Executive Functioning in Adults with Down Syndrome: Machine-Learning-Based Prediction of Inhibitory Capacity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials: Data Description
2.2. Research Instruments
2.3. Methods
2.3.1. Support Vector Machine as the Recovery Algorithm to Retrieve the Missing Data
2.3.2. Dimensionality Reduction Based on the Pearson Correlation Coefficient
Correlation Matrix Generation with All Variables
Fixing the Pearson Correlation Coefficient Threshold
Variable Elimination by Experts
Generation of New Databases to Perform the Analysis
2.3.3. Automatic Variable Selection Algorithms
Random Forest as the Variable Selection Algorithm (RF)
Logistic Regression as the Variable Selector Algorithm (LR)
Support Vector Machines as the Variable Selection Algorithm (SVM)
Feature Selection Committee of All the Algorithms
Feature Selection by Importance Index
3. Results
3.1. Data Recovery
3.2. Removal of Correlated Variables
3.3. Committee- or Intersection-Based Variable Selection
3.4. Variable Selection Based on the Importance Index
3.5. Performance of the Algorithms When Classifying the EF Variable
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Bayen, E.; Possin, K.L.; Chen, Y.; de Langavant, L.C.; Yaffe, K. Prevalence of Aging, Dementia, and Multimorbidity in Older Adults With Down Syndrome. JAMA Neurol. 2018, 75, 1399–1406, PMCID:PMC6248113. [Google Scholar] [CrossRef] [PubMed]
- Danés, C.F. Aspectos específicos del envejecimiento en el síndrome de Down. Rev. Méd. Int. Síndr. Down 2012, 16, 3–10. [Google Scholar] [CrossRef]
- Head, E.; Silverman, W.; Patterson, D.; Lott, I.T. Aging and Down Syndrome. Curr. Gerontol. Geriatr. Res. 2012, 2012, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lott, I.T.; Dierssen, M. Cognitive deficits and associated neurological complications in individuals with Down’s syndrome. Lancet Neurol. 2010, 9, 623–633. [Google Scholar] [CrossRef]
- Cipriani, G.; Danti, S.; Carlesi, C.; Di Fiorino, M. Aging with Down Syndrome: The Dual Diagnosis: Alzheimer’s Disease and Down Syndrome. Am. J. Alzheimers Dis. Other Demen. 2018, 33, 253–262. [Google Scholar] [CrossRef]
- Brown, R.; Taylor, J.; Matthews, B. Quality of life—Ageing and Down syndrome. Down Syndr. Res. Pract. 2001, 6, 111–116. [Google Scholar] [CrossRef]
- Carmeli, E.; Kessel, S.; Bar-Chad, S.; Merrick, J. A comparison between older persons with down syndrome and a control group: Clinical characteristics, functional status and sensorimotor function. Down Syndr. Res. Pract. 2004, 9, 17–24. [Google Scholar]
- Lin, J.D.; Lin, L.P.; Hsu, S.W.; Chen, W.X.; Lin, F.G.; Wu, J.L.; Chu, C. Are early onset aging conditions correlated to daily activity functions in youth and adults with Down syndrome? Res. Dev. Disabil. 2015, 36, 532–536. [Google Scholar] [CrossRef]
- Stancliffe, R.J.; Lakin, K.C.; A Larson, S.A.; Engler, J.; Taub, S.; Fortune, J.; Bershadsky, J. Demographic Characteristics, Health Conditions, and Residential Service Use in Adults with Down Syndrome in 25 U.S. States. Intellect. Dev. Disabil. 2012, 50, 92–108. [Google Scholar] [CrossRef]
- Berzosa Zaballos, G. Las Personas con Síndrome de Down y sus Familias ante el Proceso de Envejecimiento; Real Patronato Sobre Discapacidad: Madrid, Spain, 2013. [Google Scholar]
- Franceschi, C.; Garagnani, P.; Gensous, N.; Bacalini, M.G.; Conte, M.; Salvioli, S. Accelerated bio-cognitive aging in Down syndrome: State of the art and possible deceleration strategies. Aging Cell 2019, 18, e12903. [Google Scholar] [CrossRef]
- Benejam, B. Síntomas de demencia en el síndrome de Down. Rev. Méd. Int. Síndr. Down 2009, 13, 18–21. [Google Scholar] [CrossRef]
- Ghezzo, A.; Salvioli, S.; Solimando, M.C.; Palmieri, A.; Chiostergi, C.; Scurti, M.; Lomartire, L.; Bedetti, F.; Cocchi, G.; Follo, D.; et al. Age-related changes of adaptive and neuropsychological features in persons with Down Syndrome. PLoS ONE 2014, 9, e113111. [Google Scholar] [CrossRef]
- Dalton, A.J.; Fedor, B.L. Onset of dyspraxia in aging persons with Down syndrome: Longitudinal studies. J. Intellect. Dev. Disabil. 1998, 23, 13–24. [Google Scholar] [CrossRef]
- Flórez, J. La atención temprana en el síndrome de Down: Bases neurobiológicas. Rev. Síndr. Down 2006, 22, 132–142. [Google Scholar]
- Jarrold, C.; Nadel, L.; Vicari, S. Memory and neuropsychology in Down Syndrome. Down Syndr. Res. Pract. 2008, 12, 68–73. [Google Scholar] [CrossRef]
- Lanfranchi, S.; Toffanin, E.; Zilli, S.; Panzeri, B.; Vianello, R. Memory coding in individuals with Down syndrome. Child Neuropsychol. 2013, 20, 700–712. [Google Scholar] [CrossRef]
- Palmer, G.A. Neuropsychological profiles of persons with mental retardation and dementia. Res. Dev. Disabil. 2006, 27, 299–308. [Google Scholar] [CrossRef]
- Roberts, L.; Richmond, J.L. Using learning flexibly and remembering after a delay: Understanding cognitive dysfunction in adults with Down syndrome. J. Intellect. Disabil. Res. 2018, 62, 521–531. [Google Scholar] [CrossRef]
- Startin, C.M.; Hamburg, S.; Hithersay, R.; Davies, A.; Rodger, E.; Aggarwal, N.; Al-Janabi, T.; Strydom, A. The LonDownS adult cognitive assessment to study cognitive abilities and decline in Down syndrome. Wellcome Open Res. 2016, 1, 11. [Google Scholar] [CrossRef] [Green Version]
- Lao, P.J.; Betthauser, T.J.; Hillmer, A.T.; Price, J.C.; Klunk, W.E.; Mihaila, I.; Higgins, A.T.; Bulova, P.D.; Hartley, S.L.; Hardison, R.; et al. The effects of normal aging on amyloid-β deposition in nondemented adults with Down syndrome as imaged by carbon 11–labeled Pittsburgh compound B. Alzheimer Dement. 2016, 12, 380–390. [Google Scholar] [CrossRef] [Green Version]
- Pujol, J.; Fenoll, R.; Ribas-Vidal, N.; Martínez-Vilavella, G.; Blanco-Hinojo, L.; García-Alba, J.; Deus, J.; Novell, R.; Esteba-Castillo, S. A longitudinal study of brain anatomy changes preceding dementia in Down syndrome. NeuroImage Clin. 2018, 18, 160–166. [Google Scholar] [CrossRef]
- Licastro, F.; Porcellini, E. Individual Risk Detection of Developing Cognitive Decline and Dementia in Adults with Down’s Syndrome. J. Down Syndr. Chromosom. Abnorm. 2017, 3, 117. [Google Scholar] [CrossRef] [Green Version]
- McCarron, M.; Gill, M.; McCallion, P.; Begley, C. Health co-morbidities in ageing persons with Down syndrome and Alzheimer’s dementia. J. Intellect. Disabil. Res. 2005, 49, 560–566. [Google Scholar] [CrossRef]
- García-Alba, J.; Ramírez-Toraño, F.; Esteba-Castillo, S.; Bruña, R.; Moldenhauer, F.; Novell, R.; Romero-Medina, V.; Maestú, F.; Fernández, A. Neuropsychological and neurophysiological characterization of mild cognitive impairment and Alzheimer’s disease in Down syndrome. Neurobiol. Aging 2019, 84, 70–79. [Google Scholar] [CrossRef] [PubMed]
- McGlinchey, E.; Reilly, E.; McCallion, P.; Dunne, P.; Mulryan, N.; Carroll, R.; McCarron, M. Dementia and Intellectual Disability: Prevalence, Assessment and Post-Diagnostic Support. In Handbook of Intellectual Disabilities: Integrating Theory, Research and Practice; Matson, J.L., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 965–986. [Google Scholar] [CrossRef]
- Miyake, A.; Friedman, N.P.; Emerson, M.J.; Witzki, A.H.; Howerter, A.; Wager, T.D. The Unity and Diversity of Executive Functions and Their Contributions to Complex “Frontal Lobe” Tasks: A Latent Variable Analysis. Cogn. Psychol. 2000, 41, 49–100. [Google Scholar] [CrossRef] [Green Version]
- Casey, B.J.; Somerville, L.H.; Gotlib, I.H.; Ayduk, O.; Franklin, N.T.; Askren, M.K.; Jonides, J.; Berman, M.G.; Wilson, N.L.; Teslovich, T.; et al. Behavioral and neural correlates of delay of gratification 40 years later. Proc. Natl. Acad. Sci. USA 2011, 108, 14998–15003. [Google Scholar] [CrossRef] [Green Version]
- Rosas, R.; Espinoza, V.; Garolera, M.; San-Martín, P. Executive Functions at the start of kindergarten: Are they good predictors of academic performance at the end of year one? A longitudinal study. Stud. Psychol. 2017, 38, 451–472. [Google Scholar] [CrossRef]
- Petersen, I.T.; Hoyniak, C.P.; McQuillan, M.E.; Bates, J.E.; Staples, A. Measuring the development of inhibitory control: The challenge of heterotypic continuity. Dev. Rev. 2016, 40, 25–71. [Google Scholar] [CrossRef] [Green Version]
- Ball, S.L.; Holland, A.J.; Treppner, P.; Watson, P.; Huppert, F. Executive dysfunction and its association with personality and behaviour changes in the development of Alzheimer’s disease in adults with Down syndrome and mild to moderate learning disabilities. Br. J. Clin. Psychol. 2008, 47, 1–29. [Google Scholar] [CrossRef]
- Bexkens, A.; Ruzzano, L.; Escury-Koenigs, A.M.L.C.D.; Van Der Molen, M.W.; Huizenga, H.M. Inhibition deficits in individuals with intellectual disability: A meta-regression analysis. J. Intellect. Disabil. Res. 2013, 58, 3–16. [Google Scholar] [CrossRef]
- Kittler, P.; Krinsky-McHale, S.J.; Devenny, D.A. Verbal intrusions precede memory decline in adults with Down syndrome. J. Intellect. Disabil. Res. 2006, 50, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Nelson, L.; Johnson, J.K.; Freedman, M.; Lott, I.; Groot, J.; Chang, M.; Milgram, N.W.; Head, E. Learning and memory as a function of age in Down syndrome: A study using animal-based tasks. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2005, 29, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Oliver, C.; Holland, T.; Hall, S.; Crayton, L. Effects of Increasing Task Load on Memory Impairment in Adults with Down Syndrome. Am. J. Ment. Retard. 2005, 110, 339–345. [Google Scholar] [CrossRef]
- Krinsky-McHale, S.J.; Devenny, D.A.; Kittler, P.; Silverman, W. Selective Attention Deficits Associated With Mild Cognitive Impairment and Early Stage Alzheimer’s Disease in Adults with Down Syndrome. Am. J. Ment. Retard. 2008, 113, 369–386. [Google Scholar] [CrossRef]
- Danielsson, H.; Henry, L.; Rönnberg, J.; Nilsson, L.-G. Executive functions in individuals with intellectual disability. Res. Dev. Disabil. 2010, 31, 1299–1304. [Google Scholar] [CrossRef] [Green Version]
- Iacono, T.; Torr, J.; Wong, H.Y. Relationships amongst age, language and related skills in adults with Down syndrome. Res. Dev. Disabil. 2010, 31, 568–576. [Google Scholar] [CrossRef]
- Burt, D.B.; Loveland, K.A.; Cleveland, L.A.; Lewis, K.R.; Lesser, J.; Pearson, P.L.; Primeaux-Hart, S. Comparing Dementia Diagnostic Methods Used with People with Intellectual Disabilities. J. Policy Pract. Intellect. Disabil. 2005, 2, 94–115. [Google Scholar] [CrossRef]
- Das, J.; Divis, B.; Alexander, J.; Parrila, R.; Naglieri, J.A. Cognitive decline due to aging among persons with down syndrome. Res. Dev. Disabil. 1995, 16, 461–478. [Google Scholar] [CrossRef]
- Beciani, S.; Vetro, E.; Barisnikov, K.; Detraux, J.-J.; Van der Linden, M. Elaboration d’une batterie d’évaluation des signes du vieillissement dans la trisomie 21. Rev. Francoph. Défic. Intellect. 2011, 22, 129–140. [Google Scholar]
- Tomaszewski, B.; Fidler, D.; Talapatra, D.; Riley, K. Adaptive behaviour, executive function and employment in adults with Down syndrome. J. Intellect. Disabil. Res. 2018, 62, 41–52. [Google Scholar] [CrossRef]
- Iralde, L.; Roy, A.; Detroy, J.; Allain, P. A Representational Approach to Executive Function Impairments in Young Adults with Down Syndrome. Dev. Neuropsychol. 2020, 45, 263–278. [Google Scholar] [CrossRef]
- Loveall, S.J.; Conners, F.A.; Tungate, A.S.; Hahn, L.; Osso, T.D. A cross-sectional analysis of executive function in Down syndrome from 2 to 35 years. J. Intellect. Disabil. Res. 2017, 61, 877–887. [Google Scholar] [CrossRef]
- Lautarescu, B.A.; Holland, A.J.; Zaman, S.H. The Early Presentation of Dementia in People with Down Syndrome: A Systematic Review of Longitudinal Studies. Neuropsychol. Rev. 2017, 27, 31–45. [Google Scholar] [CrossRef] [Green Version]
- Margallo-Lana, M.L.; Moore, P.B.; Kay, D.W.K.; Perry, R.H.; Reid, B.E.; Berney, T.P.; Tyrer, S.P. Fifteen-year follow-up of 92 hospitalized adults with Down?s syndrome: Incidence of cognitive decline, its relationship to age and neuropathology. J. Intellect. Disabil. Res. 2007, 51, 463–477. [Google Scholar] [CrossRef]
- Carr, J.; Collins, S. Ageing and Dementia in a Longitudinal Study of a Cohort with Down Syndrome. J. Appl. Res. Intellect. Disabil. 2014, 27, 555–563. [Google Scholar] [CrossRef]
- Temple, V.; Jozsvai, E.; Konstantareas, M.M.; Hewitt, T.-A. Alzheimer dementia in Down’s syndrome: The relevance of cognitive ability. J. Intellect. Disabil. Res. 2001, 45, 47–55. [Google Scholar] [CrossRef]
- Tungate, A.S.; Conners, F.A. Executive function in Down syndrome: A meta-analysis. Res. Dev. Disabil. 2021, 108, 103802. [Google Scholar] [CrossRef]
- Li, B.; Fu, L. Exact test of goodness of fit for binomial distribution. Stat. Pap. 2018, 59, 851–860. [Google Scholar] [CrossRef]
- Signo, S. The aging Process in People with Down Syndrome: A Multicenter Study to Detect Neuropsychological Changes. Ph.D. Thesis, University Ramon Llull, Barcelona, Spain, 2020. Available online: https://www.tesisenred.net/handle/10803/352712#page=1 (accessed on 12 April 2021).
- World Medical Association. Helsinki Declaration. Ethical Principles for Medical Research Involving Human Subjects; World Medical Association: Helsinki, Finland, 2008. [Google Scholar]
- Raven, J.C. Test de Matrices Progresivas a Color; TEA Ediciones: Barcelona, Spain, 1996. [Google Scholar]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Kaufman, A.S.; Kaufman, N.L. Batería de Evaluación de Kaufman para Niños (K-ABC); TEA Ediciones: Madrid, Spain, 1997. [Google Scholar]
- Dunn, L.M.; Dunn, L.M.; Arribas, D. Peabody. Test de Vocabulario en Imágenes; (PPVT-III); TEA Ediciones: Madrid, Spain, 2006. [Google Scholar]
- Peña-Casanova, J. Programa Integrado de Exploración Neuropsicológica—PIEN: “Test Barcelona”; Masson: Barcelona, Spain, 1991. [Google Scholar]
- Kirk, S.A.; McCarthy, J.J.; Kirk, W.D. ITPA. Test Illinois de Aptitudes Psicolingüísticas; TEA Ediciones: Madrid, Spain, 1996. [Google Scholar]
- Gerstadt, C.L.; Hong, Y.J.; Diamond, A. The relationship between cognition and action: Performance of children 3 1/2−7 years old on a Stroop-like day-night test. Cognition 1994, 53, 129–153. [Google Scholar] [CrossRef]
- Cacho, J.; García, R.; Arcaya, J.; Vicente, J.L.; Lantada, N. Una propuesta de aplicación y puntuación del test del reloj en la enfermedad de Alzheimer. Rev. Neurol. 1999, 28, 648–655. [Google Scholar] [PubMed]
- Bergès, J.; Lézine, L. Test de Imitación de Gestos; Masson: Barcelona, Spain, 1975. [Google Scholar]
- Al-Tashi, Q.; Abdulkadir, S.J.; Rais, H.M.; Mirjalili, S.; Alhussian, H. Approaches to Multi-Objective Feature Selection: A Systematic Literature Review. IEEE Access 2020, 8, 125076–125096. [Google Scholar] [CrossRef]
- Smola, A.J.; Schölkopf, B. A tutorial on support vector regression. Stat. Comput. 2004, 14, 199–222. [Google Scholar] [CrossRef] [Green Version]
- Cutler, A.; Cutler, D.R.; Stevens, J.R. Random forests. In Ensemble Machine Learning, 2nd ed.; Zhang, C., Ma, Y.Q., Eds.; Springer: New York, NY, USA, 2012; pp. 157–175. [Google Scholar] [CrossRef]
- Hall, M.A. Correlation-Based Feature Selection for Machine Learning. Ph.D. Thesis, Department of Computer Science, University of Waikato, Hamilton, New Zealand, 2020. [Google Scholar]
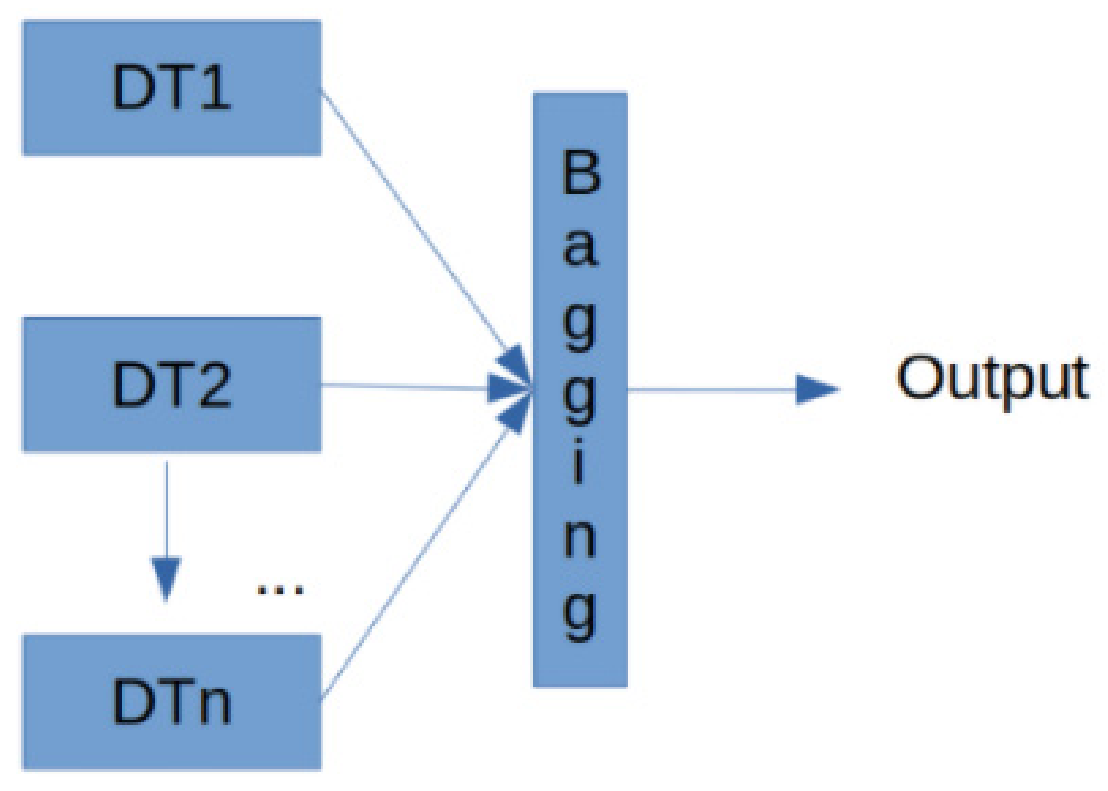
- González, S.; García, S.; Del Ser, J.; Rokach, L.; Herrera, F. A practical tutorial on bagging and boosting based ensembles for machine learning: Algorithms, software tools, performance study, practical perspectives and opportunities. Inf. Fusion 2020, 64, 205–237. [Google Scholar] [CrossRef]
- Tranmer, M.; Elliot, M. Binary Logistic Regression; The Cathie Marsh Centre for Census and Survey Research: Manchester, UK, 2020. [Google Scholar]
- Yang, J.-B.; Ong, C.-J. Feature Selection Using Probabilistic Prediction of Support Vector Regression. IEEE Trans. Neural Netw. 2011, 22, 954–962. [Google Scholar] [CrossRef]
- Lanfranchi, S.; Jerman, O.; Pont, E.D.; Alberti, A.; Vianello, R. Executive function in adolescents with Down Syndrome. J. Intellect. Disabil. Res. 2010, 54, 308–319. [Google Scholar] [CrossRef]
- Dalton, A.J.; Mehta, P.D.; Fedor, B.L.; Patti, P.J. Cognitive changes in memory precede those in praxis in aging persons with Down syndrome. J. Intellect. Dev. Disabil. 1999, 24, 169–187. [Google Scholar] [CrossRef]
- Koehl, L.; Harp, J.; Van Pelt, K.L.; Head, E.; Schmitt, F.A. Longitudinal assessment of dementia measures in Down syndrome. Alzheimers Dement. 2020, 12, e12075. [Google Scholar] [CrossRef]
- Lockrow, J.P.; Fortress, A.M.; Granholm, A.-C.E. Age-Related Neurodegeneration and Memory Loss in Down Syndrome. Curr. Gerontol. Geriatr. Res. 2012, 2012, 463909. [Google Scholar] [CrossRef]
- Tyrrell, J.; Cosgrave, M.; McCarron, M.; McPherson, J.; Calvert, J.; Kelly, A.; McLaughlin, M.; Gill, M.; Lawlor, B.A. Dementia in people with Down’s syndrome. Int. J. Geriatr. Psychiatry 2001, 16, 1168–1174. [Google Scholar] [CrossRef]
- Firth, N.C.; Startin, C.M.; Hithersay, R.; Hamburg, S.; Wijeratne, P.A.; Mok, K.Y.; Hardy, J.; Alexander, D.C.; Strydom, A.; The LonDownS Consortium; et al. Aging related cognitive changes associated with Alzheimer’s disease in Down syndrome. Ann. Clin. Transl. Neurol. 2018, 5, 741–751. [Google Scholar] [CrossRef] [Green Version]
- Ashworth, A.; Hill, C.M.; Karmiloff-Smith, A.; Dimitriou, D. The Importance of Sleep: Attentional Problems in School-Aged Children With Down Syndrome and Williams Syndrome. Behav. Sleep Med. 2014, 13, 455–471. [Google Scholar] [CrossRef]
- Rondal, J.; Comblain, A. Language in ageing persons with Down syndrome. Down Syndr. Res. Pract. 2002, 8, 1–9. [Google Scholar] [CrossRef]
- Ribes, R.; Sanuy, J. Declive cognitivo en memoria y lenguaje: Indicadores del proceso de envejecimiento psicológico en la persona con síndrome de Down. Rev. Síndr. Down 2000, 17, 54–59. [Google Scholar]
- Rondal, J.A. Dificultades del lenguaje en el síndrome de Down: Perspectiva a lo largo de la vida y principios de intervención. Rev. Síndr. Down 2006, 23, 120–128. [Google Scholar]


| Cognitive Domains | Instruments | Acronyms of Variables Names |
|---|---|---|
| General cognitive performance | Scale color progressive matrices of RAVEN (RCPM) [53] | Raven |
| Memory (immediate, verbal memory, visual memory and visual recognition memory) | Memory of images (ad hoc) | Mem_ima |
| Image recognition (ad hoc) | Mem_recog | |
| Verbal Memory 1a and 1b [54] | Mem_verbal | |
| Attention (attention and verbal short-term memory) | Direct digits (K-ABC) [55] | Direct_D |
| Language and communication (receptive vocabulary, denomination, spontaneous language and verbal fluency) | Peabody Picture Vocabulary Test (PPVT) [56] | PPVT |
| Visio-verbal denomination (ad hoc) | Total_denomin | |
| Spontaneous language: description of a sheet [57] | Spont_lang | |
| Verbal fluency: categorical evocation [58] | Verbal_flu | |
| Oral verbal comprehension (ad hoc) | OV_compr | |
| Written verbal comprehension [57] | WV_compr | |
| Executive functions (executive function, processing speed, planning and motor execution) | Cats-and-Dogs test [31,59] | EF |
| Clock test [60] | Clock_order // Clock_copy | |
| Motor execution 1 | Mot_ex1 | |
| Motor execution 2 | Mot_ex2 | |
| Overall motor execution [57] | Overall_ME | |
| Mental control—numbers | Mental_contr_num | |
| Mental control—days | Mental_contr_days | |
| Overall mental control [57] | Overall_mental_contr | |
| Praxis (visio-constructive ability, imitation of postures, ability to imitate) | Constructive praxis [57] Imitation of bilateral postures [61] Ideational praxis [57] The last item has been replaced by another, more familiar and recognizable for the DS population. | Constr_praxis Imi_post Ide_praxis |
| Orientation (time, place, person) | Orientation in person (ad hoc) | OP |
| Orientation in space (ad hoc) | OS | |
| Orientation in time (ad hoc) | OT | |
| Writing | Graphics | Graphics |
| Variable Name | Amount of Missing Information |
|---|---|
| Spont_lang | 1 |
| Direct_D | 2 |
| Span | 6 |
| Deno_obj_body | 1 |
| Graphics | 6 |
| Mem_ima | 1 |
| Mem_recog | 1 |
| Errors | 2 |
| OV_comp | 1 |
| WV_comp | 26 |
| Mental_contr_num | 1 |
| Mental_contr_days | 1 |
| Overall_mental_contr | 1 |
| Mem_verbal | 1 |
| Imi_post | 8 |
| EF | 4 |
| Secs | 5 |
| Raven | 3 |
| Verbal_flu | 3 |
| PPVT | 1 |
| Clock_copy | 3 |
| ZEF | 4 |
| PE_EF | 4 |
| Category_EF | 4 |
| Category_EF_2 | 4 |
| Z_secs_EF | 5 |
| PE_secs_EF_2 | 5 |
| Categories_secs_EF | 5 |
| Categories_secs_EF_2 | 5 |
| Description | Amount |
|---|---|
| Total complete data | 7218 |
| Total data recovered | 114 |
| Total data available for processing | 7332 |
| Total variables | 39 |
| Total registers | 188 |
| Variable Name | Amount of Recovered Data | Mean Absolute Percentage Error in Testing |
|---|---|---|
| Spont_lang | 1 | 95.77% |
| Direct_D | 2 | 98.61% |
| Span | 6 | 99.06% |
| Deno_obj_body | 1 | 95.27% |
| Graphics | 6 | 99.68% |
| Mem_ima | 1 | 96.3% |
| Mem_recog | 1 | 99.9% |
| Errors | 2 | 97.28% |
| OV_comp | 1 | 97.62% |
| WV_comp | 26 | 96.92% |
| Mental_contr_num | 1 | 95.38% |
| Mental_contr_days | 1 | 95.07% |
| Overall_mental_contr | 1 | 96.67% |
| Mem_verbal | 1 | 99.19% |
| Imi_post | 8 | 98.56% |
| EF | 4 | 95.61% |
| Secs | 5 | 95.28% |
| Raven | 3 | 97.44% |
| Verb_flu | 3 | 96.49% |
| PPVT | 1 | 95.13% |
| Clock_copy | 3 | 96.84% |
| ZEF | 4 | 98.69% |
| PE_EF | 4 | 96.91% |
| Category_EF | 4 | 96.38% |
| Category_EF_2 | 4 | 99.98% |
| Z_secs_EF | 5 | 95.56% |
| PE_secs_EF_2 | 5 | 96.38% |
| Categorias_secs_EF | 5 | 98.86% |
| Categories_secs_EF_2 | 5 | 95.2% |
| Threshold | Variable 1 | Variable 2 | Removed Variable |
|---|---|---|---|
| 0.9 | OT | OS | OT |
| Mental_contr_num | Overall_mental_contr | Mental_contr_num | |
| Mental_contr_days | Overall_mental_contr | Menta_contr_daysl | |
| 0.8 | mot_ex1 | Overall_ME | Mot_ex1 |
| Mot_ex2 | Total_EM | Mot_ex2 | |
| auto_leng_total | auto_leng_months | auto_leng_months | |
| 0.7 | Age | Age_groups | Age_groups |
| Direct_D | Span | Direct_D | |
| OS | OT | OT | |
| Total_O | OS | OS | |
| auto_leng_num | Total_leng_auto | auto_leng_num |
| Variables Selected in the Subset of Variables with <0.9 Pearson Correlation Tolerance between Variables | Variables Selected in the Subset of Variables with <0.8 Pearson Correlation Tolerance between Variables | Variables Selected in the Subset of Variables with <0.7 Pearson Correlation Tolerance between Variables |
|---|---|---|
| Imi_post | OV_compr | Overall_ME |
| Mem_verbal | Mem_verbal | OV_compr |
| Direct_D | Overall_mental_contr | Span |
| Constr_praxis | Direct_D | Clock_copy |
| Clock_copy | Constr_praxis | Const_praxis |
| Errors | Errors | Total_leng_auto |
| WV_compr | WV_compr | Ide_praxis |
| Variables with Correlation under 0.9 | Weight | Variables with Correlation under 0.8 | Weight | Variables with Correlation under 0.7 | Weight |
|---|---|---|---|---|---|
| Constr_praxis | 56 | Constr_praxis | 56 | Constr_praxis | 48 |
| Mem_verbal | 45 | Mem_verbal | 46 | Mem_verbal | 40 |
| Direct_D | 45 | VW_compr | 41 | Clock_copy | 36 |
| OP | 32 | Raven | 33 | Mem_recog | 34 |
| Mem_recog | 32 | Direct_D | 33 | OP | 33 |
| Raven | 31 | Secs | 33 | Secs | 33 |
| Secs | 31 | OP | 32 | Raven | 32 |
| Age | 28 | Mem_recog | 29 | PPVT | 32 |
| PPVT | 26 | Age | 27 | Age | 29 |
| Total_denomin | 24 | PPVT | 26 | Spont_lang | 29 |
| Clock_copy | 24 | Total_denomin | 26 | Total_denomin | 27 |
| Verbal_flu | 23 | OS | 26 | Clock_order | 26 |
| VW_compr | 22 | Clock_order | 24 | Verb_flu | 25 |
| Clock_order | 22 | Verbal_flu | 24 | VW_compr | 24 |
| Imi_post | 19 | Imi_post | 19 | Imi_post | 19 |
| Spont_lang | 19 | Clock_copy | 18 | Ide_praxis | 19 |
| Imi_post | 19 | auto_leng_num | 17 | Overall_ME | 19 |
| auto_leng_num | 17 | Imi_post | 17 | OV_compr | 17 |
| Ide_praxis | 16 | Overall_EM | 16 | Span | 17 |
| Overall_ME | 16 | Ide_praxis | 14 | Gender | 15 |
| Model | Subset Variables Correlation <0.7 | Subset Variables Correlation <0.8 | Subset Variables Correlation <0.9 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Metric | Acc | F1 | AUC | Acc | F1 | AUC | Acc | F1 | AUC |
| Random Forest | 73.6% | 68.0% | 74.4% | 86.8% | 82.5% | 87.0% | 84.2% | 81.0% | 85.4% |
| Logistic regression | 71.0% | 61.5% | 63.3% | 73.6% | 66.1% | 69.6% | 71.0% | 67.0% | 77.0% |
| Support vector machine | 57.8% | 51.0% | 55.0% | 55.2% | 47.5% | 51.6% | 60.5% | 58.1% | 70.4% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jojoa-Acosta, M.F.; Signo-Miguel, S.; Garcia-Zapirain, M.B.; Gimeno-Santos, M.; Méndez-Zorrilla, A.; Vaidya, C.J.; Molins-Sauri, M.; Guerra-Balic, M.; Bruna-Rabassa, O. Executive Functioning in Adults with Down Syndrome: Machine-Learning-Based Prediction of Inhibitory Capacity. Int. J. Environ. Res. Public Health 2021, 18, 10785. https://doi.org/10.3390/ijerph182010785
Jojoa-Acosta MF, Signo-Miguel S, Garcia-Zapirain MB, Gimeno-Santos M, Méndez-Zorrilla A, Vaidya CJ, Molins-Sauri M, Guerra-Balic M, Bruna-Rabassa O. Executive Functioning in Adults with Down Syndrome: Machine-Learning-Based Prediction of Inhibitory Capacity. International Journal of Environmental Research and Public Health. 2021; 18(20):10785. https://doi.org/10.3390/ijerph182010785
Chicago/Turabian StyleJojoa-Acosta, Mario Fernando, Sara Signo-Miguel, Maria Begoña Garcia-Zapirain, Mercè Gimeno-Santos, Amaia Méndez-Zorrilla, Chandan J. Vaidya, Marta Molins-Sauri, Myriam Guerra-Balic, and Olga Bruna-Rabassa. 2021. "Executive Functioning in Adults with Down Syndrome: Machine-Learning-Based Prediction of Inhibitory Capacity" International Journal of Environmental Research and Public Health 18, no. 20: 10785. https://doi.org/10.3390/ijerph182010785
APA StyleJojoa-Acosta, M. F., Signo-Miguel, S., Garcia-Zapirain, M. B., Gimeno-Santos, M., Méndez-Zorrilla, A., Vaidya, C. J., Molins-Sauri, M., Guerra-Balic, M., & Bruna-Rabassa, O. (2021). Executive Functioning in Adults with Down Syndrome: Machine-Learning-Based Prediction of Inhibitory Capacity. International Journal of Environmental Research and Public Health, 18(20), 10785. https://doi.org/10.3390/ijerph182010785








