Microplastics in the Center of Mediterranean: Comparison of the Two Calabrian Coasts and Distribution from Coastal Areas to the Open Sea
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sample Collection and Preparation
2.3. Microplastics Quantification and Characterization
2.4. Contamination Prevention
3. Results and Discussion
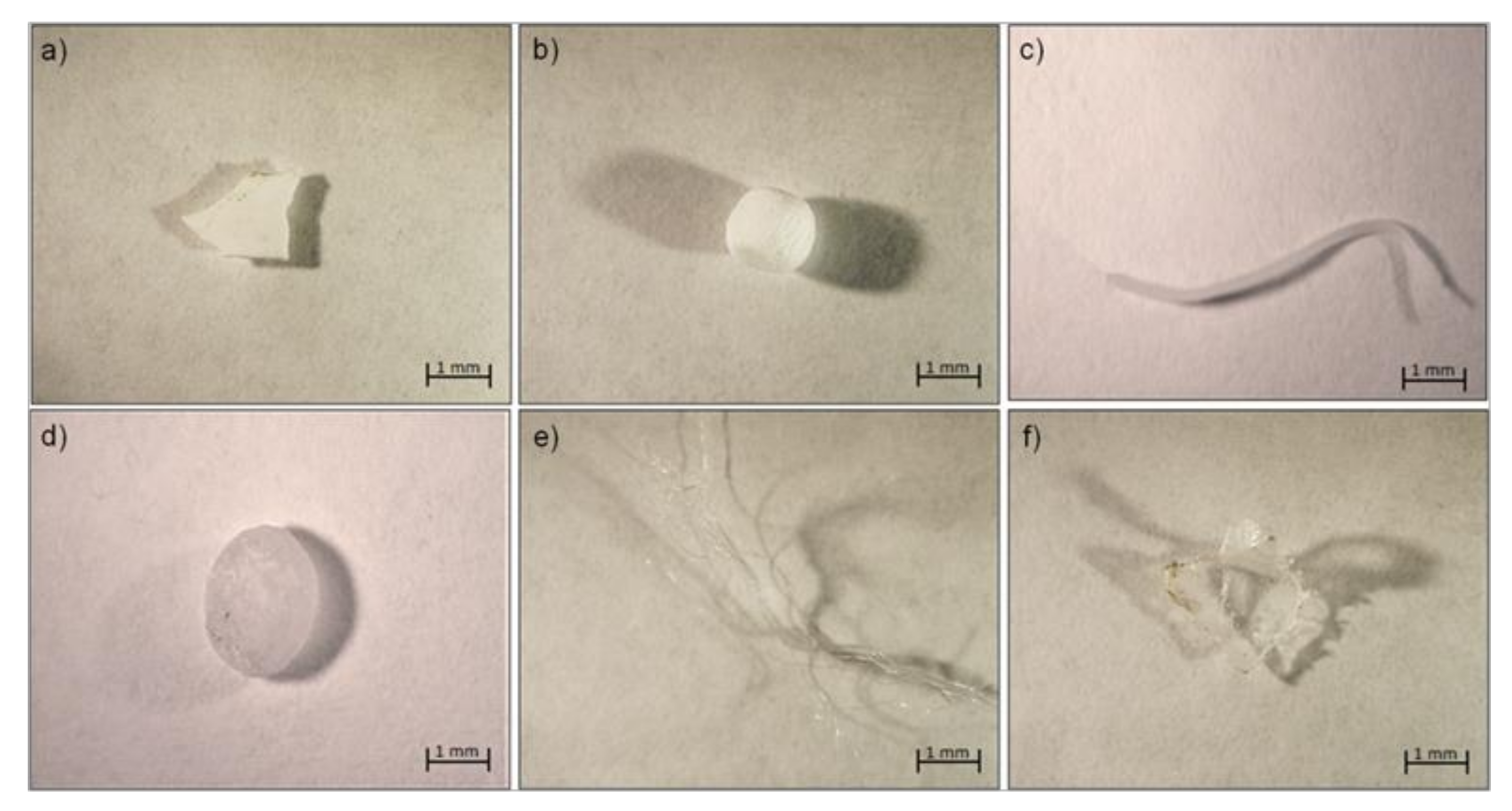
3.1. Microplastic Abundance, Size, Shape, and Color
3.2. Microplastic Composition
3.3. Microplastic Distribution
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Eriksen, M.; Lebreton, L.C.M.; Carson, H.S.; Thiel, M.; Moore, C.J.; Borerro, J.C.; Galgani, F.; Ryan, P.G.; Reisser, J. Plastic Pollution in the World’s Oceans: More than 5 Trillion Plastic Pieces Weighing over 250,000 Tons Afloat at Sea. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, G.X.; Huang, D.; Ji, J.H.; Völker, C.; Wurm, F.R. Seawater-Degradable Polymers—Fighting the Marine Plastic Pollution. Adv. Sci. 2021, 8. [Google Scholar] [CrossRef]
- Zhang, K.; Hamidian, A.H.; Tubić, A.; Zhang, Y.; Fang, J.K.H.; Wu, C.; Lam, P.K.S. Understanding plastic degradation and microplastic formation in the environment: A review. Environ. Pollut. 2021, 274. [Google Scholar] [CrossRef]
- Cai, L.; Wu, D.; Xia, J.; Shi, H.; Kim, H. Influence of physicochemical surface properties on the adhesion of bacteria onto four types of plastics. Sci. Total Environ. 2019, 671, 1101–1107. [Google Scholar] [CrossRef]
- Fotopoulou, K.N.; Karapanagioti, H.K. Surface properties of beached plastics. Environ. Sci. Pollut. Res. 2015, 22, 11022–11032. [Google Scholar] [CrossRef] [PubMed]
- Fendall, L.S.; Sewell, M.A. Contributing to marine pollution by washing your face: Microplastics in facial cleansers. Mar. Pollut. Bull. 2009, 58, 1225–1228. [Google Scholar] [CrossRef] [PubMed]
- Arthur, C.; Baker, J.; Bamford, H. Proceedings of the International Research Workshop on the Occurrence, Effects, and Fate of Microplastic Marine Debris, Tacoma, WA, USA, 9–11 September 2008; University of Washington Tacoma: Tacoma, WA, USA, 2009. [Google Scholar]
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef] [PubMed]
- Obbard, R.W.; Sadri, S.; Wong, Y.Q.; Khitun, A.A.; Baker, I.; Thompson, R.C. Global warming releases microplastic legacy frozen in Arctic Sea ice. Earth’s Futur. 2014, 2, 315–320. [Google Scholar] [CrossRef]
- Reed, S.; Clark, M.; Thompson, R.; Hughes, K.A. Microplastics in marine sediments near Rothera Research Station, Antarctica. Mar. Pollut. Bull. 2018, 133, 460–463. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Liu, X.; Huang, W.; Li, J.; Wang, C.; Zhang, D.; Zhang, C. Microplastic pollution in deep-sea sediments and organisms of the Western Pacific Ocean. Environ. Pollut. 2020, 259. [Google Scholar] [CrossRef] [PubMed]
- Graham, E.R.; Thompson, J.T. Deposit- and suspension-feeding sea cucumbers (Echinodermata) ingest plastic fragments. J. Exp. Mar. Bio. Ecol. 2009, 368, 22–29. [Google Scholar] [CrossRef]
- Nor, N.H.M.; Obbard, J.P. Microplastics in Singapore’s Coastal Mangrove Ecosystems. Mar. Pollut. Bull. 2014, 79, 278–283. [Google Scholar] [CrossRef]
- Thompson, C.R.; Olsen, Y.; Mitchell, P.R.; Davis, A.; Rowland, J.S.; John, W.G.A.; McGonigle, D.; Russell, E.A. Lost at Sea: Where Is All the Plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef] [PubMed]
- Van Cauwenberghe, L.; Vanreusel, A.; Mees, J.; Janssen, C.R. Microplastic pollution in deep-sea sediments. Environ. Pollut. 2013, 182, 495–499. [Google Scholar] [CrossRef]
- Dantas, D.V.; Barletta, M.; da Costa, M.F. The seasonal and spatial patterns of ingestion of polyfilament nylon fragments by estuarine drums (Sciaenidae). Environ. Sci. Pollut. Res. 2012, 19, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, C.; Burton, H. Origins and Biological Accumulation of Small Plastic Particles in Fur Seals from Macquarie Island. Ambio 2003, 32, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Jantz, L.A.; Morishige, C.L.; Bruland, G.L.; Lepczyk, C.A. Ingestion of plastic marine debris by longnose lancetfish (Alepisaurus ferox) in the North Pacific Ocean. Mar. Pollut. Bull. 2013, 69, 97–104. [Google Scholar] [CrossRef]
- Selvam, S.; Jesuraja, K.; Venkatramanan, S.; Roy, P.D.; Jeyanthi Kumari, V. Hazardous microplastic characteristics and its role as a vector of heavy metal in groundwater and surface water of coastal south India. J. Hazard. Mater. 2021, 402. [Google Scholar] [CrossRef]
- Sharma, S.; Sharma, V.; Chatterjee, S. Microplastics in the Mediterranean Sea: Sources, Pollution Intensity, Sea Health, and Regulatory Policies. Front. Mar. Sci. 2021, 8. [Google Scholar] [CrossRef]
- Bradney, L.; Wijesekara, H.; Palansooriya, K.N.; Obadamudalige, N.; Bolan, N.S.; Ok, Y.S.; Rinklebe, J.; Kim, K.H.; Kirkham, M.B. Particulate plastics as a vector for toxic trace-element uptake by aquatic and terrestrial organisms and human health risk. Environ. Int. 2019, 131. [Google Scholar] [CrossRef]
- Lebreton, L.C.M.; Greer, S.D.; Borrero, J.C. Numerical modelling of floating debris in the world’s oceans. Mar. Pollut. Bull. 2012, 64, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Cózar, A.; Sanz-Martín, M.; Martí, E.; González-Gordillo, J.I.; Ubeda, B.; Ágálvez, J.; Irigoien, X.; Duarte, C.M. Plastic accumulation in the mediterranean sea. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [Green Version]
- Béranger, K.; Drillet, Y.; Houssais, M.N.; Testor, P.; Bourdallé-Badie, R.; Alhammoud, B.; Bozec, A.; Mortier, L.; Bouruet-Aubertot, P.; Crépon, M. Impact of the spatial distribution of the atmospheric forcing on water mass formation in the Mediterranean Sea. J. Geophys. Res. Ocean. 2010, 115. [Google Scholar] [CrossRef] [Green Version]
- Soto-Navarro, J.; Criado-Aldeanueva, F.; García-Lafuente, J.; Sánchez-Romn, A. Estimation of the Atlantic inflow through the Strait of Gibraltar from climatological and in situ data. J. Geophys. Res. Ocean. 2010, 115. [Google Scholar] [CrossRef] [Green Version]
- Erni-Cassola, G.; Zadjelovic, V.; Gibson, M.I.; Christie-Oleza, J.A. Distribution of plastic polymer types in the marine environment; A meta-analysis. J. Hazard. Mater. 2019, 369, 691–698. [Google Scholar] [CrossRef]
- Schrank, I.; Trotter, B.; Dummert, J.; Scholz-Böttcher, B.M.; Löder, M.G.J.; Laforsch, C. Effects of microplastic particles and leaching additive on the life history and morphology of Daphnia magna. Environ. Pollut. 2019, 255. [Google Scholar] [CrossRef]
- Shahul Hamid, F.; Bhatti, M.S.; Anuar, N.; Anuar, N.; Mohan, P.; Periathamby, A. Worldwide distribution and abundance of microplastic: How dire is the situation? Waste Manag. Res. 2018, 36, 873–897. [Google Scholar] [CrossRef]
- Law, K.L.; Thompson, R.C. Oceans. Microplastics in the seas. Science 2017, 345, 144–145. [Google Scholar] [CrossRef]
- Andrady, A.L. The plastic in microplastics: A review. Mar. Pollut. Bull. 2017, 119, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Weiwei, Z.; Shoufeng, Z.; Juying, W.; Yan, W.; Jingli, M.; Ping, W.; Xinzhen, L.; Deyi, M. Microplastic pollution in the surface waters of the Bohai Sea, China. Environ. Pollut. 2017, 231, 541–548. [Google Scholar]
- Isobe, A. Percentage of microbeads in pelagic microplastics within Japanese coastal waters. Mar. Pollut. Bull. 2016, 110, 432–437. [Google Scholar] [CrossRef]
- Song, Y.K.; Hong, S.H.; Jang, M.; Kang, J.H.; Kwon, O.Y.; Han, G.M.; Shim, W.J. Large accumulation of micro-sized synthetic polymer particles in the sea surface microlayer. Environ. Sci. Technol. 2014, 48, 9014–9021. [Google Scholar] [CrossRef]
- Isobe, A.; Uchiyama-Matsumoto, K.; Uchida, K.; Tokai, T. Microplastics in the Southern Ocean. Mar. Pollut. Bull. 2017, 114, 623–626. [Google Scholar] [CrossRef]
- Gray, A.D.; Weinstein, J.E. Size- and shape-dependent effects of microplastic particles on adult daggerblade grass shrimp (Palaemonetes pugio). Environ. Toxicol. Chem. 2017, 36, 3074–3080. [Google Scholar] [CrossRef] [PubMed]
- Kögel, T.; Bjorøy, Ø.; Toto, B.; Bienfait, A.M.; Sanden, M. Micro- and nanoplastic toxicity on aquatic life: Determining factors. Sci. Total Environ. 2020, 709. [Google Scholar] [CrossRef] [PubMed]
- Engler, R.E. The complex interaction between marine debris and toxic chemicals in the ocean. Environ. Sci. Technol. 2012, 46, 12302–12315. [Google Scholar] [CrossRef] [PubMed]
- GESAMP Joint Group of Experts on the Scientific Aspects of Marine Environmental Protection Sources, fate and effects of microplastics in the marine environment: A global assessment. Rep. Stud. GESAMP 2015, 90, 96.
- Bowmer, T.; Kershaw, P. Proceedings of GESAMP International Workshop on Microplastic particles as a vector in transporting persistent, bioaccumulating and toxic substances in the ocean. GESAMP Reports Stud. 2013, 82, 1689–1699. [Google Scholar]
- Covernton, G.A.; Pearce, C.M.; Gurney-Smith, H.J.; Chastain, S.G.; Ross, P.S.; Dower, J.F.; Dudas, S.E. Size and shape matter: A preliminary analysis of microplastic sampling technique in seawater studies with implications for ecological risk assessment. Sci. Total Environ. 2019, 667, 124–132. [Google Scholar] [CrossRef]
- Martí, E.; Martin, C.; Galli, M.; Echevarría, F.; Duarte, C.M.; Cózar, A. The Colors of the Ocean Plastics. Environ. Sci. Technol. 2020, 54, 6594–6601. [Google Scholar] [CrossRef]
- Boerger, C.M.; Lattin, G.L.; Moore, S.L.; Moore, C.J. Plastic ingestion by planktivorous fishes in the North Pacific Central Gyre. Mar. Pollut. Bull. 2010, 60, 2275–2278. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.L.; Thompson, R.C.; Galloway, T.S. The physical impacts of microplastics on marine organisms: A review. Environ. Pollut. 2013, 178, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Setälä, O.; Lehtiniemi, M.; Coppock, R.; Cole, M. Microplastics in marine food webs. In Microplastic contamination in aquatic environments; Elsevier: London, UK, 2018; pp. 339–363. [Google Scholar] [CrossRef]
- Desforges, J.P.W.; Galbraith, M.; Ross, P.S. Ingestion of Microplastics by Zooplankton in the Northeast Pacific Ocean. Arch. Environ. Contam. Toxicol. 2015, 69. [Google Scholar] [CrossRef] [PubMed]
- Walkinshaw, C.; Lindeque, P.K.; Thompson, R.; Tolhurst, T.; Cole, M. Microplastics and seafood: Lower trophic organisms at highest risk of contamination. Ecotoxicol. Environ. Saf. 2020, 190. [Google Scholar] [CrossRef]
- Plastics Europe Plastics-the Facts 2019. An Analysis of European Plastics Production, Demand and Waste Data. 2019. Available online: https://www.plasticseurope.org/application/files/9715/7129/9584/FINAL_web_version_Plastics_the_facts2019_14102019.pdf (accessed on 4 August 2021).
- Tripathi, D. Practical Guide to Polypropylene; Smithers Rapra Publishing: Shawbury, UK, 2002; 104p. [Google Scholar]
- Hidalgo-Ruz, V.; Gutow, L.; Thompson, R.C.; Thiel, M. Microplastics in the marine environment: A review of the methods used for identification and quantification. Environ. Sci. Technol. 2012, 46, 3060–3075. [Google Scholar] [CrossRef]
- Frias, J.P.G.L.; Otero, V.; Sobral, P. Evidence of microplastics in samples of zooplankton from Portuguese coastal waters. Mar. Environ. Res. 2014, 95, 89–95. [Google Scholar] [CrossRef]
- Suaria, G.; Avio, C.G.; Mineo, A.; Lattin, G.L.; Magaldi, M.G.; Belmonte, G.; Moore, C.J.; Regoli, F.; Aliani, S. The Mediterranean Plastic Soup: Synthetic polymers in Mediterranean surface waters. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [Green Version]
- Enders, K.; Lenz, R.; Stedmon, C.A.; Nielsen, T.G. Abundance, size and polymer composition of marine microplastics ≥10 μm in the Atlantic Ocean and their modelled vertical distribution. Mar. Pollut. Bull. 2015, 100, 70–81. [Google Scholar] [CrossRef]
- Zhao, S.; Zhu, L.; Li, D. Microplastic in three urban estuaries, China. Environ. Pollut. 2015, 206, 597–604. [Google Scholar] [CrossRef]
- Tan, X.; Yu, X.; Cai, L.; Wang, J.; Peng, J. Microplastics and associated PAHs in surface water from the Feilaixia Reservoir in the Beijiang River, China. Chemosphere 2019, 221, 834–840. [Google Scholar] [CrossRef]
- Cellini, E.P.L. I dati della regione mar ionio mediterraneo centrale. Ecoscienza 2020, 1, 32, ISSN 2039-0432. [Google Scholar]
- Mani, T.; Hauk, A.; Walter, U.; Burkhardt-Holm, P. Microplastics profile along the Rhine River. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef]
- Poulain, P.M.; Bussani, A.; Gerin, R.; Jungwirth, R.; Mauri, E.; Menna, M.; Notarstefano, G. Mediterranean surface currents measured with drifters from basin to subinertial scales. Oceanography 2013, 26, 38–47. [Google Scholar] [CrossRef] [Green Version]
- Ducrocq, V.; Drobinski, P.; Gualdi, S.; Raimbault, P. Sub-chapter 1.2.1. The water cycle in the Mediterranean. Mediterr. Reg. under Clim. Chang. 2018, 73–81. [Google Scholar] [CrossRef]
- Pierdomenico, M.; Casalbore, D.; Chiocci, F.L. Massive benthic litter funnelled to deep sea by flash-flood generated hyperpycnal flows. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef] [Green Version]
- Canals, M.; Pham, C.K.; Bergmann, M.; Gutow, L.; Hanke, G.; van Sebille, E.; Angiolillo, M.; Buhl-Mortensen, L.; Cau, A.; Ioakeimidis, C.; et al. The quest for seafloor macrolitter: A critical review of background knowledge, current methods and future prospects. Environ. Res. Lett. 2020. [Google Scholar] [CrossRef]
- van der Hal, N.; Ariel, A.; Angel, D.L. Exceptionally high abundances of microplastics in the oligotrophic Israeli Mediterranean coastal waters. Mar. Pollut. Bull. 2017, 116, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Pedrotti, M.L.; Petit, S.; Elineau, A.; Bruzaud, S.; Crebassa, J.C.; Dumontet, B.; Martí, E.; Gorsky, G.; Cózar, A. Changes in the floating plastic pollution of the mediterranean sea in relation to the distance to land. PLoS ONE 2016, 11. [Google Scholar] [CrossRef] [Green Version]
- Kazour, M.; Jemaa, S.; Issa, C.; Khalaf, G.; Amara, R. Microplastics pollution along the Lebanese coast (Eastern Mediterranean Basin): Occurrence in surface water, sediments and biota samples. Sci. Total Environ. 2019, 696. [Google Scholar] [CrossRef]





| Study Area | Net Mesh | Mean Density | Reference |
|---|---|---|---|
| Mediterranean | 200 µm | 0.243 particles/m2 | (Cózar et al., 2015) |
| North Western Mediterranean | 333 µm | 0.116 particles/m2 | (Collignon et al., 2012) |
| Western Mediterranean | 333 µm | 0.135 particles/m2 | (Faure et al., 2016) |
| Western Mediterranean-Adriatic | 200 µm | 0.40 (±0.74) particles/m2 | (Suaria et al., 2016) |
| Mediterranean-Corsica | 200 µm | 0.062 particles/m2 | (Collignon et al., 2014) |
| Central-Western Mediterranean | 500 µm | 0.15 particles/m3 | (de Lucia et al., 2014) |
| Central-Western Mediterranean | 333 µm | 0.147 particles/m2 | (Ruiz-Orejón et al., 2016) |
| Sardinian Sea | 200 µm | 0.16 (±0.31) particles/m3 | (Fossi et al., 2016) |
| Sardinian Sea | 200 µm | 0.17 (±0.32) particles/m3 | (Panti et al., 2016) |
| Ligurian Sea | 200 µm | 0.49 (±1.66) particles/m3 | (Fossi et al., 2016) |
| Ligurian Sea | 333 µm | 0.103 particles/m3 | (Pedrotti et al., 2014) |
| Calabrian costs | 333 µm | 0.13 (±0.194) particles/m2 | This study |
| 0.52 (±0.778) particles/m3 | |||
| North Atlantic | 350 µm | 1.70 particles/m3 | (Eriksen et al., 2014) |
| North-east Atlantic | 250 µm | 2.46 particles/m3 | (Lusher et al., 2014) |
| East Asian seas | 335 µm | 3.70 (±10.4) particles/m3 | (Isobe et al., 2015) |
| Seto Inland Sea | 335 µm | 0.39 particles/m3 | (Isobe et al., 2014) |
| Arctic polar waters | 333 µm | 0.34 (±0.31) particles/m3 | (Lusher et al., 2015) |
| Bohai Sea | 330 µm | 0.33 (±0.36) particles/m3 | (Weiwei et al., 2017) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marrone, A.; La Russa, M.F.; Randazzo, L.; La Russa, D.; Cellini, E.; Pellegrino, D. Microplastics in the Center of Mediterranean: Comparison of the Two Calabrian Coasts and Distribution from Coastal Areas to the Open Sea. Int. J. Environ. Res. Public Health 2021, 18, 10712. https://doi.org/10.3390/ijerph182010712
Marrone A, La Russa MF, Randazzo L, La Russa D, Cellini E, Pellegrino D. Microplastics in the Center of Mediterranean: Comparison of the Two Calabrian Coasts and Distribution from Coastal Areas to the Open Sea. International Journal of Environmental Research and Public Health. 2021; 18(20):10712. https://doi.org/10.3390/ijerph182010712
Chicago/Turabian StyleMarrone, Alessandro, Mauro F. La Russa, Luciana Randazzo, Daniele La Russa, Emilio Cellini, and Daniela Pellegrino. 2021. "Microplastics in the Center of Mediterranean: Comparison of the Two Calabrian Coasts and Distribution from Coastal Areas to the Open Sea" International Journal of Environmental Research and Public Health 18, no. 20: 10712. https://doi.org/10.3390/ijerph182010712









