Role of SatO2, PaO2/FiO2 Ratio and PaO2 to Predict Adverse Outcome in COVID-19: A Retrospective, Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Settings
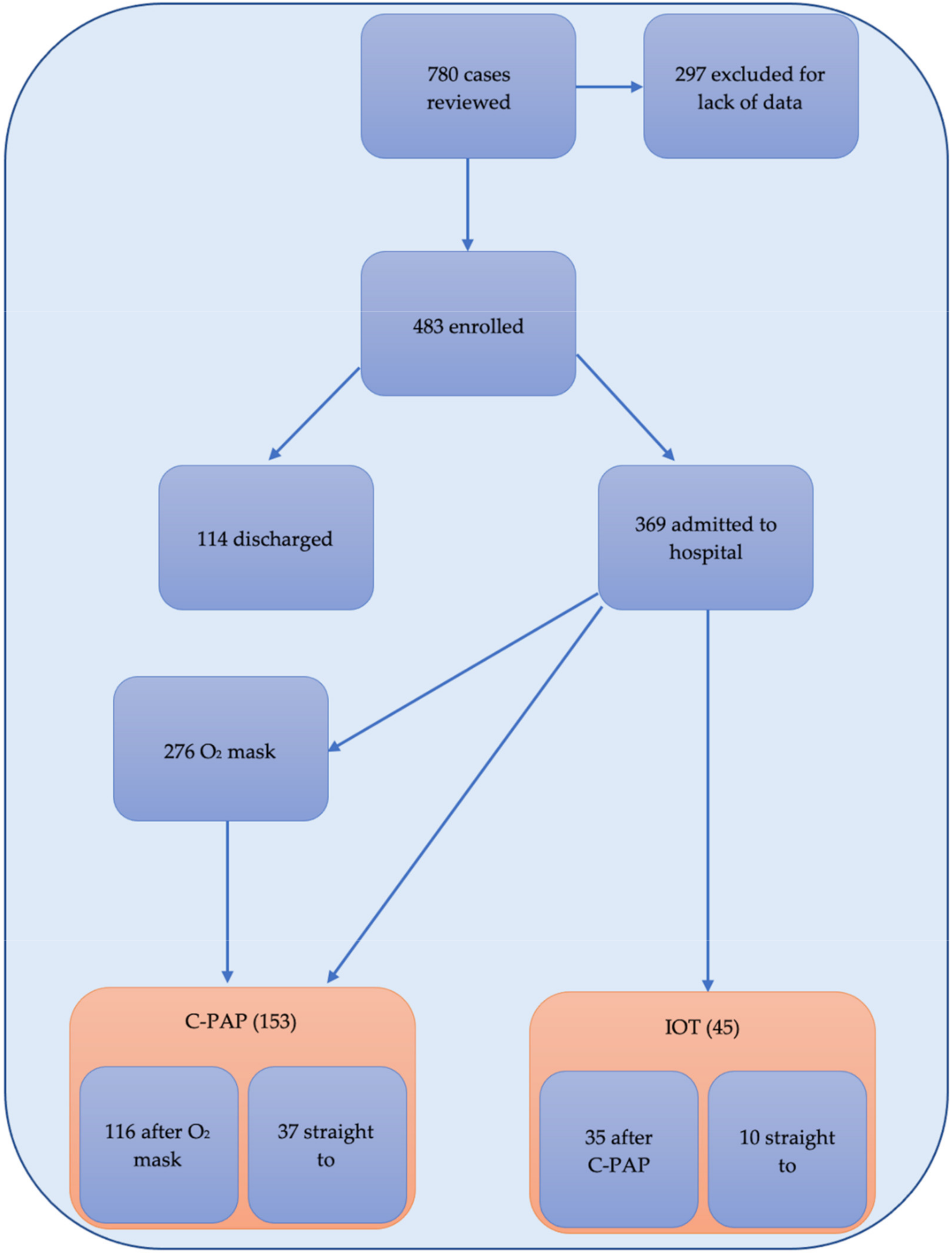
2.2. Particitants and Data Collection
- -
- Age;
- -
- Gender;
- -
- Coexisting disorder (hypertension, smoke, hypercholesterolemia, heart failure, COPD, pulmonary restrictive diseases, coagulopathies, immunodepression, diabetes, vascular-artery disease, chronic kidney disease, active solid cancer, active hematological disorder);
- -
- Medications (ACE inhibitors, steroids, oral anticoagulant);
- -
- Vital parameters at admission (systolic pressure, diastolic pressure, SatO2%, heart rate, respiratory rate, temperature);
- -
- Laboratory test at admission (white cell count, neutrophils, lymphocytes, platelets, aPTT, INR, d-dimer, fibrinogen, CRP, procalcitonin, lactate deydrogenase, IL-6, creatine-kinase, ferritin, troponin, creatinine, NT-probnp);
- -
- Arterial blood gas analysis: pH, pCO2, PaO2, PaO2/FiO2 ratio;
- -
- Number of patients requiring supplemental oxygen via face mask and those requiring non-invasive ventilation/C-PAP helmet.
2.3. Outcomes Measures
- SatO2 < 94% versus SatO2 ≥ 94% (value chosen on the basis of WHO indication).
- PaO2/FiO2 ratio subdivided using the threshold of 100–200–300 according to the Berlin criteria of ARDS.
- PaO2 < 60 and >100 mmHG (out of normal range) versus PaO2 60–100 (in range).
2.4. Data Analysis and Statistical Methods
3. Results
3.1. Characteristic of Patients
3.2. Outcome and Blood Gas Analysis
Logistic Analysis Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ARDS | acute respiratory distress syndrome |
| HFNC | high flow nasal cannula |
| C-PAP | continuous positive air pressure |
| NIV | non-invasive ventilation |
| ICU | Intensive Care Unit |
| DO2 | tissue oxygen delivery |
| VO2 | cellular oxygen consumption |
| ROS | reactive oxygen species |
| SatO2 | Peripheral oxygen saturation |
| ABG | arterial blood gas |
| PaO2 | partial pressure of arterial oxygen |
| PaO2/FiO2 | partial pressure of arterial oxygen/fraction of inspired oxygen rate |
| RT-PCR | reverse transcription of polymerase chain reaction |
| COPD | Chronic obstructive pulmonary disease |
| ACE | angiotensin-converting enzyme |
| aPTT | activated partial thromboplastin time |
| INR | International Normalized Ratio |
| NT-proBNP | N-Terminal Fragment of the Prohormone Brain-Type Natriuretic Peptide |
| IOT | mechanical ventilation |
| IQR | interquartile range |
| OR | odds ratio |
| CIs | confidence intervals |
References
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Moghadas, S.M.; Shoukat, A.; Fitzpatrick, M.; Wells, C.R.; Sah, P.; Pandey, A.; Sachs, J.D.; Wang, Z.; Meyers, L.A.; Singer, B.H.; et al. Projecting hospital utilization during the COVID-19 outbreaks in the United States. Proc. Natl. Acad. Sci. USA 2020, 117, 9122–9126. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Fang, X.; Cai, Z.; Wu, X.; Gao, X.; Min, J.; Wang, F. Comorbid Chronic Diseases and Acute Organ Injuries Are Strongly Correlated with Disease Severity and Mortality among COVID-19 Patients: A Systemic Review and Meta-Analysis. Research 2020, 2020, 2402961. [Google Scholar] [CrossRef] [Green Version]
- Grasselli, G.; Pesenti, A.; Cecconi, M. Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: Early experience and forecast during an emergency response. Jama 2020, 323, 1545–1546. [Google Scholar] [CrossRef] [Green Version]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- World Health Organization. Clinical Management COVID-19: Interim Guidance, 27 May 2020. Available online: https://apps.who.int/iris/handle/10665/332196 (accessed on 10 November 2020).
- Qureshi, A.I.; Baskett, W.I.; Huang, W.; Shyu, D.; Myers, D.; Raju, M.; Shyu, C.R. Acute ischemic stroke and COVID-19: An analysis of 27 676 patients. Stroke 2021, 52, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Long, B.; Brady, W.J.; Koyfman, A.; Gottlieb, M. Cardiovascular complications in COVID-19. Am. J. Emerg. Med. 2020, 38, 1504–1507. [Google Scholar] [CrossRef]
- Kellawan, J.M.; Harrell, J.W.; Roldan-Alzate, A.; Wieben, O.; Schrage, W.G. Regional hypoxic cerebral vasodilation facilitated by diameter changes primarily in anterior versus posterior circulation. J. Cereb. Blood Flow Metab. 2017, 37, 2025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casas, A.I.; Geuss, E.; Kleikers, P.W.M.; Mencl, S.; Herrmann, A.M.; Buendia, I.; Egea, J.; Meuth, S.G.; Lopez, M.G.; Kleinschnitz, C.; et al. NOX4-dependent neuronal autotoxicity and BBB breakdown explain the superior sensitivity of the brain to ischemic damage. Proc. Natl. Acad. Sci. USA 2017, 114, 12315–12320. [Google Scholar] [CrossRef] [Green Version]
- Girardis, M.; Busani, S.; Damiani, E.; Donati, A.; Rinaldi, L.; Marudi, A.; Morelli, A.; Antonelli, M.; Singer, M. Effect of conservative vs conventional oxygen therapy on mortality among patients in an intensive care unit: The oxygen-ICU randomized clinical trial. JAMA 2016, 316, 1583–1589. [Google Scholar] [CrossRef]
- Del Vecchio, L.; Locatelli, F. Hypoxia response and acute lung and kidney injury: Possible implications for therapy of COVID-19. Clin. Kidney J. 2020, 13, 494–499. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ma, X. Acute respiratory failure in COVID-19: Is it “typical” ARDS? Crit. Care 2020, 24, 1–5. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, G.M.; Mitchell, R.D.; Wu, J.; Rajiv, P.; Bannon-Murphy, H.; Amos, T.; Brichko, L.; Brennecke, H.; Noonan, M.P.; Mitra, B.; et al. Epidemiology and clinical features of emergency department patients with suspected COVID-19: Initial results from the COVID-19 Emergency Department Quality Improvement Project (COVED-1). Emerg. Med. Australas. 2020, 32, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Lazzerini, M.; Barbi, E.; Apicella, A.; Marchetti, F.; Cardinale, F.; Trobia, G. Delayed access or provision of care in Italy resulting from fear of COVID-19. Lancet Child Adolesc. Health 2020, 4, e10–e11. [Google Scholar] [CrossRef]
- Sapey, E.; Gallier, S.; Mainey, C.; Nightingale, P.; McNulty, D.; Crothers, H.; Evison, F.; Reeves, K.; Pagano, D.; Denniston, A.K. Ethnicity and risk of death in patients hospitalised for COVID-19 infection in the UK: An observational cohort study in an urban catchment area. BMJ Open Respir. Res. 2020, 7, e000644. [Google Scholar] [CrossRef]
- Samaja, M.; Milano, G. Adaptation to Hypoxia: A Chimera? Int. J. Mol. Sci. 2020, 21, 1527. [Google Scholar] [CrossRef] [Green Version]
- McKenna, H.T.; Murray, A.J.; Martin, D.S. Human adaptation to hypoxia in critical illness. J. Appl. Physiol. 2020, 129, 656–663. [Google Scholar] [CrossRef]
- Connett, R.J.; Honig, C.R.; Gayeski, T.E.; Brooks, G.A. Defining hypoxia: A systems view of VO2, glycolysis, energetics, and intracellular PO2. J. Appl. Physiol. 1990, 68, 833–842. [Google Scholar] [CrossRef]
- Jackson, R.M. Pulmonary oxygen toxicity. Chest 1985, 88, 900–905. [Google Scholar] [CrossRef]
- Machado, H.S. Paradigms of oxygen therapy in critically Ill patients. J. Intensive Crit. Care 2017, 3, 3. [Google Scholar]
- Zaytoun, T.M.; Elsayed, H.E.; Elsayed, S.E. The relation between arterial hyperoxia and mortality among intensive care unit patients with septic shock. Res. Opin. Anesth. Intensive Care 2020, 7, 41. [Google Scholar]
- LaForge, M.; Elbim, C.; Frère, C.; Hémadi, M.; Massaad, C.; Nuss, P.; Benoliel, J.-J.; Becker, C. Tissue damage from neutrophil-induced oxidative stress in COVID-19. Nat. Rev. Immunol. 2020, 20, 515–516. [Google Scholar] [CrossRef]
- Force, A.D.T.; Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute respiratory distress syndrome. JAMA 2012, 307, 2526–2533. [Google Scholar]
- Duan, J.; Han, X.; Bai, L.; Zhou, L.; Huang, S. Assessment of heart rate, acidosis, consciousness, oxygenation, and respiratory rate to predict noninvasive ventilation failure in hypoxemic patients. Intensive Care Med. 2017, 43, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Suliman, L.A.; Abdelgawad, T.T.; Farrag, N.S.; Abdelwahab, H.W. Validity of ROX index in prediction of risk of intubation in patients with COVID-19 pneumonia. Adv. Respir. Med. 2021, 89, 1–7. [Google Scholar] [CrossRef]
- World Health Organization. Clinical Care for Severe Acute Respiratory Infection: Toolkit. WHO/2019-nCoV/SARI_toolkit/2020.1. Available online: https://www.who.int/publications-detail-redirect/clinical-care-of-severe-acute-respiratory-infections-tool-kit (accessed on 11 April 2020).
- Becerra-Muñoz, V.M.; Núñez-Gil, I.J.; Eid, C.M.; García Aguado, M.; Romero, R.; Huang, J.; Mulet, A.; Ugo, F.; Rametta, F.; Liebetrau, C.; et al. Clinical profile and predictors of in-hospital mortality among older patients hospitalised for COVID-19. Age Ageing 2021, 50, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Mikami, T.; Miyashita, H.; Yamada, T.; Harrington, M.; Steinberg, D.; Dunn, A.; Siau, E. Risk Factors for Mortality in Patients with COVID-19 in New York City. J. Gen. Intern. Med. 2020, 36, 17–26. [Google Scholar] [CrossRef]
- Grasselli, G.; Tonetti, T.; Protti, A.; Langer, T.; Girardis, M.; Bellani, G.; Laffey, J.; Carrafiello, G.; Carsana, L.; Rizzuto, C.; et al. Pathophysiology of COVID-19-associated acute respiratory distress syndrome: A multicentre prospective observational study. Lancet Respir. Med. 2020, 8, 1201–1208. [Google Scholar] [CrossRef]
- Aliberti, S.; Radovanovic, D.; Billi, F.; Sotgiu, G.; Costanzo, M.; Pilocane, T.; Saderi, L.; Gramegna, A.; Rovellini, A.; Perotto, L.; et al. Helmet CPAP treatment in patients with COVID-19 pneumonia: A multicentre cohort study. Eur. Respir. J. 2020, 56, 2001935. [Google Scholar] [CrossRef] [PubMed]
- De Vita, N.; Scotti, L.; Cammarota, G.; Racca, F.; Pissaia, C.; Maestrone, C.; Colombo, D.; Olivieri, C.; Della Corte, F.; Barone-Adesi, F.; et al. Predictors of intubation in COVID-19 patients treated with out-of-ICU continuous positive airway pressure. Pulmonology 2021. [Google Scholar] [CrossRef]
- Ferrando, C.; Suarez-Sipmann, F.; Mellado-Artigas, R.; Hernández, M.; Gea, A.; Arruti, E.; Aldecoa, C.; Martínez-Pallí, G.; Martínez-González, M.A.; Slutsky, A.S.; et al. Clinical features, ventilatory management, and outcome of ARDS caused by COVID-19 are similar to other causes of ARDS. Intensive Care Med. 2020, 46, 2200–2211. [Google Scholar] [CrossRef]
- Bellani, G.; Laffey, J.G.; Pham, T.; Madotto, F.; Fan, E.; Brochard, L.; Esteban, A.; Gattinoni, L.; Bumbasirevic, V.; Piquilloud, L.; et al. Noninvasive Ventilation of Patients with Acute Respiratory Distress Syndrome. Insights from the LUNG SAFE Study. Am. J. Respir. Crit. Care Med. 2017, 195, 67–77. [Google Scholar] [CrossRef] [Green Version]
- Villar, J.; Blanco, J.; Del Campo, R.; Andaluz-Ojeda, D.; Díaz-Domínguez, F.J.; Muriel, A.; Córcoles, V.; Suarez-Sipmann, F.; Tarancón, C.; González-Higueras, E.; et al. Assessment of PaO2/FiO2 for stratification of patients with moderate and severe acute respiratory distress syndrome. BMJ Open 2015, 5, e006812. [Google Scholar] [CrossRef]
- Feiner, J.R.; Weiskopf, R.B. Evaluating pulmonary function: An assessment of PaO2/FIO2. Crit. Care Med. 2017, 45, e40–e48. [Google Scholar] [CrossRef] [PubMed]
- Santus, P.; Radovanovic, D.; Saderi, L.; Marino, P.; Cogliati, C.; De Filippis, G.; Rizzi, M.; Franceschi, E.; Pini, S.; Giuliani, F.; et al. Severity of respiratory failure at admission and in-hospital mortality in patients with COVID-19: A prospective observational multicentre study. BMJ Open 2020, 10, e043651. [Google Scholar] [CrossRef]
- West, J.B. State of the art: Ventilation-perfusion relationships. Am. Rev. Respir. Dis. 1977, 116, 919–943. [Google Scholar] [CrossRef] [PubMed]
- Dantzker, D.R. Gas Exchange in the Adult Respiratory Distress Syndrome. Clin. Chest Med. 1982, 3, 57–67. [Google Scholar] [CrossRef]
- Gowda, M.S.; Klocke, R.A. Variability of indices of hypoxemia in adult respiratory distress syndrome. Crit. Care Med. 1997, 25, 41–45. [Google Scholar] [CrossRef]
- Karbing, D.S.; Kjærgaard, S.; Smith, B.W.; Espersen, K.; Allerød, C.; Andreassen, S.; Rees, S.E. Variation in the PaO 2/FiO 2 ratio with FiO 2: Mathematical and experimental description, and clinical relevance. Crit. Care 2007, 11, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Wiersinga, W.J.; Rhodes, A.; Cheng, A.C.; Peacock, S.J.; Prescott, H.C. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): A review. JAMA 2020, 324, 782–793. [Google Scholar] [CrossRef]
- Hatipoğlu, U.; Chatburn, R.; Duggal, A. Optimal Respiratory Assistance Strategy for Patients with COVID-19. Ann. Am. Thorac. Soc. 2021, 18, 916–917. [Google Scholar] [CrossRef]
- Grimaldi, D.; Hraiech, S.; Boutin, E.; Lacherade, J.C.; Boissier, F.; Pham, T.; Richard, J.C.; Thille, A.W.; Ehrmann, S.; Lascarrou, J.B.; et al. Hypoxemia in the ICU: Prevalence, treatment, and outcome. Ann. Intensive Care 2018, 8, 1–11. [Google Scholar]
- Kashani, K.B. Hypoxia in COVID-19: Sign of Severity or Cause for Poor Outcomes. Mayo Clin. Proc. 2020, 95, 1094–1096. [Google Scholar] [CrossRef]
- Rincon, F.; Kang, J.; Maltenfort, M.; Vibbert, M.; Urtecho, J.; Athar, M.K.; Jallo, J.; Pineda, C.; Tzeng, D.; McBride, W.; et al. Association between hyperoxia and mortality after stroke: A multicenter cohort study. Crit. Care Med. 2014, 42, 387–396. [Google Scholar] [CrossRef]
- Chu, D.; Kim, L.H.-Y.; Young, P.; Zamiri, N.; Almenawer, S.A.; Jaeschke, R.; Szczeklik, W.; Schünemann, H.J.; Neary, J.D.; Alhazzani, W. Mortality and morbidity in acutely ill adults treated with liberal versus conservative oxygen therapy (IOTA): A systematic review and meta-analysis. Lancet 2018, 391, 1693–1705. [Google Scholar] [CrossRef]
- De Graaff, A.E.; Dongelmans, D.A.; Binnekade, J.M.; de Jonge, E. Clinicians’ response to hyperoxia in ventilated patients in a Dutch ICU depends on the level of FiO 2. Intensive Care Med. 2011, 37, 46–51. [Google Scholar] [CrossRef] [Green Version]
- Martin, D.S.; Grocott, M.P.W. Oxygen therapy in critical illness: Precise control of arterial oxygenation and permissive hypoxemia. Crit. Care Med. 2013, 41, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.; Chandel, N.S.; Simon, M.C. Cellular adaptation to hypoxia through hypoxia inducible factors and beyond. Nat. Rev. Mol. Cell Biol. 2020, 21, 268–283. [Google Scholar] [CrossRef]
- Oliynyk, O.V.; Rorat, M.; Barg, W. Oxygen metabolism markers as predictors of mortality in severe COVID-19. Int. J. Infect. Dis. 2021, 103, 452–456. [Google Scholar] [CrossRef]
- Levy, M.M. Pathophysiology of Oxygen Delivery in Respiratory Failure. Chest 2005, 128, 547S–553S. [Google Scholar] [CrossRef]
| All Participants (n = 483) | GROUP A Admitted to Hospital Not Requiring C-PAP (n = 217) | GROUP B Admitted to Hospital on C-PAP (n = 140) | |
|---|---|---|---|
| Age, years | 74 (61–83) | 77 (61–85) | 69 (61–77) |
| Gender | |||
| Men Woman | 278/483 (57.56%) 205/483 (42.44%) | 170/330 (51.52%) 160/333 (48.48%) | 108/153 (70.59%) 45/153 (29.41%) |
| Coexisting Disorders | |||
| Hypertension | 205/483 (42.44%) | 132/330 (40.00%) | 73/153 (47.71%) |
| Smoke | 17/483 (3.52%) | 10/330 (3.03%) | 7/153 (4.58%) |
| Hypercholesterolemia | 58/483 (12.01%) | 42/330 (12.73%) | 16/153 (10.46%) |
| Heart failure with EF < 50% | 18/483 (3.73%) | 16/330 (4.85%) | 2/153 (1.31%) |
| COPD | 28/483 (5.80%) | 21/330 (6.36%) | 7/153 (4.58%) |
| Pulmonary restrictive diseases | 2/483 (0.41%) | 1/330 (0.30%) | 1/153 (0.65%) |
| Coagulopathy | 3/483 (0.62%) | 2/330 (0.61%) | 1/153 (0.65%) |
| Immunodepression (acquired or congenital) | 22/483 (4.55%) | 13/330 (3.94%) | 9/153 (5.88%) |
| Diabetes | 49/483 (10.14%) | 26/330 (7.88%) | 23/153 (15.03%) |
| Vascular artery diseases | 86/483 (17.81%) | 66/330 (20.00%) | 20/153 (13.07%) |
| Chronic kidney diseases | 22/483 (4.55%) | 13/330 (3.94%) | 9/153 (5.88%) |
| Medications | |||
| ACE-inhibitors | 104/483 (21.53%) | 63/330 (19.09%) | 41/153 (26.80%) |
| Steroids | 25/483 (5.18%) | 12/330 (3.64%) | 13/153 (8.50%) |
| Active solid cancer | 50/483 (10.35%) | 37/330 (11.21%) | 13/153 (8.50%) |
| Active hematological disorders | 27/483 (5.59%) | 18/330 (5.45%) | 9/153 (5.88%) |
| Vital parameters at admission | |||
| Systolic, mmHg | 130 (117–145) | 130 (116–145) | 130 (120–150) |
| Diastolic, mmHg | 75 (65–84) | 75 (65–82) | 77 (68–87) |
| SatO2, % | 95 (91–97) | 95 (92–97) | 94 (89–97) |
| Heart rate, per minute | 85 (75–99) | 85 (75–99) | 86 (75–98) |
| Respiratory rate, per minute | 20 (18–25) | 20 (18–24) | 22 (18–30) |
| Temperature, Celsius | 36.9 (36.5–37.7) | 36.8 (36.5–37.7) | 37 (36.5–37.7) |
| Laboratory test at admission | |||
| White cell count, 10⁹ cells/L | 6.98 (4.94–10.5) | 7.18 (4.96–11.42) | 6.77 (4.94–9.76) |
| Neutrophil, 10⁹ cells/L | 5.3 (3.6–8.5) | 5.4 (3.5–9) | 5.25 (3.8–8.1) |
| Lymphocyte, 10⁹ cells/L | 0.9 (0.6–1.2) | 0.9 (0.6–1.3) | 0.8 (0.5–1.05) |
| Hemoglobin, g/L | 135.5 (122–147) | 133 (119.5–144.5) | 141 (125–150) |
| Platelets, 10⁹ cells/L | 200 (153–266) | 212 (156–275) | 182 (139–243) |
| aPTT, second | 33.1 (30.6–35.5) | 32.7 (30.3–35.7) | 33.45 (31.35–35.4) |
| INR | 1.2 (1.12–1.32) | 1.2 (1.11–1.34) | 1.21 (1.14–1.29) |
| D-dimer, ng/mL | 1027.5 (613.15–1636) | 1054 (615.7–1969) | 988 (612.6–1388) |
| Fibrinogen, g/L | 5.47 (4.43–6.88) | 5.2 (4.23–6.62) | 6.26 (5.04–7.6) |
| C-reactive protein, µg/dL | 75.4 (34.6–130) | 64.7 (27.7–124) | 99.15 (54.1–140) |
| Procalcitonin, ng/mL | 0.15 (0.06–0.38) | 0.12 (0.05–0.35) | 0.175 (0.1–0.415) |
| Lactic dehydrogenase, u/L | 307 (236–408) | 289 (221–380) | 339.5 (272.5–459.5) |
| IL-6, ng/L | 638.85 (618.7–674.2) | 632.2 (615.9–663.3) | 655.8 (629.3–699) |
| Creatine-kinase, u/L | 101.5 (59–204) | 89 (54–160) | 130 (68–238) |
| Ferritin, mg/mL | 569 (264–1115) | 497 (234–924) | 758 (365–1425) |
| Troponin, µg/L | 0.015 (0.015–0.043) | 0.015 (0.015–0.066) | 0.015 (0.015–0.026) |
| NT-proBNP, ng/L | 350 (92–2076) | 399 (91–2915) | 302.5 (92–781) |
| Arterial blood gas analysis at admission | |||
| pH | 7.46 (7.42–7.49) | 7.45 (7.42–7.49) | 7.46 (7.43–7.49) |
| PaO2, mmHg | 67 (58–80) | 70 (61–83) | 60 (52–73) |
| PaO2/FiO2 ratio | 285 (203–340) | 304.5 (232–357) | 246 (150–294) |
| Outcomes | |||
| Mechanical ventilation (IOT) | 45/483 (9.26%) | 10/330 (3.03%) | 35/153 (22.88%) |
| Intra-hospital mortality | 185/483 (38.07%) | 123/330 (37.5274%) | 60/153 (39.22%) |
| C-PAP failure | 70/483 (14.40%) | 0/330 (0%) | 70/153 (45.75%) |
| Hospital readmission within 30 days from discharge | 40/483 (8.23%) | 25/330 (7.58%) | 15/153 (9.80%) |
| Length of hospital stay, days | 13.99 (3.98–23.14) | 12.14 (1.95–22.05) | 17.65 (11.14–24.04) |
| Time 0 | Group A | Group B | ||||
|---|---|---|---|---|---|---|
| At least one adverse outcome | No | Yes | No | Yes | No | Yes |
| SatO2 < 94% | 59 | 113 | 18 | 75 | 1 | 34 |
| SatO2 ≥ 94% | 158 | 153 | 49 | 58 | 36 | 64 |
| p-value | 0.000 | 0.000 | 0.000 | |||
| Time 0 | Group A | Group B | ||||
|---|---|---|---|---|---|---|
| At least one adverse outcome | No | Yes | No | Yes | No | Yes |
| PaO2/FiO2 < 100 | 7 | 30 | 12 | 60 | 2 | 41 |
| PaO2/FiO2 100–200 | 20 | 59 | 30 | 68 | 23 | 44 |
| PaO2/FiO2 200–300 | 65 | 96 | 17 | 14 | 6 | 14 |
| PaO2/FiO2 > 300 | 125 | 81 | 10 | 6 | 6 | 4 |
| p-value | 0.000 | 0.000 | 0.000 | |||
| Time 0 | Group A | Group B | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| pO2 | Best pO2 | Worst pO2 | Best pO2 | Worst pO2 | ||||||
| At least one adverse outcome | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes |
| pO2 < 60 mmHg | 43 | 100 | 2 | 27 | 23 | 84 | 0 | 6 | 3 | 38 |
| pO2 60–100 mmHg | 158 | 137 | 35 | 60 | 41 | 42 | 4 | 24 | 19 | 35 |
| pO2 > 100 mmHg | 16 | 29 | 29 | 47 | 3 | 8 | 32 | 68 | 14 | 25 |
| p-value | 0.000 | 0.005 | 0.000 | 0.055 | 0.003 | |||||
| At Least One Adverse Outcome | In-Hospital Mortality | |||
|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| SatO2 < 94% | ||||
| at Time 0 | 1.98 (1.34–2.91) | 0.001 | 2.12 (1.45–3.11) | 0.000 |
| in Group A | 3.52 (1.86–6.67) | 0.000 | 4.10 (2.25–7.47) | 0.000 |
| in Group B | 19.12 (2.51–145.63) | 0.004 | 10.12 (4.08–25.14) | 0.000 |
| PaO2/FiO2 at Time 0 | ||||
| <100 vs. ≥100 | 3.81 (1.64–8.86) | 0.002 | 3.33 (1.65–6.72) | 0.001 |
| <200 vs. ≥200 | 3.54 (2.19–5.70) | 0.000 | 3.10 (2.02–4.77) | 0.000 |
| <300 vs. ≥300 | 3.10 (2.13–4.51) | 0.000 | 3.40 (2.27–5.10) | 0.000 |
| PaO2/FiO2 in Group A | ||||
| <100 vs. ≥100 | 3.24 (1.60–6.55) | 0.001 | 3.38 (1.88–6.09) | 0.000 |
| <200 vs. ≥200 | 4.11 (2.09–8.08) | 0.000 | 2.47 (1.20–5.09) | 0.014 |
| <300 vs. ≥300 | 4.01 (1.39–11.54) | 0.010 | 1.61 (0.54–4.81) | 0.392 |
| PaO2/FiO2 in Group B | ||||
| <100 vs. ≥100 | 11.57 (2.64–50.76) | 0.001 | 11.94 (5.07–28.13) | 0.000 |
| <200 vs. ≥200 | 2.27 (0.96–5.33) | 0.061 | 12.55 (2.85–55.28) | 0.001 |
| <300 vs. ≥300 | 4.79 (1.27–18.07) | 0.021 | ** | |
| PaO2 < 60 or PaO2 > 100 | ||||
| at Time 0 | 2.52 (1.72–3.70) | 0.000 | 2.59 (1.77–3.79) | 0.000 |
| Worst in Group A | 3.45 (1.87–6.37) | 0.000 | 3.37 (1.81–6.26) | 0.000 |
| Best in Group A | 1.39 (0.77–2.51) | 0.272 | 1.13 (0.64–1.99) | 0.671 |
| Worst in Group B | 2.01 (0.93–4.36) | 0.077 | 3.72 (1.68–8.23) | 0.001 |
| Best in Group B | 0.38 (0.12–1.20) | 0.100 | 0.41 (0.17–0.95) | 0.039 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sartini, S.; Massobrio, L.; Cutuli, O.; Campodonico, P.; Bernini, C.; Sartini, M.; Cristina, M.L.; Castellani, L.; Ceschi, L.; Spadaro, M.; et al. Role of SatO2, PaO2/FiO2 Ratio and PaO2 to Predict Adverse Outcome in COVID-19: A Retrospective, Cohort Study. Int. J. Environ. Res. Public Health 2021, 18, 11534. https://doi.org/10.3390/ijerph182111534
Sartini S, Massobrio L, Cutuli O, Campodonico P, Bernini C, Sartini M, Cristina ML, Castellani L, Ceschi L, Spadaro M, et al. Role of SatO2, PaO2/FiO2 Ratio and PaO2 to Predict Adverse Outcome in COVID-19: A Retrospective, Cohort Study. International Journal of Environmental Research and Public Health. 2021; 18(21):11534. https://doi.org/10.3390/ijerph182111534
Chicago/Turabian StyleSartini, Stefano, Laura Massobrio, Ombretta Cutuli, Paola Campodonico, Cristina Bernini, Marina Sartini, Maria Luisa Cristina, Luca Castellani, Ludovica Ceschi, Marzia Spadaro, and et al. 2021. "Role of SatO2, PaO2/FiO2 Ratio and PaO2 to Predict Adverse Outcome in COVID-19: A Retrospective, Cohort Study" International Journal of Environmental Research and Public Health 18, no. 21: 11534. https://doi.org/10.3390/ijerph182111534






