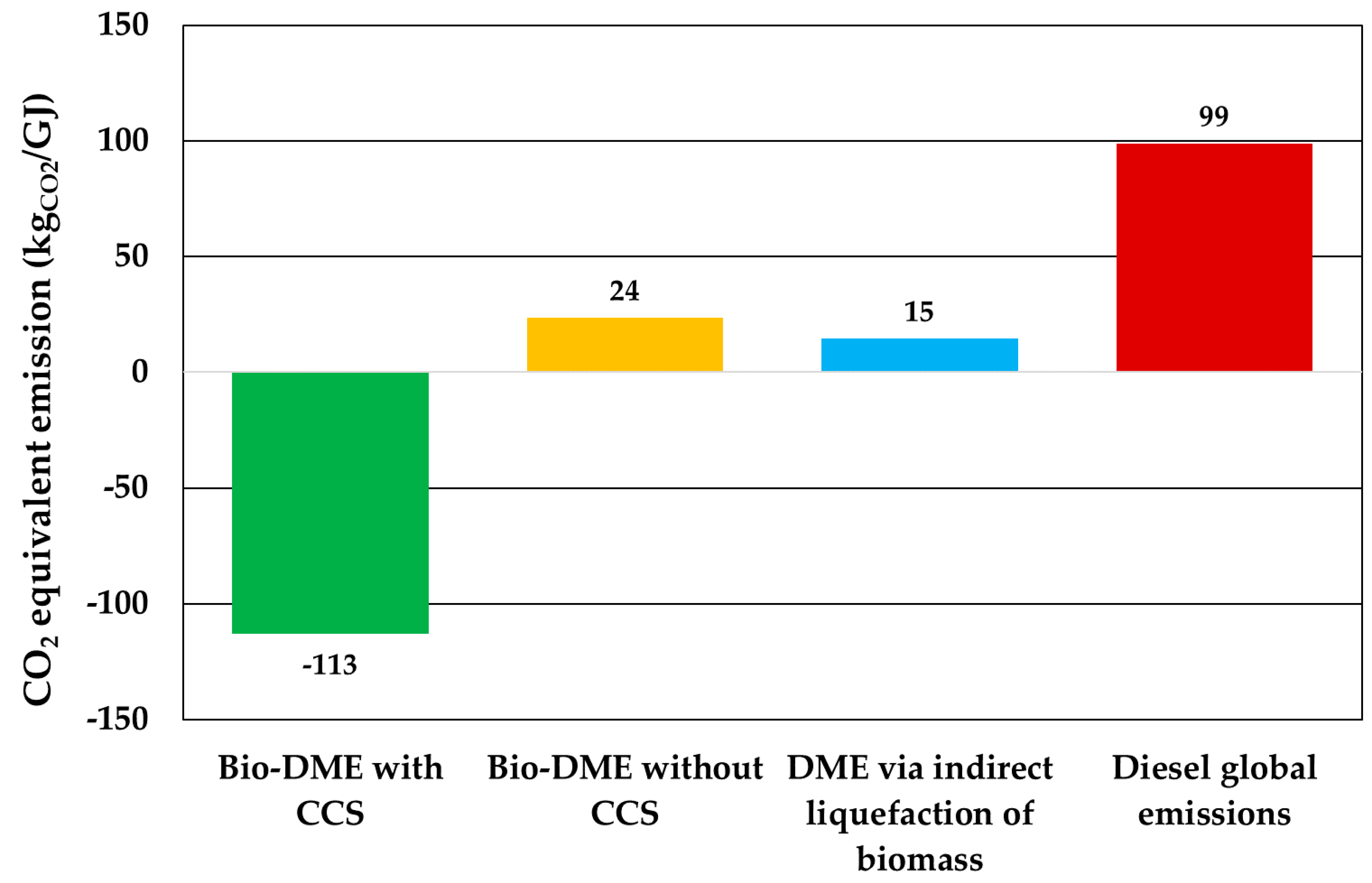
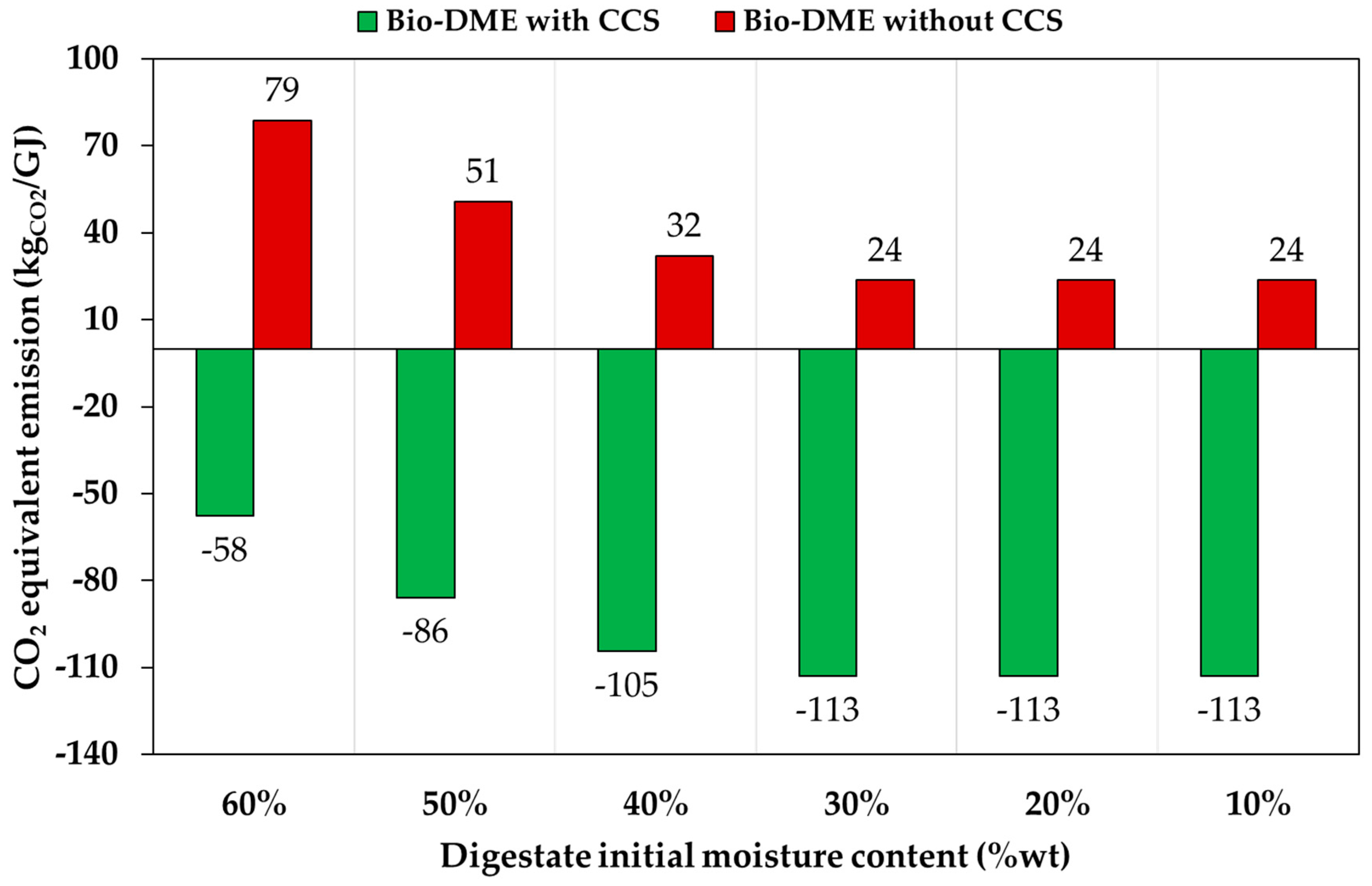
3.4. Environmental Assessment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Wang, L.; Vo, X.V.; Shahbaz, M.; Ak, A. Globalization and carbon emissions: Is there any role of agriculture value-added, financial development, and natural resource rent in the aftermath of COP21? J. Environ. Manag. 2020, 268, 110712. [Google Scholar] [CrossRef] [PubMed]
- Quadrelli, E.A.; Centi, G.; Duplan, J.L.; Perathoner, S. Carbon dioxide recycling: Emerging large-scale technologies with industrial potential. ChemSusChem 2011, 4, 1194–1215. [Google Scholar] [CrossRef] [PubMed]
- Demirbas, A. Waste management, waste resource facilities and waste conversion processes. Energy Convers. Manag. 2011, 52, 1280–1287. [Google Scholar] [CrossRef]
- Hoornweg, D.; Bhada-Tata, P. What a Waste: A Global Review of Solid Waste Management. Available online: https://openknowledge.worldbank.org/handle/10986/17388 (accessed on 5 November 2020).
- Migliori, M.; Catizzone, E.; Giordano, G.; Le Pera, A.; Sellaro, M.; Lista, A.; Zanardi, G.; Zoia, L. Pilot Plant Data Assessment in Anaerobic Digestion of Organic Fraction of Municipal Waste Solids. Processes 2019, 7, 54. [Google Scholar] [CrossRef] [Green Version]
- Ramachandran, A.; Rustum, R.; Adeloye, A.J. Review of anaerobic digestion modeling and optimization using nature-inspired techniques. Processes 2019, 7, 953. [Google Scholar] [CrossRef] [Green Version]
- Banks, C.J.; Chesshire, M.; Heaven, S.; Arnold, R. Anaerobic digestion of source-segregated domestic food waste: Performance assessment by mass and energy balance. Bioresour. Technol. 2011, 102, 612–620. [Google Scholar] [CrossRef] [Green Version]
- Monlau, F.; Francavilla, M.; Sambusiti, C.; Antoniou, N.; Solhy, A.; Libutti, A.; Zabaniotou, A.; Barakat, A.; Monteleone, M. Toward a functional integration of anaerobic digestion and pyrolysis for a sustainable resource management. Comparison between solid-digestate and its derived pyrochar as soil amendment. Appl. Energy 2016, 169, 652–662. [Google Scholar] [CrossRef]
- Antoniou, N.; Monlau, F.; Sambusiti, C.; Ficara, E.; Barakat, A.; Zabaniotou, A. Contribution to Circular Economy options of mixed agricultural wastes management: Coupling anaerobic digestion with gasification for enhanced energy and material recovery. J. Clean. Prod. 2019, 209, 505–514. [Google Scholar] [CrossRef]
- Califano, F.; Mongiello, C.; Freda, C. Combined Heat and Power Production from Meat and Bone Meal via Gasification and Gas Turbine: Technical and Economic Analysis. Waste Biomass Valoriz. 2017, 8, 975–986. [Google Scholar] [CrossRef]
- Kumar, A.; Jones, D.D.; Hanna, M.A. Thermochemical biomass gasification: A review of the current status of the technology. Energies 2009, 2, 556–581. [Google Scholar] [CrossRef] [Green Version]
- Abdoulmoumine, N.; Adhikari, S.; Kulkarni, A.; Chattanathan, S. A review on biomass gasification syngas cleanup. Appl. Energy 2015, 155, 294–307. [Google Scholar] [CrossRef]
- Chen, G.; Guo, X.; Cheng, Z.; Yan, B.; Dan, Z.; Ma, W. Air gasification of biogas-derived digestate in a downdraft fixed bed gasifier. Waste Manag. 2017, 69, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Gnanendra, P.M.; Ramesha, D.K.; Dasappa, S. Preliminary investigation on the use of biogas sludge for gasification. Int. J. Sustain. Energy 2012, 31, 251–267. [Google Scholar] [CrossRef]
- Freda, C.; Nanna, F.; Villone, A.; Barisano, D.; Brandani, S.; Cornacchia, G. Air gasification of digestate and its co-gasification with residual biomass in a pilot scale rotary kiln. Int. J. Energy Environ. Eng. 2019, 10, 335–346. [Google Scholar] [CrossRef] [Green Version]
- Abe, J.O.; Popoola, A.P.I.; Ajenifuja, E.; Popoola, O.M. Hydrogen energy, economy and storage: Review and recommendation. Int. J. Hydrogen Energy 2019, 44, 15072–15086. [Google Scholar] [CrossRef]
- Matzen, M.; Demirel, Y. Methanol and dimethyl ether from renewable hydrogen and carbon dioxide: Alternative fuels production and life-cycle assessment. J. Clean. Prod. 2016, 139, 1068–1077. [Google Scholar] [CrossRef] [Green Version]
- Cai, G.; Liu, Z.; Shi, R.; He, C.; Yang, L.; Sun, C.; Chang, Y. Light alkenes from syngas via dimethyl ether. Appl. Catal. A Gen. 1995, 125, 29–38. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, C.; Wang, G.; Wang, Q.; Cai, G. New progress in R&D of lower olefin synthesis. Fuel Process. Technol. 2000, 62, 161–172. [Google Scholar] [CrossRef]
- Adachi, Y.; Komoto, M.; Watanabe, I.; Ohno, Y.; Fujimoto, K. Effective utilization of remote coal through dimethyl ether synthesis. Fuel 2000, 79, 229–234. [Google Scholar] [CrossRef]
- Catizzone, E.; Cirelli, Z.; Aloise, A.; Lanzafame, P.; Migliori, M.; Giordano, G. Methanol conversion over ZSM-12, ZSM-22 and EU-1 zeolites: From DME to hydrocarbons production. Catal. Today 2018, 304, 39–50. [Google Scholar] [CrossRef]
- Azizi, Z.; Rezaeimanesh, M.; Tohidian, T.; Rahimpour, M.R. Dimethyl ether: A review of technologies and production challenges. Chem. Eng. Process. Process Intensif. 2014, 82, 150–172. [Google Scholar] [CrossRef]
- Ju, F.; Chen, H.; Ding, X.; Yang, H.; Wang, X.; Zhang, S.; Dai, Z. Process simulation of single-step dimethyl ether production via biomass gasification. Biotechnol. Adv. 2009, 27, 599–605. [Google Scholar] [CrossRef]
- Clausen, L.R.; Elmegaard, B.; Houbak, N. Technoeconomic analysis of a low CO2 emission dimethyl ether (DME) plant based on gasification of torrefied biomass. Energy 2010, 35, 4831–4842. [Google Scholar] [CrossRef] [Green Version]
- Ogawa, T.; Inoue, N.; Shikada, T.; Ohno, Y. Direct Dimethyl Ether Synthesis. J. Nat. Gas Chem. 2003, 12, 219–227. [Google Scholar]
- Huang, M.H.; Lee, H.M.; Liang, K.C.; Tzeng, C.C.; Chen, W.H. An experimental study on single-step dimethyl ether (DME) synthesis from hydrogen and carbon monoxide under various catalysts. Int. J. Hydrogen Energy 2015, 40, 13583–13593. [Google Scholar] [CrossRef]
- Moradi, G.R.; Ahmadpour, J.; Yaripour, F. Intrinsic kinetics study of LPDME process from syngas over bi-functional catalyst. Chem. Eng. J. 2008, 144, 88–95. [Google Scholar] [CrossRef]
- Peng, X.D.; Toseland, B.A.; Tijm, P.J.A. Kinetic understanding of the chemical synergy under LPDME(TM) conditions-once-through applications. Chem. Eng. Sci. 1999, 54, 2787–2792. [Google Scholar] [CrossRef]
- Nakyai, T.; Saebea, D. Exergoeconomic comparison of syngas production from biomass, coal, and natural gas for dimethyl ether synthesis in single-step and two-step processes. J. Clean. Prod. 2019, 241. [Google Scholar] [CrossRef]
- Catizzone, E.; Bonura, G.; Migliori, M.; Frusteri, F.; Giordano, G. CO2 recycling to dimethyl ether: State-of-the-art and perspectives. Molecules 2018, 23, 31. [Google Scholar] [CrossRef] [Green Version]
- Perathoner, S.; Centi, G. CO2 recycling: A key strategy to introduce green energy in the chemical production chain. ChemSusChem 2014, 7, 1274–1282. [Google Scholar] [CrossRef]
- De Falco, M.; Capocelli, M.; Centi, G. Dimethyl ether production from CO2 rich feedstocks in a one-step process: Thermodynamic evaluation and reactor simulation. Chem. Eng. J. 2016, 294, 400–409. [Google Scholar] [CrossRef]
- Catizzone, E.; Migliori, M.; Purita, A.; Giordano, G. Ferrierite vs. γ-Al2O3: The superiority of zeolites in terms of water-resistance in vapour-phase dehydration of methanol to dimethyl ether. J. Energy Chem. 2019, 30, 162–169. [Google Scholar] [CrossRef] [Green Version]
- Bozzano, G.; Manenti, F. Efficient methanol synthesis: Perspectives, technologies and optimization strategies. Prog. Energy Combust. Sci. 2016, 56, 71–105. [Google Scholar] [CrossRef]
- Giuliano, A.; Catizzone, E.; Freda, C.; Cornacchia, G. Valorization of OFMSW Digestate-Derived Syngas toward Methanol, Hydrogen, or Electricity: Process Simulation and Carbon Footprint Calculation. Processes 2020, 8, 526. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, H.; Ying, W.; Fang, D. Modeling and Simulation of Production Process on Dimethyl Ether Synthesized from Coal-based Syngas by One-step Method. Chin. J. Chem. Eng. 2009, 17, 108–112. [Google Scholar] [CrossRef]
- Giuliano, A.; Poletto, M.; Barletta, D. Pure hydrogen co-production by membrane technology in an IGCC power plant with carbon capture. Int. J. Hydrogen Energy 2018, 43, 19279–19292. [Google Scholar] [CrossRef]
- Giuliano, A.; Cerulli, R.; Poletto, M.; Raiconi, G.; Barletta, D. Optimization of a Multiproduct Lignocellulosic Biorefinery Using a MILP Approximation; Elsevier: Amsterdam, The Netherlands, 2014; Volume 33. [Google Scholar]
- Giuliano, A.; Barletta, D.; De Bari, I.; Poletto, M. Techno-economic assessment of a lignocellulosic biorefinery co-producing ethanol and xylitol or furfural. Comput. Aided Chem. Eng. 2018, 43, 585–590. [Google Scholar] [CrossRef]
- Sofia, D.; Giuliano, A.; Poletto, M.; Barletta, D. Techno-economic analysis of power and hydrogen co-production by an IGCC plant with CO2 capture based on membrane technology. Comput. Aided Chem. Eng. 2015, 37, 1373–1378. [Google Scholar] [CrossRef]
- Giuliano, A.; Freda, C.; Catizzone, E. Techno-economic assessment of bio-syngas production for methanol synthesis: A focus on the water–gas shift and carbon capture sections. Bioengineering 2020, 7, 70. [Google Scholar] [CrossRef]
- Giuliano, A.; De Bari, I.; Motola, V.; Pierro, N.; Giocoli, A.; Barletta, D. Techno-environmental Assessment of Two Biorefinery Systems to Valorize the Residual Lignocellulosic Biomass of the Basilicata Region. Math. Model. Eng. Probl. 2019, 6, 317–323. [Google Scholar] [CrossRef]
- Rodrigues Gurgel da Silva, A.; Giuliano, A.; Errico, M.; Rong, B.-G.; Barletta, D. Economic value and environmental impact analysis of lignocellulosic ethanol production: Assessment of different pretreatment processes. Clean Technol. Environ. Policy 2019, 21, 637–654. [Google Scholar] [CrossRef]
- Perez, J.; De Andres, M.J.; Lumbreras, J.; Rodriguez, E. Evaluating carbon footprint of municipal solid waste treatment: Methodological proposal and application to a case study. J. Clean. Prod. 2018, 205, 419–431. [Google Scholar] [CrossRef]
- Giuliano, A.; Catizzone, E.; Barisano, D.; Nanna, F.; Villone, A.; Bari, I. De Towards Methanol Economy: A Techno-environmental Assessment for a Bio-methanol OFMSW/Biomass/Carbon Capture-based Integrated Plant. Int. J. Heat Technol. 2019, 37, 665–674. [Google Scholar] [CrossRef]
- Venvik, H.J.; Yang, J. Catalysis in microstructured reactors: Short review on small-scale syngas production and further conversion into methanol, DME and Fischer-Tropsch products. Catal. Today 2017, 285, 135–146. [Google Scholar] [CrossRef]
- Frusteri, F.; Bonura, G.; Cannilla, C.; Drago Ferrante, G.; Aloise, A.; Catizzone, E.; Migliori, M.; Giordano, G. Stepwise tuning of metal-oxide and acid sites of CuZnZr-MFI hybrid catalysts for the direct DME synthesis by CO2 hydrogenation. Appl. Catal. B Environ. 2015, 176–177, 522–531. [Google Scholar] [CrossRef]
- Bonura, G.; Cannilla, C.; Frusteri, L.; Catizzone, E.; Todaro, S.; Migliori, M.; Giordano, G.; Frusteri, F. Interaction effects between CuO-ZnO-ZrO2 methanol phase and zeolite surface affecting stability of hybrid systems during one-step CO2 hydrogenation to DME. Catal. Today 2020, 345, 175–182. [Google Scholar] [CrossRef]
- Catizzone, E.; Bonura, G.; Migliori, M.; Braccio, G.; Frusteri, F.; Giordano, G. Direct CO2-to-dimethyl Ether Hydrogenation over CuZnZr/zeolite Hybrid Catalyst: New Evidences on the Interaction Between Acid and Metal Sites. Ann. Chim. Sci. Matér. 2019, 43, 141–149. [Google Scholar] [CrossRef]
- Jung, J.W.; Lee, Y.J.; Um, S.H.; Yoo, P.J.; Lee, D.H.; Jun, K.-W.; Bae, J.W. Effect of copper surface area and acidic sites to intrinsic catalytic activity for dimethyl ether synthesis from biomass-derived syngas. Appl. Catal. B Environ. 2012, 126, 1–8. [Google Scholar] [CrossRef]
- Bae, J.W.; Potdar, H.S.; Kang, S.H.; Jun, K.W. Coproduction of methanol and dimethyl ether from biomass-derived syngas on a Cu-ZnO-Al2O3/ γ-A2O3 hybrid catalyst. Energy Fuels 2008, 22, 223–230. [Google Scholar] [CrossRef]
- Naik, S.P.; Ryu, T.; Bui, V.; Miller, J.D.; Drinnan, N.B.; Zmierczak, W. Synthesis of DME from CO2/H2 gas mixture. Chem. Eng. J. 2011, 167, 362–368. [Google Scholar] [CrossRef]
- Ren, S.; Shoemaker, W.R.; Wang, X.; Shang, Z.; Klinghoffer, N.; Li, S.; Yu, M.; He, X.; White, T.A.; Liang, X. Highly active and selective Cu-ZnO based catalyst for methanol and dimethyl ether synthesis via CO2 hydrogenation. Fuel 2019, 239, 1125–1133. [Google Scholar] [CrossRef]
- Galanopoulos, C.; Giuliano, A.; Barletta, D.; Zondervan, E. An integrated methodology for the economic and environmental assessment of a biorefinery supply chain. Chem. Eng. Res. Des. 2020, 160, 199–215. [Google Scholar] [CrossRef]
- Uddin, M.; Simson, A.; Wright, M.M. Techno-economic and greenhouse gas emission analysis of dimethyl ether production via the bi-reforming pathway for transportation fuel. Energy 2020, 211, 119031. [Google Scholar] [CrossRef]
- Tan, E.C.; Talmadge, M.; Dutta, A.; Hensley, J.; Snowden-Swan, L.J.; Humbird, D.; Schaidle, J.; Biddy, M. Conceptual process design and economics for the production of high-octane gasoline blendstock via indirect liquefaction of biomass through methanol/dimethyl ether intermediates. Biofuels Bioprod. Biorefining 2016, 10, 17–35. [Google Scholar] [CrossRef]
- ECN Phyllis Classification. Available online: https://phyllis.nl/Browse/Standard/ECN-Phyllis#digestat (accessed on 18 December 2020).
- Giaconia, A.; Caputo, G.; Ienna, A.; Mazzei, D.; Schiavom, B.; Scialdone, O.; Galia, A. Biorefinery process for hydrothermal liquefaction of microalgae powered by a concentrating solar plant: A conceptual study. Appl. Energy 2017, 208, 1139–1149. [Google Scholar] [CrossRef]


| Process Parameter | Units | Value/Range | Reference | |
|---|---|---|---|---|
| Syngas composition | % | CO2 | 12.9 | [15] |
| C2H6 | 1.3 | |||
| H2 | 11.9 | |||
| CH4 | 4.5 | |||
| CO | 13.7 | |||
| N2 | 55.7 | |||
| Syngas flowrate | t/h | 16.3 | [15] | |
| WGS conversion | % | 0–95 | - | |
| CO2 capture | % | 0–95 | - | |
| Purge split ratio | % | 10–90 | - | |
| HT-WGS temperature | °C | 400 | [35] | |
| LT-WGS temperature | °C | 200 | [35] | |
| DME synthesis equilibrium temperature | °C | 200–300 | [22] | |
| DME synthesis pressure | bar | 20–80 | [32] | |
| Digestate flowrate | t/y | 100,000 | [35] | |
| DME purification columns pressure | bar | 2 | [36] | |
| Selexol® separation temperature | °C | 35 | [37] | |
| H2O/DME absorption column ratio | mol/mol | 33 | [36] | |
| Process Stream | Units | Value | Reference |
|---|---|---|---|
| Electricity required | kgCO2eq/MWhe | 600 | [45] |
| MeOH | kgCO2eq/t | −1643 | [45] |
| Digestate | kgCO2eq/t | −1821 | [44] |
| Wastewater | kgCO2eq/t | 500 | [45] |
| Solid gasification residues | kgCO2eq/t | 1821 | [44] |
| Process water | kgCO2eq/t | 6.5 | [35] |
| Process Parameter | Units | Value |
|---|---|---|
| WGS conversion | % | 0 |
| CO2 capture | % | 85 |
| Purge split ratio | % | 10 |
| DME reactor outlet temperature | °C | 244 |
| DME reactor outlet pressure | bar | 78 |
| Pure DME flowrate | t/y | 7496 |
| Pure methanol flowrate | t/y | 82 |
| Wastewater | t/y | 97,153 |
| Process water | t/y | 97,200 |
| CO2 captured and stored | t/y | 30,923 |
| Solid residues to disposal | t/y | 39,000 |
| DEPG flowrate | t/h | 9.9 |
| Flue gases | t/h | 36 |
| Recycle/fresh syngas stream | mol/mol | 5.8 |
| Energy power produced | MWe | 4.8 |
| Energy power consumed | MWe | 5.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giuliano, A.; Catizzone, E.; Freda, C. Process Simulation and Environmental Aspects of Dimethyl Ether Production from Digestate-Derived Syngas. Int. J. Environ. Res. Public Health 2021, 18, 807. https://doi.org/10.3390/ijerph18020807
Giuliano A, Catizzone E, Freda C. Process Simulation and Environmental Aspects of Dimethyl Ether Production from Digestate-Derived Syngas. International Journal of Environmental Research and Public Health. 2021; 18(2):807. https://doi.org/10.3390/ijerph18020807
Chicago/Turabian StyleGiuliano, Aristide, Enrico Catizzone, and Cesare Freda. 2021. "Process Simulation and Environmental Aspects of Dimethyl Ether Production from Digestate-Derived Syngas" International Journal of Environmental Research and Public Health 18, no. 2: 807. https://doi.org/10.3390/ijerph18020807





