Phytotoxic Effects of Polyethylene Microplastics on the Growth of Food Crops Soybean (Glycine max) and Mung Bean (Vigna radiata)
Abstract
:1. Introduction
- (1)
- How MPs with different sizes (6.5 μm and 13 μm) affect seed germination characteristics of (a) soybean (Glycine max) and (b) mung bean (Vigna radiata)?
- (2)
- What are the differences between two studied species in response to applied MPs?
2. Materials and Methods
2.1. Preparation of Suspensions of MPs
2.2. Germination Test
2.3. Statistical Analysis
3. Results and Discussion
3.1. Effect of PE-MPs on Seed Vigor of Soybean and Mung Bean
3.1.1. Effect of PE-MPs on Germination Energy of Soybean and Mung Bean
3.1.2. Effect of PE-MPs on Germination Energy of Soybean and Mung Bean
3.1.3. Effect of PE-MPs on Mean Germination Speed of Soybean and Mung Bean
3.1.4. Effect of PE-MPs on Germination Rate of Soybean and Mung Bean
3.2. Effect of PE-MPs on the Growth of Soybean and Mung Bean
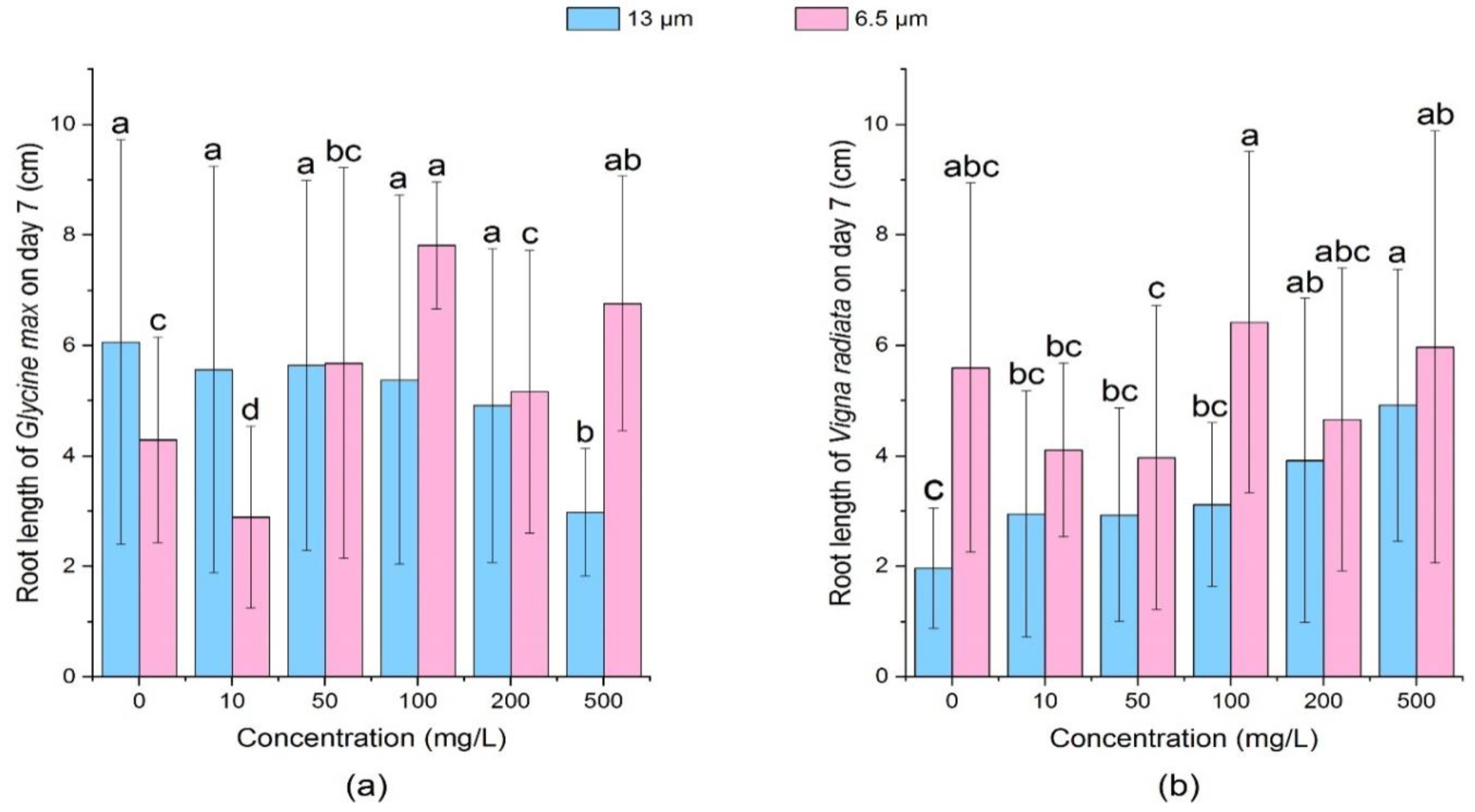
3.2.1. Effect of PE-MPs on the Root Length and Shoot Length
3.2.2. Effects of PE-MPs on the Dry Weight
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barnes, D.K.A.; Galgani, F.; Thompson, R.C.; Barlaz, M. Accumulation and fragmentation of plastic debris in global environments. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1985–1998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, X.; Wang, J. The chemical behaviors of microplastics in marine environment: A Review. Mar. Pollut. Bull. 2019, 142, 1–14. [Google Scholar] [CrossRef]
- Liu, G.; Zhu, Z.; Yang, Y.; Sun, Y.; Yu, F.; Ma, J. Sorption behavior and mechanism of hydrophilic organic chemicals to virgin and aged microplastics in freshwater and seawater. Environ. Pollut. 2019, 246, 26–33. [Google Scholar] [CrossRef]
- Rujnić-Sokele, M.; Pilipović, A. Challenges and opportunities of biodegradable plastics: A mini review. Waste Manag. Res. J. Sustain. Circ. Econ. 2017, 35, 132–140. [Google Scholar] [CrossRef]
- Batel, A.; Linti, F.; Scherer, M.; Erdinger, L.; Braunbeck, T. Transfer of benzo[a]pyrene from microplastics to Artemia nauplii and further to zebrafish via a trophic food web experiment: CYP1A induction and visual tracking of persistent organic pollutants: Trophic transfer of microplastics and associated POPs. Environ. Toxicol. Chem. 2016, 35, 1656–1666. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Peng, J.; Wang, J.; Wang, K.; Bao, S. Occurrence of microplastics in the beach sand of the Chinese Inner Sea: The Bohai Sea. Environ. Pollut. 2016, 214, 722–730. [Google Scholar] [CrossRef] [PubMed]
- Chae, Y.; An, Y.-J. Current research trends on plastic pollution and ecological impacts on the soil ecosystem: A Review. Environ. Pollut. 2018, 240, 387–395. [Google Scholar] [CrossRef]
- Yang, C.Z.; Yaniger, S.I.; Jordan, V.C.; Klein, D.J.; Bittner, G.D. Most plastic products release estrogenic chemicals: A potential health problem that can be solved. Environ. Health Perspect. 2011, 119, 989–996. [Google Scholar] [CrossRef]
- Bhatt, P.; Pathak, V.M.; Bagheri, A.R.; Bilal, M. Microplastic contaminants in the aqueous environment, fate, toxicity consequences, and remediation strategies. Environ. Res. 2021, 200, 111762. [Google Scholar] [CrossRef]
- Eerkes-Medrano, D.; Thompson, R.C.; Aldridge, D.C. Microplastics in freshwater systems: A review of the emerging threats, identification of knowledge gaps and prioritisation of research needs. Water Res. 2015, 75, 63–82. [Google Scholar] [CrossRef]
- Avio, C.G.; Gorbi, S.; Regoli, F. Plastics and microplastics in the oceans: From emerging pollutants to emerged threat. Mar. Environ. Res. 2017, 128, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.C.; Moore, C.J.; vom Saal, F.S.; Swan, S.H. Plastics, the environment and human health: Current consensus and future trends. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2153–2166. [Google Scholar] [CrossRef] [PubMed]
- Santana, M.F.M.; Moreira, F.T.; Turra, A. Trophic transference of microplastics under a low exposure scenario: Insights on the likelihood of particle cascading along marine food-webs. Mar. Pollut. Bull. 2017, 121, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Santana, M.F.M.; Ascer, L.G.; Custódio, M.R.; Moreira, F.T.; Turra, A. Microplastic contamination in natural mussel beds from a Brazilian urbanized coastal region: Rapid evaluation through bio-assessment. Mar. Pollut. Bull. 2016, 106, 183–189. [Google Scholar] [CrossRef]
- Rummel, C.D.; Jahnke, A.; Gorokhova, E.; Kühnel, D.; Schmitt-Jansen, M. Impacts of biofilm formation on the fate and potential effects of microplastic in the aquatic environment. Environ. Sci. Technol. Lett. 2017, 4, 258–267. [Google Scholar] [CrossRef] [Green Version]
- Auta, H.S.; Emenike, C.U.; Fauziah, S.H. Screening of Bacillus strains isolated from Mangrove ecosystems in Peninsular Malaysia for microplastic degradation. Environ. Pollut. 2017, 231, 1552–1559. [Google Scholar] [CrossRef]
- de Paiva Pinheiro, S.K.; de Medeiros Chaves, M.; Rangel Miguel, T.B.A.; de Freitas Barros, F.C.; Farias, C.P.; Ferreira, O.P.; de Castro Miguel, E. Toxic effects of silver nanoparticles on the germination and root development of Lettuce (Lactuca sativa). Aust. J. Bot. 2020, 68, 127. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, J.; Wu, W.-M.; Zhao, J.; Song, Y.; Gao, L.; Yang, R.; Jiang, L. Biodegradation and mineralization of polystyrene by plastic-eating Mealworms: Part 1. Chemical and physical characterization and isotopic tests. Environ. Sci. Technol. 2015, 49, 12080–12086. [Google Scholar] [CrossRef]
- Hermabessiere, L.; Dehaut, A.; Paul-Pont, I.; Lacroix, C.; Jezequel, R.; Soudant, P.; Duflos, G. Occurrence and effects of plastic additives on marine environments and organisms: A review. Chemosphere 2017, 182, 781–793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hahladakis, J.N.; Velis, C.A.; Weber, R.; Iacovidou, E.; Purnell, P. An overview of chemical additives present in plastics: Migration, release, fate and environmental impact during their use, disposal and recycling. J. Hazard. Mater. 2018, 344, 179–199. [Google Scholar] [CrossRef]
- Hofmann, U.; Schlosser, D. Biochemical and physicochemical processes contributing to the removal of endocrine-disrupting chemicals and pharmaceuticals by the aquatic ascomycete Phoma sp. UHH 5-1-03. Appl. Microbiol. Biotechnol. 2016, 100, 2381–2399. [Google Scholar] [CrossRef]
- Im, J.; Löffler, F.E. Fate of bisphenol A in terrestrial and aquatic environments. Environ. Sci. Technol. 2016, 50, 8403–8416. [Google Scholar] [CrossRef] [PubMed]
- Vom Saal, F.S.; Myers, J.P. BisphenolA and risk of metabolic disorders. JAMA 2008, 300, 1353–1355. [Google Scholar] [CrossRef] [PubMed]
- Hirai, H.; Takada, H.; Ogata, Y.; Yamashita, R.; Mizukawa, K.; Saha, M.; Kwan, C.; Moore, C.; Gray, H.; Laursen, D.; et al. Organic micropollutants in marine plastics debris from the open ocean and remote and urban beaches. Mar. Pollut. Bull. 2011, 62, 1683–1692. [Google Scholar] [CrossRef] [PubMed]
- Rehse, S.; Kloas, W.; Zarfl, C. Microplastics reduce short-term effects of environmental contaminants. Part I: Effects of bisphenol A on freshwater zooplankton are lower in presence of polyamide particles. Int. J. Environ. Res. Public. Health 2018, 15, 280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.; Tian, J.-Y.; Xu, R.; Zhang, Z.-Y.; Yang, G.-P.; Wang, X.-D.; Lai, J.-G.; Chen, R. Effects of microplastics exposure on ingestion, fecundity, development, and dimethylsulfide production in Tigriopus japonicus (Harpacticoida, Copepod). Environ. Pollut. 2020, 267, 115429. [Google Scholar] [CrossRef] [PubMed]
- Khalid, N.; Aqeel, M.; Noman, A. Microplastics could be a threat to plants in terrestrial systems directly or indirectly. Environ. Pollut. 2020, 267, 115653. [Google Scholar] [CrossRef]
- Chen, G.; Fu, Z.; Yang, H.; Wang, J. An overview of analytical methods for detecting microplastics in the atmosphere. TrAC Trends Anal. Chem. 2020, 130, 115981. [Google Scholar] [CrossRef]
- Tsang, Y.Y.; Mak, C.W.; Liebich, C.; Lam, S.W.; Sze, E.T.P.; Chan, K.M. Spatial and temporal variations of coastal microplastic pollution in Hong Kong. Mar. Pollut. Bull. 2020, 161, 111765. [Google Scholar] [CrossRef]
- Shah, Z.; Krumholz, L.; Aktas, D.F.; Hasan, F.; Khattak, M.; Shah, A.A. Degradation of polyester polyurethane by a newly isolated soil bacterium, Bacillus subtilis Strain MZA-75. Biodegradation 2013, 24, 865–877. [Google Scholar] [CrossRef]
- Qi, Y.; Yang, X.; Pelaez, A.M.; Huerta Lwanga, E.; Beriot, N.; Gertsen, H.; Garbeva, P.; Geissen, V. Macro- and micro- plastics in soil-plant system: Effects of plastic mulch film residues on wheat (Triticum aestivum) growth. Sci. Total Environ. 2018, 645, 1048–1056. [Google Scholar] [CrossRef]
- de Souza Machado, A.A.; Lau, C.W.; Kloas, W.; Bergmann, J.; Bachelier, J.B.; Faltin, E.; Becker, R.; Görlich, A.S.; Rillig, M.C. Microplastics can change soil properties and affect plant performance. Environ. Sci. Technol. 2019, 53, 6044–6052. [Google Scholar] [CrossRef] [Green Version]
- Bosker, T.; Bouwman, L.J.; Brun, N.R.; Behrens, P.; Vijver, M.G. Microplastics accumulate on pores in seed capsule and delay germination and root growth of the terrestrial vascular plant Lepidium sativum. Chemosphere 2019, 226, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.-R.; Wang, C.-X.; Dong, F.-Q.; Gao, Z.-Y.; Zhang, C.-J.; Zhang, X.-J.; Fu, L.-M.; Wang, Y.; Zhang, J.-P. Uptake and translocation of styrene maleic anhydride nanoparticles in Murraya exotica plants as revealed by noninvasive, real-time optical bio-imaging. Environ. Sci. Technol. 2019, 53, 1471–1481. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Chen, H.; Liao, Y.; Ye, Z.; Li, M.; Klobučar, G. Ecotoxicity and genotoxicity of polystyrene microplastics on higher plant Vicia faba. Environ. Pollut. 2019, 250, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Liu, Y.; Song, Z. Effects of polyethylene microplastic on the phytotoxicity of di-n-butyl phthalate in Lettuce (Lactuca sativa L. Var. Ramosa Hort). Chemosphere 2019, 237, 124482. [Google Scholar] [CrossRef] [PubMed]
- Boots, B.; Russell, C.W.; Green, D.S. Effects of microplastics in soil ecosystems: Above and below ground. Environ. Sci. Technol. 2019, 53, 11496–11506. [Google Scholar] [CrossRef]
- Sun, X.-D.; Yuan, X.-Z.; Jia, Y.; Feng, L.-J.; Zhu, F.-P.; Dong, S.-S.; Liu, J.; Kong, X.; Tian, H.; Duan, J.-L.; et al. Differentially charged nanoplastics demonstrate distinct accumulation in Arabidopsis thaliana. Nat. Nanotechnol. 2020, 15, 755–760. [Google Scholar] [CrossRef]
- Rochman, C.M. Microplastics research—From sink to source. Science 2018, 360, 28–29. [Google Scholar] [CrossRef]
- Xue, W.; Berendse, F.; Bezemer, T.M. Spatial heterogeneity in plant–soil feedbacks alters competitive interactions between two grassland plant species. Funct. Ecol. 2018, 32, 2085–2094. [Google Scholar] [CrossRef] [Green Version]
- Maity, S.; Pramanick, K. Perspectives and challenges of micro/nanoplastics-induced toxicity with special reference to phytotoxicity. Glob. Change Biol. 2020, 26, 3241–3250. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, X.; Hu, J.; Peng, J.; Qu, J. Ecotoxicity of polystyrene microplastics to submerged carnivorous Utricularia vulgaris plants in freshwater ecosystems. Environ. Pollut. 2020, 114830. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Bento, C.P.M.; Chen, H.; Zhang, H.; Xue, S.; Lwanga, E.H.; Zomer, P.; Ritsema, C.J.; Geissen, V. Influence of microplastic addition on glyphosate decay and soil microbial activities in Chinese loess soil. Environ. Pollut. 2018, 242, 338e347. [Google Scholar] [CrossRef] [PubMed]
- Fei, Y.; Huang, S.; Zhang, H.; Tong, Y.; Wen, D.; Xia, X. Response of soil enzyme activities and bacterial communities to the accumulation of microplastics in an acid cropped soil. Sci. Total Environ. 2020, 707, 135634. [Google Scholar] [CrossRef]
- Liu, H.; Yang, X.; Liu, G.; Liang, C.; Xue, S.; Chen, H.; Ritsema, C.J.; Geissen, V. Response of soil dissolved organic matter to microplastic addition in Chinese loess soil. Chemosphere 2017, 185, 907–917. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhao, Y.; Wang, J.; Zhang, M.; Jia, W.; Qin, X. LDPE microplastic films alter microbial community composition and enzymatic activities in soil. Environ. Pollut. 2019, 254, 112983. [Google Scholar] [CrossRef]
- Amaral-Zettler, L.A.; Zettler, E.R.; Mincer, T.J. Ecology of the plastisphere. Nat. Rev. Microbiol. 2020, 18, 139–151. [Google Scholar] [CrossRef]
- Wright, R.J.; Erni-Cassola, G.; Zadjelovic, V.; Latva, M.; Christie-Oleza, J.A. Marine plastic debris: A new surface for microbial colonization. Environ. Sci. Technol. 2020, 54, 11657–11672. [Google Scholar] [CrossRef]
- Wu, X.; Lyu, X.; Li, Z.; Gao, B.; Zeng, X.; Wu, J.; Sun, Y. Transport of polystyrene nanoplastics in natural soils: Effect of soil properties, ionic strength and cation type. Sci. Total Environ. 2020, 707, 136065. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, X.; Zhang, S.; Zhang, S.; Sun, Y. Interactions of microplastics and cadmium on plant growth and arbuscular mycorrhizal fungal communities in an agricultural soil. Chemosphere 2020, 254, 126791. [Google Scholar] [CrossRef]
- Zang, H.; Zhou, J.; Marshall, M.R.; Chadwick, D.R.; Wen, Y.; Jones, D.L. Microplastics in the agroecosystem: Are they an emerging threat to the plant-soil system? Soil Biol. Biochem. 2020, 148, 107926. [Google Scholar] [CrossRef]
- Powell, J.R.; Rillig, M.C. Biodiversity of arbuscular mycorrhizal fungi and ecosystem function. New Phytol. 2018, 220, 1059–1075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Wang, Y.; Sun, X.; Peng, Y.; Xiao, L. Mixing effect of polylactic acid microplastic and straw residue on soil property and ecological function. Chemosphere 2020, 243, 125271. [Google Scholar] [CrossRef] [PubMed]
- Bender, S.F.; Wagg, C.; van der Heijden, M.G.A. An underground revolution: Biodiversity and soil ecological engineering for agricultural sustainability. Trends Ecol. Evol. 2016, 31, 440–452. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.; Bafana, A.; Naoghare, P.K.; Krishnamurthi, K.; Sivanesan, S. Environmental prevalence, fate, impacts, and mitigation of microplastics—A critical review on present understanding and future research scope. Environ. Sci. Pollut. Res. 2021, 28, 4951–4974. [Google Scholar] [CrossRef] [PubMed]
- Aliyar, Z.B.; Shafiei, A.B.; Seyedi, N.; Rezapour, S.; Moghanjugi, S.M. Effect of traffic-induced air pollution on seed germination of Arizona Cypress(Cupressus arizonica Green) and Black Pine (Pinus nigra Arnold). Urban For. Urban Green. 2020, 55, 126841. [Google Scholar] [CrossRef]
- Bai, Y.; Zhang, Q.; Wang, B.; Zhang, M.; Xu, Y.; Li, S.; Zhao, Y.; Yu, Z. Plasma pharmacokinetics, bioavailability, and tissue distribution of four c-glycosyl flavones from mung bean (Vigna radiata L.) seed extracts in rat by ultrahigh-performance liquid chromatography–tandem mass spectrometry. J. Agric. Food Chem. 2017, 65, 5570–5580. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, M.; Feng, F.; Tian, Z. Toward a “Green Revolution” for soybean. Mol. Plant 2020, 13, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Xin, X.; Zhao, F.; Rho, J.Y.; Goodrich, S.L.; Sumerlin, B.S.; Hea, Z. Use of polymeric nanoparticles to improve seed germination and plant growth under copper stress. Sci. Total Environ. 2020, 745, 141055. [Google Scholar] [CrossRef] [PubMed]
- Debeaujon, I.; Leon-Kloosterziel, K.M.; Koornneef, M. Influence of testa on seed dormany, germination and longevity in Arabidopsis. Plant Physiol. 2000, 122, 403–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calero, E.; West, S.H.; Hinson, K. Water absorption of soyabean seeds and associated causal factors. Crop Sci. 1981, 21, 926–933. [Google Scholar] [CrossRef]
- Mahakham, W.; Sarmah, A.K.; Maensiri, S.; Theerakulpisut, P. Nanopriming technology for enhancing germination and starch metabolism of aged rice seeds using phytosynthesized silver nanoparticles. Sci. Rep. 2017, 7, 1–21. [Google Scholar] [CrossRef]
- Fincheira, P.; Tortella, G.; Duran, N.; Seabra, A.B.; Rubilar, O. Current applications of nanotechnology to develop plant growth inducer agents as an innovation strategy. Crit. Rev. Biotechnol. 2020, 40, 15–30. [Google Scholar] [CrossRef]
- Xin, X.; He, Z.; Hill, M.R.; Niedz, R.P.; Jiang, X.; Sumerlin, B.S. Efficiency of biodegradable and ph-responsive polysuccinimide nanoparticles (PSI-NPs) as smart nanodelivery systems in grapefruit: In vitro cellular investigation. Macromol. Biosci. 2018, 18, 1800159. [Google Scholar] [CrossRef] [PubMed]
- Pignattelli, S.; Broccoli, A.; Renzi, M. Physiological responses of Garden Cress (L. sativum) to different types of microplastics. Sci. Total Environ. 2020, 727, 138609. [Google Scholar] [CrossRef] [PubMed]
- Rillig, M.C.; Leifheit, E.; Lehmann, J. Micro-plastic effects on carbon cycling processes in soils. PLoS Biol. 2021, 19, e3001130. [Google Scholar] [CrossRef] [PubMed]
- Amelia, T.S.M.; Khalik, W.M.A.W.M.; Ong, M.C.; Shao, Y.T.; Pan, H.J.; Bhubalan, K. Marine microplastics as vectors of major ocean pollutants and its hazards to the marine ecosystem and humans. Prog. Earth Planet Sci. 2021, 8, 12. [Google Scholar] [CrossRef]
- Lian, J.; Wu, J.; Xiong, H.; Zeb, A.; Yang, T.; Su, X.; Su, L.; Liu, W. Impact of polystyrene nanaoplastics (PSNPs) on seed germination and seedling growth of wheat (Triticum aestivum L.). J. Hazard. Mater. 2019, 38, 121620. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, J.; Zou, M.; Jia, Z.; Zhou, S.; Li, Y. Microplastics in soils: A review of methods, occurrence, fate, transport, ecological and environmental risks. Sci. Total Environ. 2020, 748, 141368. [Google Scholar] [CrossRef]









| Index | Formula |
|---|---|
| Germination Rate (GR) | |
| Germination Index (GI) | |
| Mean Germination Time (MGT) (d) | |
| Germination Energy (GE) |
| PE (µm) | Concentration (mg/L) | Root Length on Day 3 (cm) | Root Length on Day 7 (cm) | Shoot Length on Day 3 (cm) | Shoot Length on Day 7 (cm) | Dry Weight on Day 3 (g) | Dry Weight on Day 7 (g) |
|---|---|---|---|---|---|---|---|
| 13 | 0 | 2.27 ± 1.67 a | 6.05 ± 5.21 a | 3.24 ± 1.43 a | 6.25 ± 2.19 a | 1.44 ± 0.83 a | 1.30 ± 0.04 a |
| 10 | 1.84 ± 1.41 a | 5.56 ± 5.41 ab | 2.50 ± 0.96 bc | 5.36 ± 1.39 ab | 1.25 ± 0.35 a | 0.92 ± 0.54 a | |
| 50 | 2.13 ± 1.27 a | 5.64 ± 5.42 ab | 2.95 ± 0.99 ab | 5.80 ± 2.05 ab | 1.41 ± 0.01 a | 1.57 ± 0.77 a | |
| 100 | 1.83 ± 1.41 a | 5.38 ± 5.16 ab | 2.35 ± 0.94 bc | 4.72 ± 1.81 b | 1.45 ± 0.05 a | 1.14 ± 0.23 a | |
| 200 | 2.21 ± 1.19 a | 4.91 ± 3.46 ab | 2.68 ± 0.59 abc | 5.05 ± 1.78 b | 1.44 ± 0.09 a | 1.27 ± 0.01 a | |
| 500 | 1.70 ± 1.35 a | 3.02 ± 1.45 b | 2.23 ± 0.76 c | 3.40 ± 1.37 c | 1.35 ± 0.05 a | 0.90 ± 0.54 a | |
| 6.5 | 0 | 1.57 ± 1.18 a | 4.29 ± 2.91 bc | 2.22 ± 0.66 b | 6.08 ± 2.09 ab | 1.53 ± 0.06 a | 0.92 ± 0.57 a |
| 10 | 1.83 ± 1.54 a | 2.99 ± 3.02 c | 2.37 ± 0.83 ab | 6.26 ± 2.62 a | 0.89 ± 0.42 b | 1.34 ± 0.17 a | |
| 50 | 1.84 ± 0.77 a | 5.68 ± 4.98 abc | 2.42 ± 0.90 ab | 6.52 ± 3.03 ab | 1.26 ± 0.45 ab | 1.34 ± 0.29 a | |
| 100 | 1.38 ± 1.46 a | 7.84 ± 5.34 a | 2.68 ± 0.73 ab | 7.50 ± 3.09 ab | 1.29 ± 0.13 ab | 1.29 ± 0.39 a | |
| 200 | 1.70 ± 1.46 a | 5.16 ± 4.60 abc | 2.74 ± 0.96 ab | 5.52 ± 2.78 b | 1.54 ± 0.48 a | 1.24 ± 0.11 a | |
| 500 | 1.84 ± 0.94 a | 6.80 ± 3.96 ab | 2.95 ± 0.61 a | 7.00 ± 2.17 ab | 1.46 ± 0.08 ab | 1.40 ± 0.09 a |
| PE (µm) | Concentration (mg/L) | Root Length on day 3 (cm) | Root Length on Day 7 (cm) | Shoot Length on Day 3 (cm) | Shoot Length on Day 7 (cm) | Dry Weight on Day 3 (g) | Dry Weight on Day 7 (g) |
|---|---|---|---|---|---|---|---|
| 13 | 0 | 1.88 ± 1.02 ab | 1.97 ± 1.09 c | 3.25 ± 1.24 bc | 9.73 ± 2.79 cd | 0.55 ± 0.03 a | 0.36 ± 0.02 ab |
| 10 | 2.21 ± 0.82 a | 2.95 ± 2.23 bc | 3.01 ± 0.74 cd | 10.03 ± 4.01 cd | 0.53 ± 0.22 ab | 0.50 ± 0.16 a | |
| 50 | 1.52 ± 0.99 b | 2.93 ± 1.93 bc | 3.04 ± 1.37 cd | 10.46 ± 3.66 bc | 0.52 ± 0.17 ab | 0.34 ± 0.33 ab | |
| 100 | 2.4 ± 1.01 a | 3.12 ± 1.48 bc | 3.69 ± 1.14 ab | 8.28 ± 2.75 d | 0.45 ± 0.03 c | 0.32 ± 0.10 b | |
| 200 | 2.48 ± 1.15 a | 3.92 ± 2.93 ab | 3.90 ± 0.84 a | 11.85 ± 2.82 ab | 0.48 ± 0.02 bc | 0.38 ± 0.01 ab | |
| 500 | 2.05 ± 1.35 ab | 4.92 ± 2.46 a | 2.59 ± 1.04 d | 12.50 ± 3.38 a | 0.50 ± 0.28 bc | 0.36 ± 0.04 ab | |
| 6.5 | 0 | 2.35 ± 1.26 a | 5.60 ± 3.34 abc | 2.90 ± 0.86 cd | 9.76 ± 3.74 c | 0.48 ± 0.02 ab | 0.36 ± 0.01 a |
| 10 | 2.68 ± 1.61 a | 4.11 ± 1.57 bc | 3.19 ± 0.94 bcd | 8.90 ± 2.71 c | 0.48 ± 0.02 ab | 0.39 ± 0.01 a | |
| 50 | 2.48 ± 1.36 a | 3.97 ± 2.76 c | 3.37 ± 0.91 abc | 10.48 ± 2.95 bc | 0.48 ± 0.01 ab | 0.38 ± 0.04 a | |
| 100 | 2.74 ± 1.02 a | 6.42 ± 3.09 a | 3.79 ± 0.89 a | 12.23 ± 2.60 ab | 0.48 ± 0.02 a | 0.40 ± 0.02 a | |
| 200 | 2.09 ± 0.95 a | 4.66 ± 2.74 abc | 2.83 ± 0.94 d | 11.76 ± 2.20 ab | 0.45 ± 0.01 b | 0.42 ± 0.04 a | |
| 500 | 2.58 ± 1.40 a | 5.97 ± 3.91 ab | 3.52 ± 0.97 ab | 12.59 ± 3.40 a | 0.47 ± 0.01 ab | 0.34 ± 0.10 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Liu, Y.; Kaur, M.; Yao, Z.; Chen, T.; Xu, M. Phytotoxic Effects of Polyethylene Microplastics on the Growth of Food Crops Soybean (Glycine max) and Mung Bean (Vigna radiata). Int. J. Environ. Res. Public Health 2021, 18, 10629. https://doi.org/10.3390/ijerph182010629
Wang L, Liu Y, Kaur M, Yao Z, Chen T, Xu M. Phytotoxic Effects of Polyethylene Microplastics on the Growth of Food Crops Soybean (Glycine max) and Mung Bean (Vigna radiata). International Journal of Environmental Research and Public Health. 2021; 18(20):10629. https://doi.org/10.3390/ijerph182010629
Chicago/Turabian StyleWang, Lin, Yi Liu, Mandeep Kaur, Zhisheng Yao, Taizheng Chen, and Ming Xu. 2021. "Phytotoxic Effects of Polyethylene Microplastics on the Growth of Food Crops Soybean (Glycine max) and Mung Bean (Vigna radiata)" International Journal of Environmental Research and Public Health 18, no. 20: 10629. https://doi.org/10.3390/ijerph182010629
APA StyleWang, L., Liu, Y., Kaur, M., Yao, Z., Chen, T., & Xu, M. (2021). Phytotoxic Effects of Polyethylene Microplastics on the Growth of Food Crops Soybean (Glycine max) and Mung Bean (Vigna radiata). International Journal of Environmental Research and Public Health, 18(20), 10629. https://doi.org/10.3390/ijerph182010629








