Enhanced Phytoremediation of Bisphenol A in Polluted Lake Water by Seedlings of Ceratophyllum demersum and Myriophyllum spicatum from In Vitro Culture
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Reagent
2.3. Experimental Set-Up
2.4. Determination of Residual BPA in Water
2.5. Data Collection and Analysis
3. Results
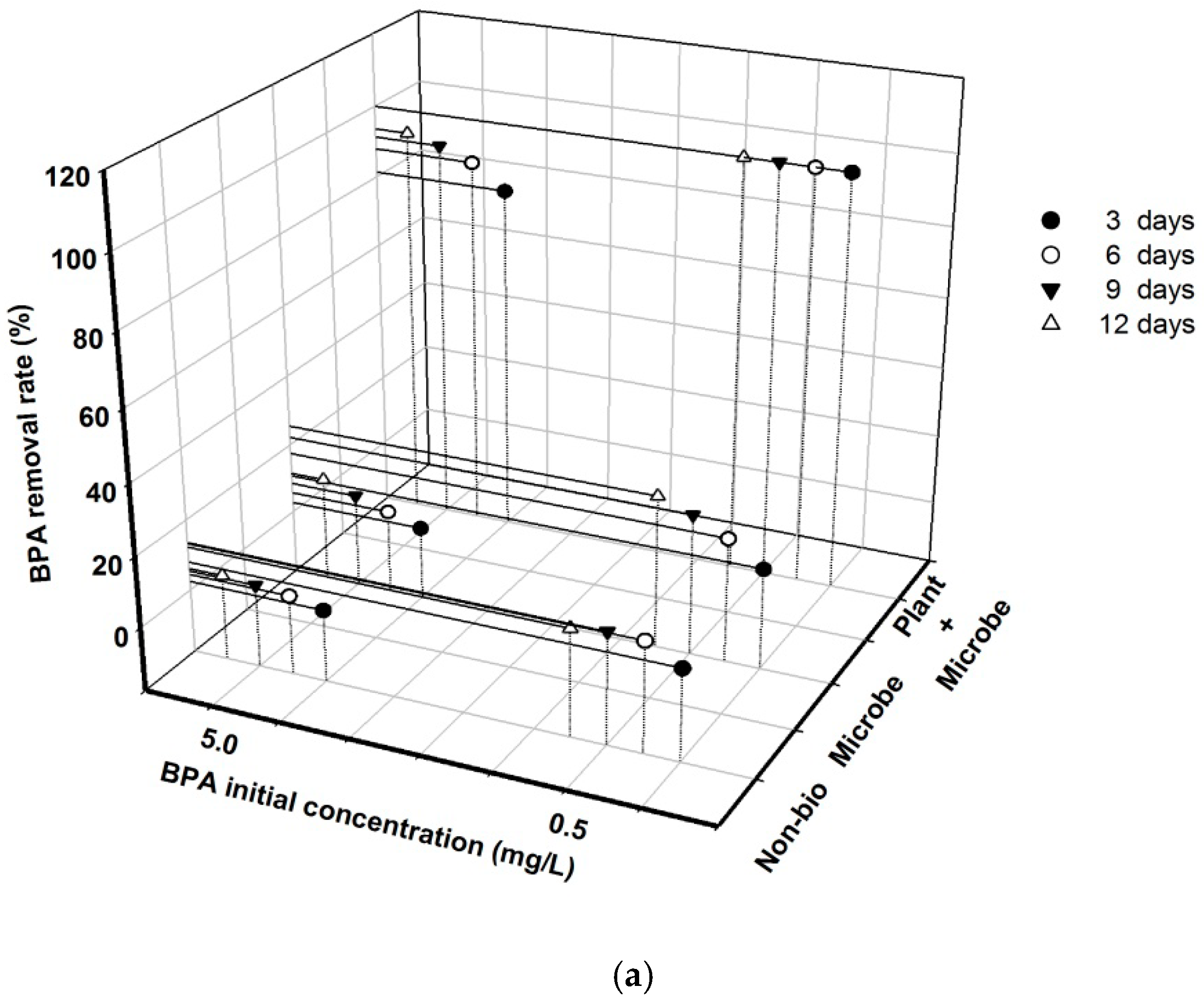
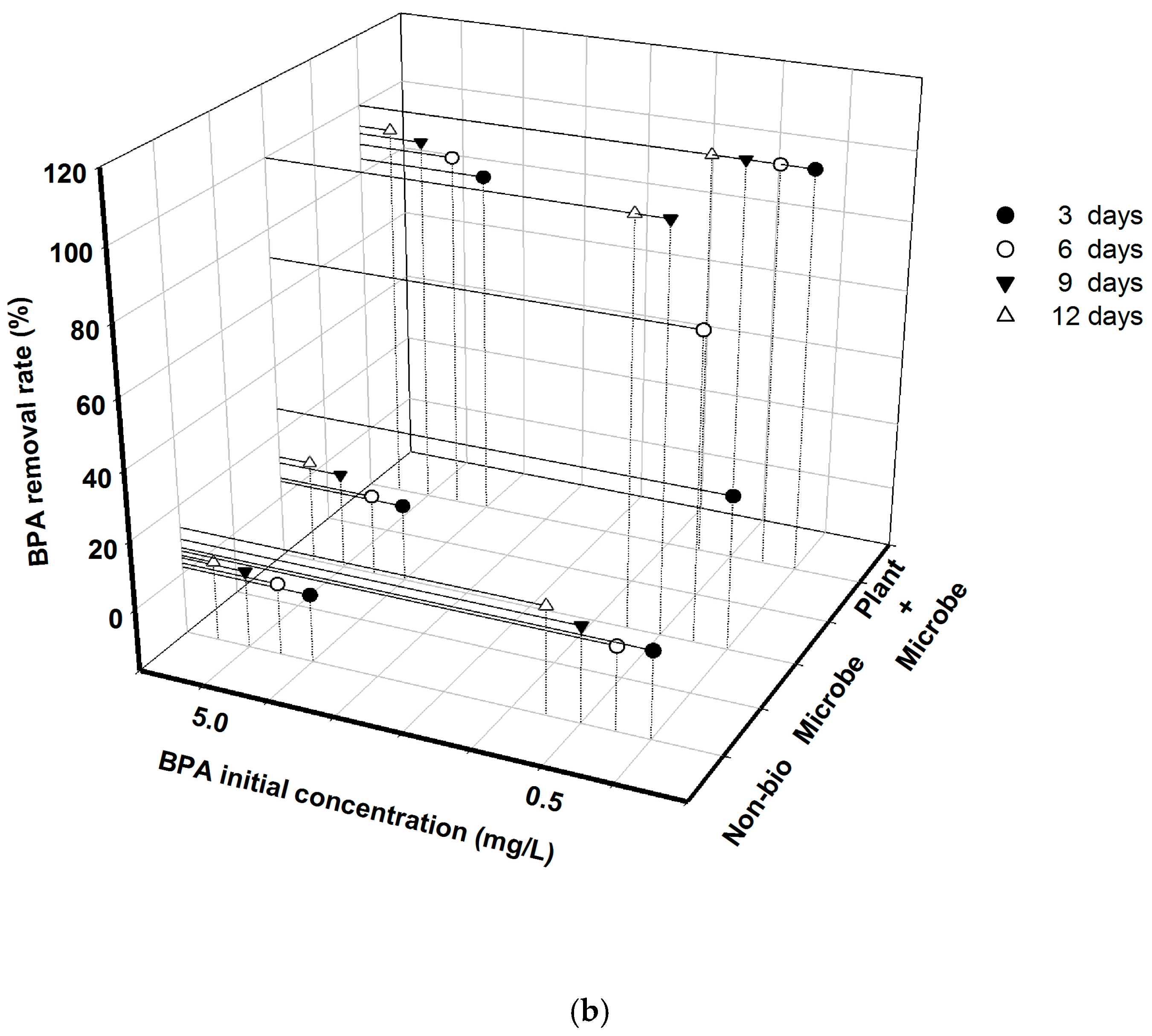
3.1. BPA Removal Was Greatly Affected by Initial Concentration and Biota Treatment under Different Nutrient Conditions
3.2. Species Type, Initial BPA Concentration and Biomass Density Affected BPA Removal in Plant + Microbe Groups under Different Nutrient Conditions
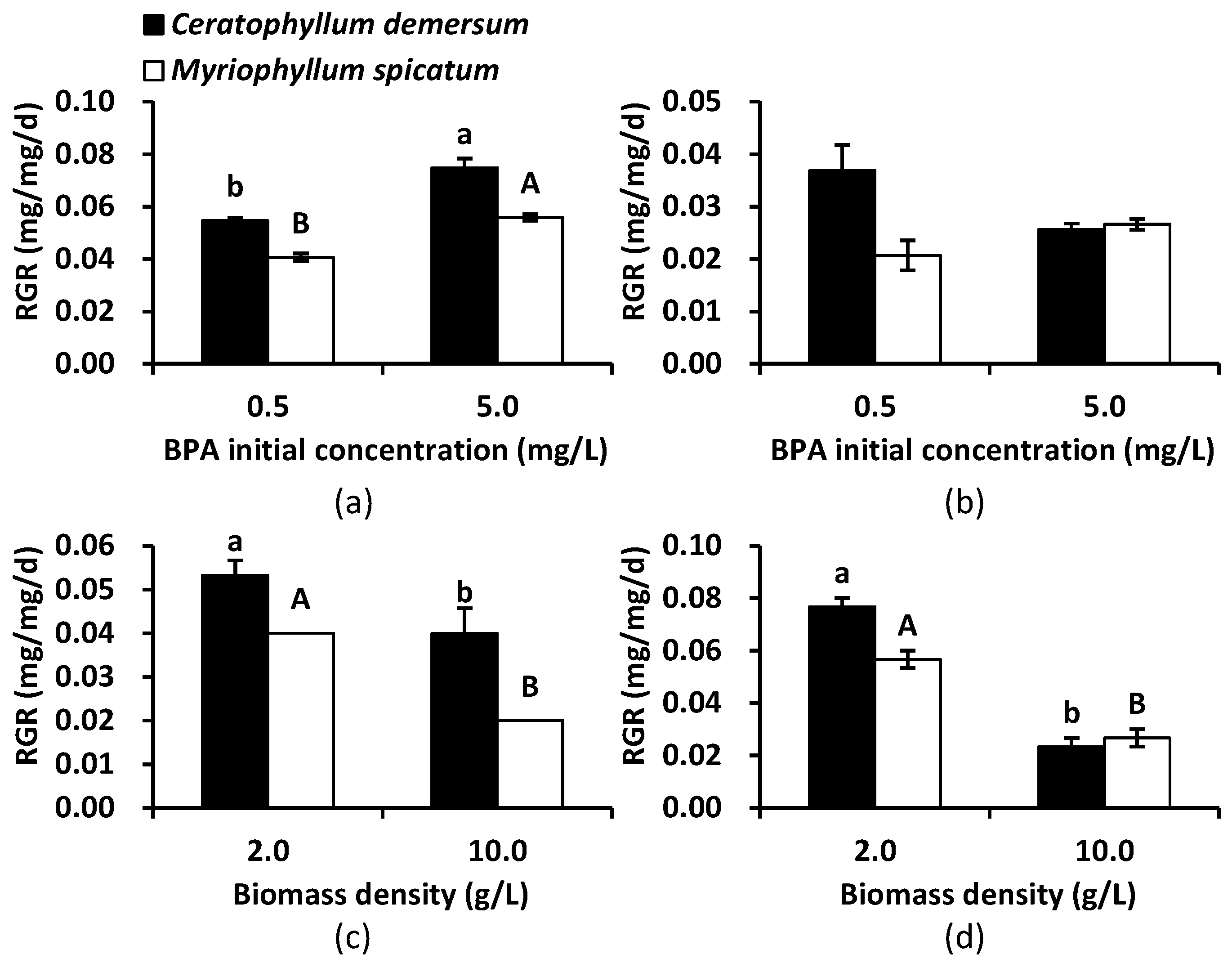
3.3. BPA Tolerance of Aseptic Seedlings of C. demersum and M. spicatum
4. Discussion
4.1. Indigenous Microbes Showed Limited Ability for BPA Removal
4.2. Aseptic Seedlings of C. demersum and M. spicatum Greatly Enhanced BPA Removal from Lake Water
4.3. C. demersum Was Preferred in BPA Phytoremediation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Usman, A.; Ahmad, M. From BPA to its analogues: Is it a safe journey. Chemosphere 2016, 158, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Lu, G.; Liu, J.; Jin, S. An integrated assessment of estrogenic contamination and feminization risk in fish in taihu lake, China. Ecotoxicol. Environ. Saf. 2012, 84, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Mathieu-Denoncourt, J.; Wallace, S.J.; de Solla, S.R.; Langlois, V.S. Plasticizer endocrine disruption: Highlighting developmental and reproductive effects in mammals and non-mammalian aquatic species. Gen. Comp. Endocr. 2015, 219, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiang, Z.; Dong, H.; Zhu, B.; Qu, J.; Nie, Y. A comparison of various rural wastewater treatment processes for the removal of endocrine-disrupting chemicals (edcs). Chemosphere 2013, 92, 986–992. [Google Scholar] [CrossRef] [PubMed]
- Salgueiro-González, N.; Turnes-Carou, I.; Viñas-Diéguez, L.; Muniategui-Lorenzo, S.; López-Mahía, P.; Prada-Rodríguez, D. Occurrence of endocrine disrupting compounds in five estuaries of the northwest coast of spain: Ecological and human health impact. Chemosphere 2015, 131, 241–247. [Google Scholar] [CrossRef]
- Gorga, M.; Insa, S.; Petrovic, M.; Barceló, D. Occurrence and spatial distribution of edcs and related compounds in waters and sediments of iberian rivers. Sci. Total Environ. 2015, 503, 69–86. [Google Scholar] [CrossRef]
- Jonkers, N.; Sousa, A.; Galanteoliveira, S.; Barroso, C.M.; Kohler, H.P.; Giger, W. Occurrence and sources of selected phenolic endocrine disruptors in ria de aveiro, Portugal. Environ. Sci. Pollut. Res. 2010, 17, 834–843. [Google Scholar] [CrossRef] [Green Version]
- Xiong, J.; An, T.; Zhang, C.; Li, G. Pollution profiles and risk assessment of pbdes and phenolic brominated flame retardants in water environments within a typical electronic waste dismantling region. Environ. Geochem. Health 2015, 37, 457–473. [Google Scholar] [CrossRef]
- Jin, S.; Yang, F.; Xu, Y.; Dai, H.; Liu, W. Risk assessment of xenoestrogens in a typical domestic sewage-holding lake in China. Chemosphere 2013, 93, 892–898. [Google Scholar] [CrossRef]
- Yamamoto, T.; Yasuhara, A.; Shiraishi, H.; Nakasugi, O. Bisphenol a in hazardous waste landfill leachates. Chemosphere 2001, 42, 415. [Google Scholar] [CrossRef]
- De Kermoysan, G.; Joachim, S.; Baudoin, P.; Lonjaret, M.; Tebby, C.; Lesaulnier, F.; Lestremau, F.; Chatellier, C.; Akrour, Z.; Pheron, E.; et al. Effects of bisphenol a on different trophic levels in a lotic experimental ecosystem. Aquat. Toxicol. 2013, 144, 186–198. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.H.; Chen, Y.J.; Chang, Y.J.; Shih, Y.H. Biodegradation of bisphenol a with diverse microorganisms from river sediment. J. Hazard. Mater. 2015, 286, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, Z.; Xie, S. Aerobic biodegradation of bisphenol a in river sediment and associated bacterial community change. Sci. Total Environ. 2014, 470, 1184–1188. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, Y.; Jiang, J.; Yang, S. Bisphenol A removal by submerged macrophytes and the contribution of epiphytic microorganisms to the removal process. Bull. Environ. Contam. Toxicol. 2017, 98, 770–775. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Kondo, F. Bisphenol a degradation in seawater is different from that in river water. Chemosphere 2005, 60, 1288–1292. [Google Scholar] [CrossRef]
- Sakai, K.; Yamanaka, H.; Moriyoshi, K.; Ohmoto, T.; Ohe, T. Biodegradation of bisphenol a and related compounds by sphingomonas sp. strain bp-7 isolated from seawater. Biosci. Biotechnol. Biochem. 2007, 71, 51–57. [Google Scholar] [CrossRef] [Green Version]
- Syranidou, E.; Christofilopoulos, S.; Politi, M.; Weyens, N.; Venieri, D.; Vangronsveld, J.; Kalogerakis, N. Bisphenol-a removal by the halophyte juncus acutus in a phytoremediation pilot: Characterization and potential role of the endophytic community. J. Hazard. Mater. 2016, 323, 350–358. [Google Scholar] [CrossRef] [Green Version]
- Loffredo, E.; Gattullo, E.; Traversa, A.; Senesi, N. Potential of various herbaceous species to remove the endocrine disruptor bisphenol a from aqueous media. Chemosphere 2010, 80, 1274–1280. [Google Scholar] [CrossRef]
- Gattullo, C.E.; Bährs, H.; Steinberg, C.E.; Loffredo, E. Removal of bisphenol a by the freshwater green alga monoraphidium braunii and the role of natural organic matter. Sci. Total Environ. 2012, 416, 501–506. [Google Scholar] [CrossRef]
- Chi, J.; Gao, J. Effects of potamogeton crispus l.-bacteria interactions on the removal of phthalate acid esters from surface water. Chemosphere 2015, 119, 59–64. [Google Scholar] [CrossRef]
- Reis, A.R.; Tabei, K.; Sakakibara, Y. Oxidation mechanism and overall removal rates of endocrine disrupting chemicals by aquatic plants. J. Hazard. Mater. 2014, 265, 79–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Yin, K.; Chen, L. Bacteria-mediated bisphenol a degradation. Appl. Microbiol. Biotechnol. 2013, 97, 5681–5689. [Google Scholar] [CrossRef] [PubMed]
- Phillips, G.; Willby, N.; Moss, B. Submerged macrophyte decline in shallow lakes: What have we learnt in the last forty years. Aquat. Bot. 2016, 135, 37–45. [Google Scholar] [CrossRef] [Green Version]
- Zhou, C.; An, S.; Jiang, J.; Yin, D.; Wang, Z.; Fang, C.; Sun, Z.; Qian, C. An in vitro propagation protocol of two submerged macrophytes for lake revegetation in east china. Aquat. Bot. 2006, 85, 44–52. [Google Scholar] [CrossRef]
- Kang, J.H.; Kondo, F. Bisphenol a degradation by bacteria isolated from river water. Arch. Environ. Contam. Toxicol. 2002, 43, 265–269. [Google Scholar] [CrossRef]
- Nakada, N.; Tanishima, T.; Shinohara, H.; Kiri, K.; Takada, H. Pharmaceutical chemicals and endocrine disrupters in municipal wastewater in tokyo and their removal during activated sludge treatment. Water Res. 2006, 40, 3297–3303. [Google Scholar] [CrossRef]
- Zhang, C.; Zeng, G.; Yuan, L.; Yu, J.; Li, J.; Huang, G.; Xi, B.; Liu, H. Aerobic degradation of bisphenol a by achromobacter xylosoxidans, strain b-16 isolated from compost leachate of municipal solid waste. Chemosphere 2007, 68, 181–190. [Google Scholar] [CrossRef]
- Klecka, G.M.; Gonsior, S.J.; West, R.J.; Goodwin, P.A.; Markham, D.A. Biodegradation of bisphenol a in aquatic environments: River die-away. Environ. Toxicol. Chem. 2001, 20, 2725–2735. [Google Scholar] [CrossRef]
- Saiyood, S.; Vangnai, A.S.; Thiravetyan, P.; Inthorn, D. Bisphenol a removal by the dracaena plant and the role of plant-associating bacteria. J. Hazard. Mater. 2010, 178, 777–785. [Google Scholar] [CrossRef]
- Okuhata, H.; Ikeda, K.; Miyasaka, H.; Takahashi, S.; Matsui, T.; Nakayama, H.; Kato, K.; Hirata, K. Floricultural salvia plants have a high ability to eliminate bisphenol a. J. Biosci. Bioeng. 2010, 110, 99–101. [Google Scholar] [CrossRef]
- Zazouli, M.A.; Mahdavi, Y.; Bazrafshan, E.; Balarak, D. Phytodegradation potential of bisphenol A from aqueous solution by Azolla filiculoides. J. Environ. Health Sci. 2014, 12, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berhane, T.M.; Levy, J.; Krekeler, M.P.S.; Danielson, N.D. Adsorption of bisphenol a and ciprofloxacin by palygorskite-montmorillonite: Effect of granule size, solution chemistry and temperature. Appl. Clay Sci. 2016, 132, 518–527. [Google Scholar] [CrossRef]
- Van Donk, E.; van de Bund, W.J. Impact of submerged macrophytes including charophytes on phyto- and zooplankton communities: Allelopathy versus other mechanisms. Aquat. Bot. 2002, 72, 261–274. [Google Scholar] [CrossRef]
- Noureddin, M.I.; Furumoto, T.; Ishida, Y.; Fukui, H. Absorption and metabolism of bisphenol a, a possible endocrine disruptor, in the aquatic edible plant, water convolvulus (Ipomoea aquatica). Biosci. Biotechnol. Biochem. 2004, 68, 1398–1402. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Ono, H.; Mori, Y.; Chuda, Y.; Mori, M. Oxygenation of bisphenol A to quinones by polyphenol oxidase in vegetables. J. Agric. Food Chem. 2002, 50, 4377–4381. [Google Scholar] [CrossRef]
- Skledar, D.G.; Mašič, L.P. Bisphenol A and its analogs: Do their metabolites have endocrine activity? Environ. Toxicol. Pharmacol. 2016, 47, 182–199. [Google Scholar] [CrossRef]
- Nakajima, N.; Ohshima, Y.; Serizawa, S.; Kouda, T.; Edmonds, J.S.; Shiraishi, F.; Aono, M.; Kubo, A.; Tamaoki, M.; Saji, H.; et al. Processing of bisphenol A by plant tissues: Glucosylation by cultured BY-2 cells and glucosylation/translocation by plants of Nicotiana tabacum. Plant Cell Physiol. 2002, 43, 1036–1042. [Google Scholar] [CrossRef] [Green Version]
- Sakuyama, H.; Endo, Y.; Fujimoto, K.; Hatana, Y. Oxidative degradation of alkylphenols by horseradish peroxidase. J. Biosci. Bioeng. 2003, 96, 227–231. [Google Scholar] [CrossRef]
- Imai, S.; Shiraishi, A.; Gamo, K.; Watanabe, I.; Okuhata, H.; Miyasaka, H.; Ikeda, K.; Bamba, T.; Hirata, K. Removal of phenolic endocrine disruptors by Portulaca oleracea. J. Biosci. Bioeng. 2007, 103, 420–426. [Google Scholar] [CrossRef]
- Skene, K.R. The evolution of physiology and development in the cluster root: Teaching an old dog new tricks? Plant Soil 2003, 248, 21–30. [Google Scholar] [CrossRef]


| Nutrient Conditions | Experimental Time | BPA Initial Concentration | Biota Treatment | BPA initial Concentration × Biota Treatment |
|---|---|---|---|---|
| Original condition | 3 | 5.27 * | 216.99 ** | 1.09 |
| 6 | 10.73 ** | 422.83 ** | 0.09 | |
| 9 | 13.75 ** | 612.51** | 0.37 | |
| 12 | 14.75 ** | 864.96 ** | 1.17 | |
| Nutrient addition condition | 3 | 10.24 ** | 215.36 ** | 0.85 |
| 6 | 43.55 ** | 226.59 ** | 19.97 ** | |
| 9 | 124.05 ** | 352.14 ** | 72.94 ** | |
| 12 | 190.93 ** | 544.61 ** | 113.16 ** |
| Nutrient Condition | Experimental Time | Species Type | BPA Initial Concentration | Biomass Density | Species Type × BPA Initial Concentration | Species Type × Biomass Density | BPA Initial Concentration × Biomass Density | Species Type × BPA Initial Concentration × Biomass Density |
|---|---|---|---|---|---|---|---|---|
| Original nutrient condition | 3 | 55.49 ** | 274.56 ** | 177.53 ** | 55.49 ** | 17.67 ** | 177.53 ** | 17.67 ** |
| 6 | 75.93 ** | 259.22 ** | 176.47 ** | 75.93 ** | 34.78 ** | 176.47 ** | 34.78 ** | |
| 9 | 56.43 ** | 164.25 ** | 137.32 ** | 56.43 ** | 41.14 ** | 137.32 ** | 41.14 ** | |
| 12 | 136.78 ** | 311.06 ** | 281.59 ** | 136.78 ** | 117.48 ** | 281.59 ** | 117.48 ** | |
| Nutrient addition condition | 3 | 223.35 ** | 1860.43 ** | 1860.43 ** | 223.35 ** | 223.35 ** | 1860.43 ** | 223.35 ** |
| 6 | 200.49 ** | 750.98 ** | 750.98 ** | 200.49 ** | 200.49 ** | 750.98 ** | 200.49 ** | |
| 9 | 109.38 ** | 215.51 ** | 215.57 ** | 111.21 ** | 111.17 ** | 213.02 ** | 113.01 ** | |
| 12 | 25.02 ** | 41.23 ** | 41.23 ** | 25.02 ** | 25.02 ** | 41.23 ** | 25.02 ** |
| Nutrient Condition | Plant Species | BPA Initial Concentration | Biomass Density | BPA Initial Concentration × Biomass Density |
|---|---|---|---|---|
| Original nutrient condition | C. demersum | 2.004 | 117.936 ** | 26.050 ** |
| M. spicatum | 35.122 ** | 188.311 ** | 6.906 * | |
| Nutrient addition condition | C. demersum | 29.584 ** | 113.300 ** | 1.412 |
| M. spicatum | 112.590 ** | 225.467 ** | 9.205 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, C.; Zhang, G.; Jiang, J. Enhanced Phytoremediation of Bisphenol A in Polluted Lake Water by Seedlings of Ceratophyllum demersum and Myriophyllum spicatum from In Vitro Culture. Int. J. Environ. Res. Public Health 2021, 18, 810. https://doi.org/10.3390/ijerph18020810
Zhao C, Zhang G, Jiang J. Enhanced Phytoremediation of Bisphenol A in Polluted Lake Water by Seedlings of Ceratophyllum demersum and Myriophyllum spicatum from In Vitro Culture. International Journal of Environmental Research and Public Health. 2021; 18(2):810. https://doi.org/10.3390/ijerph18020810
Chicago/Turabian StyleZhao, Chong, Guosen Zhang, and Jinhui Jiang. 2021. "Enhanced Phytoremediation of Bisphenol A in Polluted Lake Water by Seedlings of Ceratophyllum demersum and Myriophyllum spicatum from In Vitro Culture" International Journal of Environmental Research and Public Health 18, no. 2: 810. https://doi.org/10.3390/ijerph18020810





