Interactions with Arsenic: Mechanisms of Toxicity and Cellular Resistance in Eukaryotic Microorganisms
Abstract
1. By Way of Introduction
2. Arsenic, a Metalloid with Complex Chemistry and Biogeochemistry
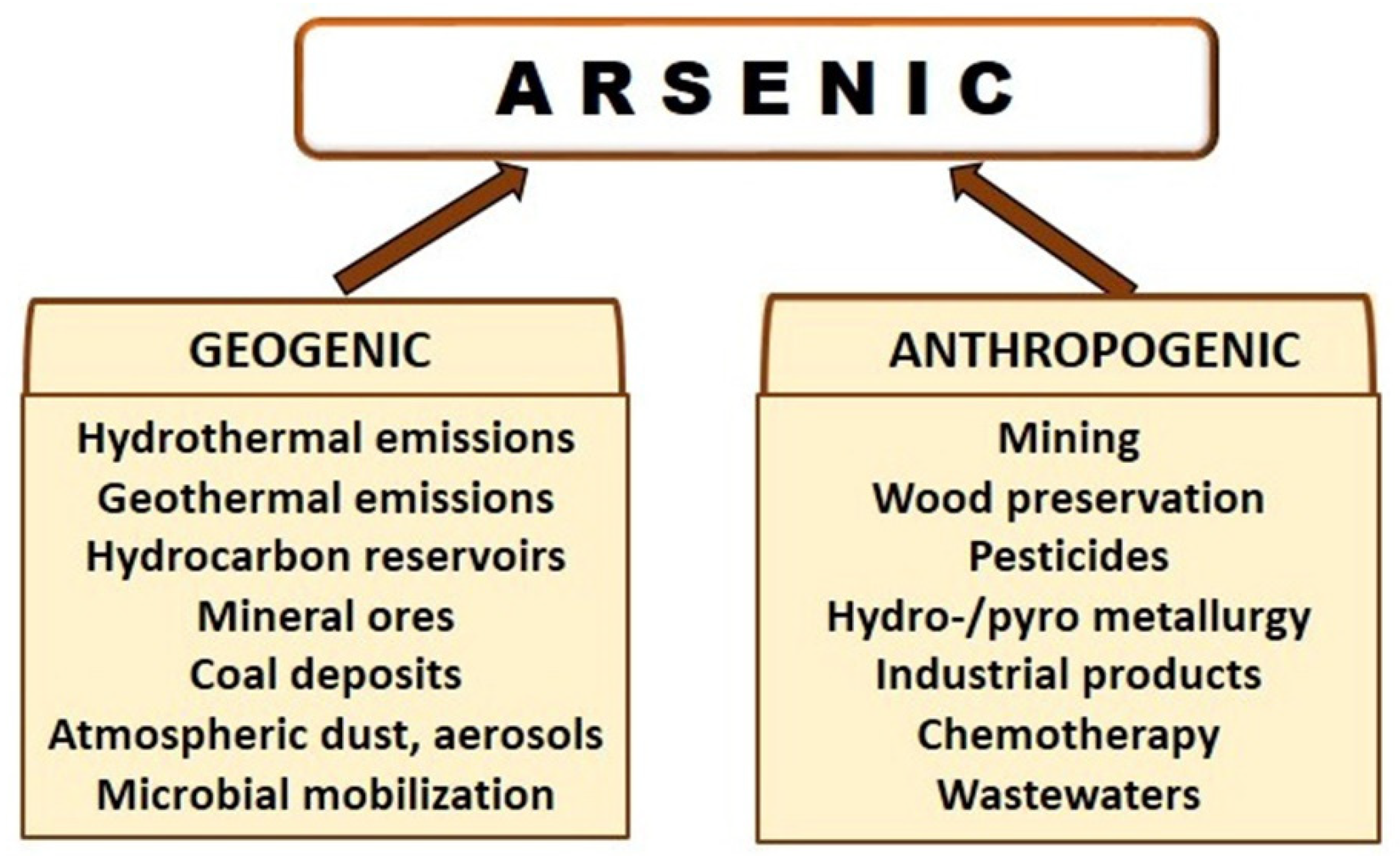
3. Arsenic Sources and Emissions in the Biosphere and Atmosphere
4. Anthropogenic As, a Global Environmental Problem with Health Risks
5. Microbial Biotransformations: Impacts on Arsenic and Arsenic Methylation Cycles
5.1. Oxidation and Reduction
5.2. Biomethylation As Methylation Cycle
5.3. Immobilization and Liberation of Arsenicals
6. Arsenic Toxicity in Eukaryotic Microorganisms: Main Effects and Targets
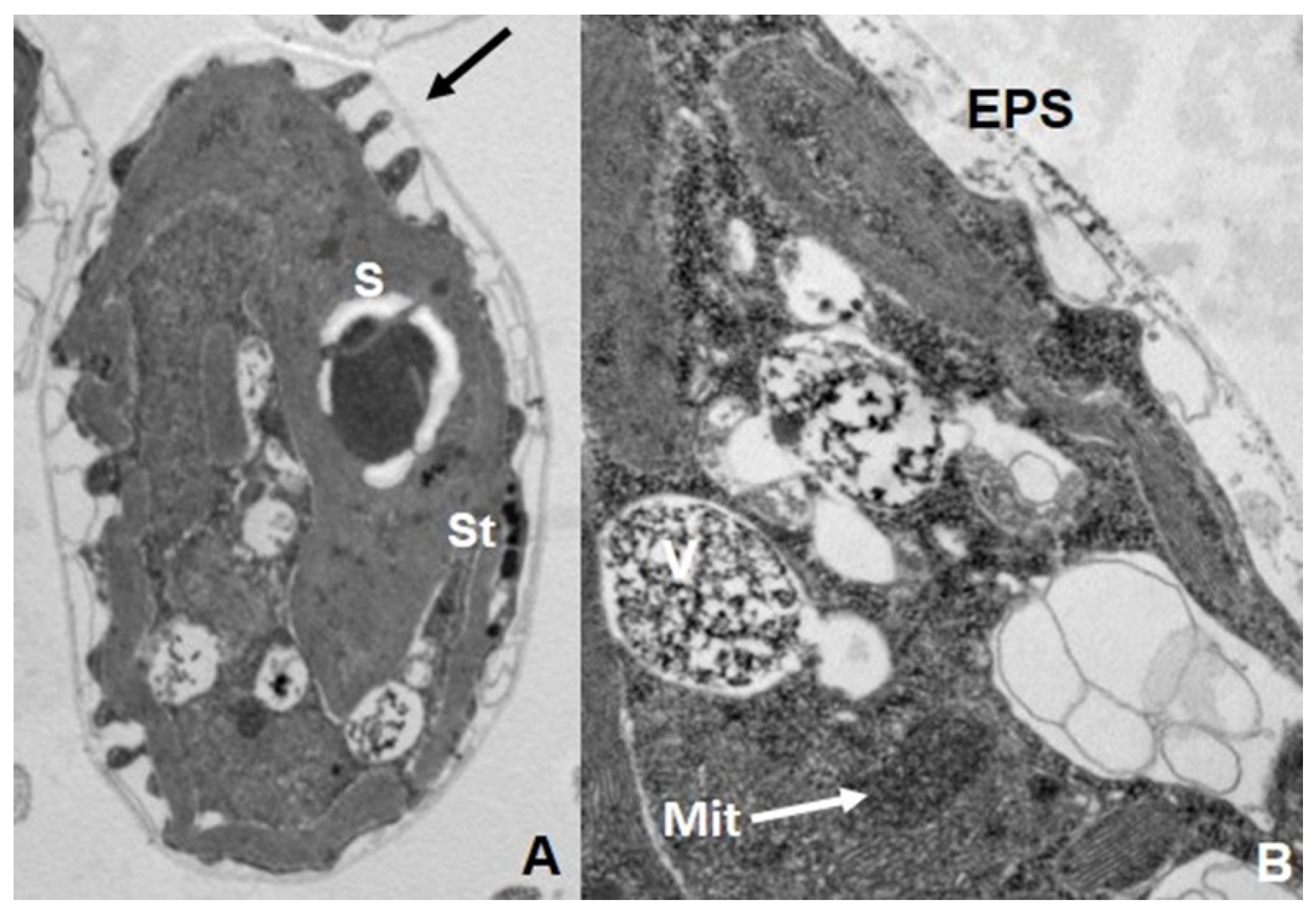
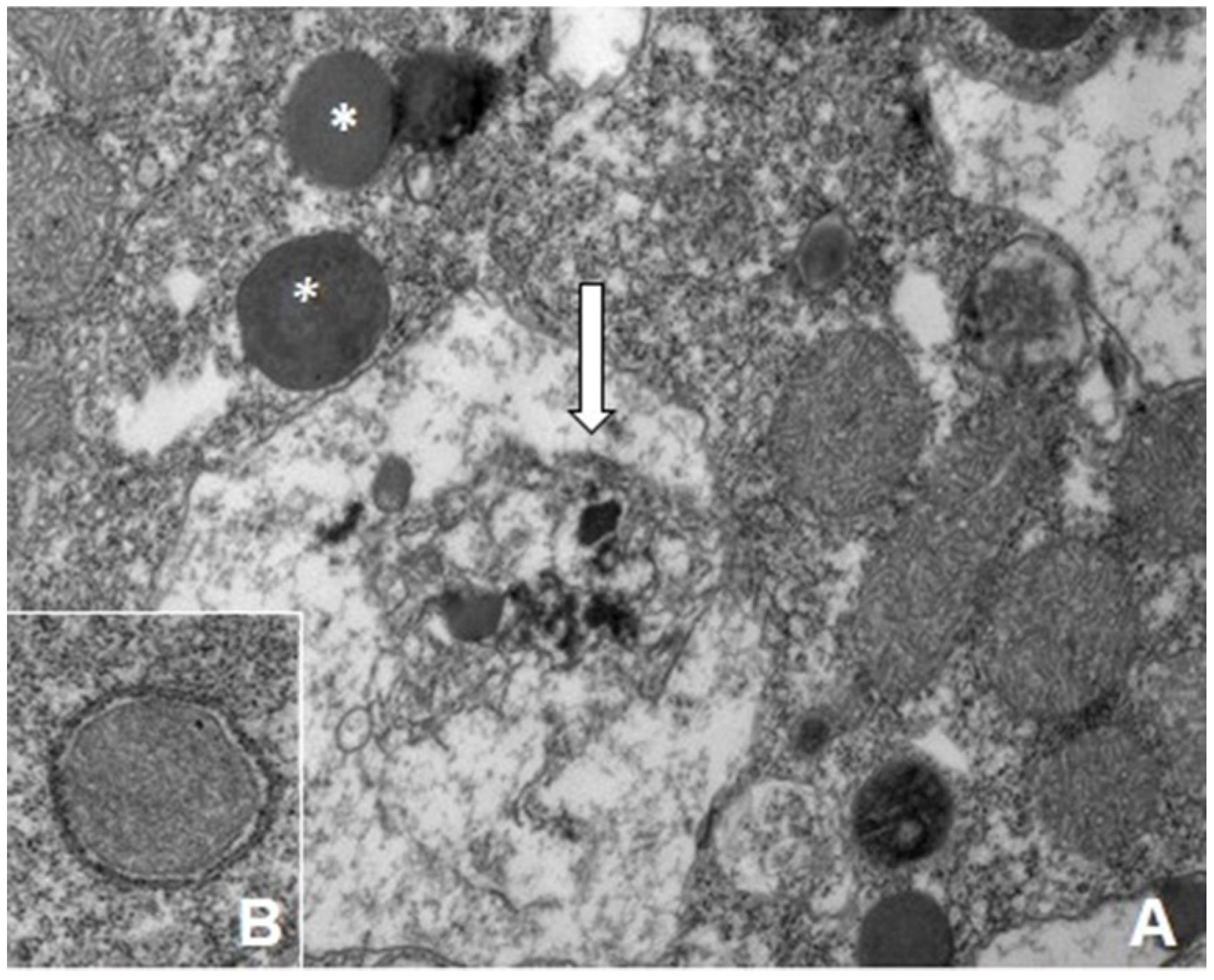
7. Biotransformation and Resistance/Tolerance to As in Fungi and Protists
8. Outlook of Eukaryotic Microorganism Applications in As Bioremediation
9. Conclusions
- Arsenic contamination is a relevant environmental problem, with global distribution.
- In most ecosystems, biotransformations of As have been carried out mainly by microorganisms, establishing physiological interactions among them.
- Eukaryotic microorganisms present many different As tolerance/resistance mechanisms, some of them are applicable in bioremediation.
- New molecular studies, using eukaryotic microorganisms (microalgae, ciliates, filamentous fungi) are necessary, before developing more efficient strategies of bioremediation.
- Due to the existence of complex microbial interactions in As polluted ecosystems, Systems Microbiology could be an innovative and appropriate approach to reduce the contamination with this metalloid.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Al-Makihah, N.H.; Taleb, M.A.; Bakarat, M.A. Arsenic bioaccumulation in arsenic-contaminated soil: A review. Chem. Pap. 2020, 74, 2743–2757. [Google Scholar] [CrossRef]
- Khalid, M.S.; Niazi, N.K.; Rafiq, M.; Bakhat, H.F.; Imran, M.; Abbas, T.; Bibi, I.; Dumat, C. Arsenic Behaviour in Soil-Plant System: Biogeochemical Reactions and Chemical Speciation Influences. In Enhancing Cleanup of Environmental Pollutants; Najum, N.A., Gill, S.S., Tuteja, N., Eds.; Non-Biological Approaches; Springer: Cham, Switzerland, 2017; Volume 2, pp. 97–140. [Google Scholar]
- Abbas, G.; Murtaza, B.; Bibi, I.; Shahid, M.; Niazi, N.K.; Khan, N.I.; Amjad, M.; Hussain, M.; Nathasha. Arsenic Uptake, Toxicity, Detoxification, and Speciation in Plants: Physiological, Biochemical, and Molecular Aspects. Int. J. Environ. Res. Public Health 2018, 15, 59. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.Y.; Costa, M. Arsenic: A Global Environmental Challenge. Annu. Rev. Pharmacol. Toxicol. 2021, 61, 47–63. [Google Scholar] [CrossRef] [PubMed]
- Duker, A.A.; Carranza, E.J.M.; Hale, M. Arsenic geochemistry and health. Environ. Int. 2005, 31, 631–641. [Google Scholar] [CrossRef]
- Polya, D.A.; Lawson, M. Geogenic and Anthropogenic Arsenic Hazard in Groundwaters and Soils: Distribution, Nature, Origin, and Human Exposure Routes. In Arsenic: Exposure Sources, Health Risks, and Mechanisms of Toxicity; States, E., Ed.; John Wiley and Sons: Hoboken, NJ, USA, 2016; pp. 23–60. [Google Scholar]
- Matschullat, J. Arsenic in the geosphere—A review. Sci. Total Environ. 2000, 249, 297–312. [Google Scholar] [CrossRef]
- O´Day, P.A. Chemistry and Mineralogy of Arsenic. Elements 2006, 2, 77–83. [Google Scholar] [CrossRef]
- Ng, J.C. Environmental Contamination of Arsenic and its Toxicological Impacts on Humans. Environ. Chem. 2005, 2, 146–160. [Google Scholar] [CrossRef]
- Sharma, V.K.; Sohn, M. Aquatic arsenic: Toxicity, speciation, transformations, and remediation. Environ. Int. 2009, 35, 743–759. [Google Scholar] [CrossRef] [PubMed]
- Caumette, G.; Koch, I.; Reimer, K.J. Arsenobetaine formation in plankton: A review of studies at the base of the aquatic food chain. J. Environ. Monit. 2012, 14, 2841–2853. [Google Scholar] [CrossRef]
- Hoffmann, T.; Warmbold, B.; Smits, S.H.J.; Tschapek, B.; Ronzheimer, S.; Bashir, A.; Chen, C.; Rolbetzki, A.; Pittelkow, M.; Jebbar, M.; et al. Arsenobetaine: An ecophysiologically important organoarsenical confers cytoprotection against osmotic stress and growth temperature extremes. Environ. Microbiol. 2018, 20, 305–323. [Google Scholar] [CrossRef]
- Xue, X.-M.; Xiong, C.; Yoshinaga, M.; Rosen, B.; Zhu, Y.-Z. The enigma of environmental organoarsenicals: Insights and implications. Crit. Rev. Environ. Sci. Technol. 2021. [Google Scholar] [CrossRef]
- Byeon, E.; Kang, H.-M.; Yoon, C.; Lee, J.-S. Toxicity mechanisms of arsenic compounds in aquatic organisms. Aquat. Toxicol. 2021, 235, 105901. [Google Scholar] [CrossRef] [PubMed]
- Lloyds, J.; Omerland, R. Microbial Transformations of Arsenic in the Environment: From Soda Lakes to Aquifers. Elements 2006, 2, 85–90. [Google Scholar] [CrossRef]
- Ye, J.; Rensing, C.; Rosen, B.P.; Zhu, Y.-G. Arsenic biomethylation by photosynthetic organisms. Trends Plant Sci. 2012, 17, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Smedley, P.L.; Kinniburgh, D.G. A review of the source, behaviour and distribution of arsenic in natural waters. Appl. Geochem. 2002, 17, 517–568. [Google Scholar] [CrossRef]
- Flora, S.J.S. Arsenic: Chemistry, Occurrence, and Exposure. In Handbook of Arsenic Toxicology; Flora, S.J.S., Ed.; Academic Press: New York, NY, USA, 2016; pp. 1–49. [Google Scholar] [CrossRef]
- Turpeinen, R.; Pantsar-Kallio, M.; Kairesalo, T. Role of microbes in controlling the speciation of arsenic and production of arsines in contaminated soils. Sci. Total Environ. 2002, 285, 133–145. [Google Scholar] [CrossRef]
- Gorny, J.; Billon, G.; Lesven, L.; Dumoulin, D.; Madé, B.; Noiriel, G. Arsenic behavior in river sediments under redox gradient: A review. Sci. Total Environ. 2015, 505, 423–434. [Google Scholar] [CrossRef]
- Thomas, D.J. The Chemistry and Metabolism of Arsenic. In Arsenic: Exposure Sources, Health Risks, and Mechanisms of Toxicity; States, E., Ed.; John Wiley and Sons: Hoboken, NJ, USA, 2016; pp. 81–110. [Google Scholar]
- Rahman, M.A.; Hassler, C. Is arsenic biotransformation a detoxification mechanism for microorganisms? Aquat. Toxicol. 2014, 146, 212–219. [Google Scholar] [CrossRef]
- Barral-Fraga, L.; Barral, M.T.; MacNeill, K.L.; Martiñá-Prieto, D.; Morín, S.; Rodriguez-Castro, M.C.; Tuulaikhuu, B.-A.; Guasch, E. Biotic and Abiotic Factors Influencing Arsenic Biogeochemistry and Toxicity in Fluvial Ecosystems: A Review. Int. J. Environ. Res. Public Health 2020, 17, 2331. [Google Scholar] [CrossRef] [PubMed]
- Hasewaga, H.; Rahman, M.A.; Matsuda, T.; Kitahara, T.; Maki, T.; Ueda, K. Effect of eutrophication on the distribution of arsenic species in eutrophic and mesotrophic lakes. Sci. Total Environ. 2009, 407, 1418–1425. [Google Scholar] [CrossRef]
- Pigna, M.; Caporale, A.G.; Cavalca, L.; Sommella, A.; Violante, A. Arsenic in the Soil Environment: Mobility and Phytoavailability. Environ. Engin. Sci. 2015, 32, 1–13. [Google Scholar] [CrossRef]
- Drewniak, L.; Sklodowska, A. Arsenic-transforming microbes and their role in biomining processes. Environ. Sci. Pollut. Res. 2013, 20, 7728–7739. [Google Scholar] [CrossRef] [PubMed]
- Bundschuh, J.; Schneider, J.; Alam, M.A.; Niazi, N.K.; Herath, I.; Parvez, F.; Tomaszewska, B.; Guilherme, L.R.G.; Maity, J.P.; López, D.L.; et al. Seven potential sources of arsenic pollution in Latin America and their environmental and health impacts. Sci. Total Environ. 2021, 780, 146274. [Google Scholar] [CrossRef]
- Matschullat, J. The global arsenic cycle revisited. In Arsenic: Natural and Antrophogenic; Deschams, E., Matschullat, J., Eds.; CRC Press: Boca Ratón, FL, USA, 2011; pp. 3–27. [Google Scholar]
- Yamamura, S.; Amachi, S. Microbiology of inorganic arsenic: From metabolism to bioremediation. J. Biosci. Bioeng. 2014, 118, 1–9. [Google Scholar] [CrossRef]
- Ozturk, M.; Metin, M.; Altay, V.; Baht, R.A.; Ejaz, M.; Gul, A.; Unal, B.T.; Hassanuzzaman, M.; Nibir, L.; Nahar, K.; et al. Arsenic and Human Health: Genotoxicity, Epigenomic Effects, and Cancer Signaling. Biol. Trace Elem. Res. 2021. [Google Scholar] [CrossRef]
- Nazarí, A.M.; Radzinski, R.; Ghahreman, A. Review of arsenic metallurgy: Treatment of arsenical minerals and the immobilization of arsenic. Hydrometallurgy 2017, 174, 258–281. [Google Scholar] [CrossRef]
- Gupta, D.K.; Tiwari, S.; Razanfindrabe, B.H.N.; Chatterjee, S. Arsenic Contamination from Historical Aspects to the Present. In Arsenic Contamination in the Environment; Gupta, D.K., Chatterjee, S., Eds.; Springer: Cham, Switzerland, 2017; pp. 1–13. [Google Scholar]
- Barraj, L.M.; Scrafford, C.G.; Eaton, W.C.; Rogers, R.E.; Jeng, C.-J. Arsenic levels in wipe samples collected from play structures constructed with CCA-treated wood: Impact on exposure estimates. Sci. Total Environ. 2009, 407, 2586–2592. [Google Scholar] [CrossRef] [PubMed]
- Matos, R.C.; Bessa, M.; Oliveira, H.; Gonsalves, F.; Pereira, M.L.; Nunes, B. Mechanisms of kidney toxicity for chromium-and arsenic-based preservatives: Potential involvement of a pro-oxidative pathway. Environ. Toxicol. Pharmacol. 2013, 36, 929–936. [Google Scholar] [CrossRef]
- Hughes, M.F.; Beck, B.D.; Chen, Y.; Lewis, A.S.; Thomas, D.J. Arsenic Exposure and Toxicology: A Historical Perspective. Toxicol. Sci. 2011, 123, 305–322. [Google Scholar] [CrossRef]
- Hughes, M.F. History of Arsenic as a Poison and a Medicinal Agent. In Arsenic: Exposure Sources, Health Risks, and Mechanisms of Toxicity; States, E., Ed.; John Wiley and Sons: Hoboken, NJ, USA, 2016; pp. 3–22. [Google Scholar]
- Frith, J. Arsenic—The “Poison of Kings” and the “Saviour of Syphilis”. J. Mil. Veterans’ Health 2013, 21, 11–17. [Google Scholar]
- Dilda, P.J.; Hogg, P.J. Arsenical-based cancer drugs. Cancer Treat. Rev. 2007, 33, 542–564. [Google Scholar] [CrossRef]
- Doyle, D. Notoriety to respectability: A short history of arsenic prior to its present day use in hematology. Br. J. Haematol. 2009, 145, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Meharg, A.A.; Meharg, C. The Pedosphere as a Sink, Source, and Record of Anthropogenic and Natural Arsenic Atmospheric Deposition. Environ. Sci. Technol. 2021, 55, 7757–7769. [Google Scholar] [CrossRef] [PubMed]
- Wai, K.M.; Wu, S.; Li, X.; Jaffe, D.A.; Perry, K.D. Global Atmospheric Transport and Source-Receptor Relationships for Arsenic. Environ. Sci. Technol. 2016, 50, 3714–3720. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Gao, Y.; Wu, S.; Zhang, S.; Smith, K.R.; Yao, X.; Gao, Y. Global impact of atmospheric arsenic on health risk: 2005 to 2015. Proc. Natl. Acad. Sci. USA 2020, 117, 13975–13982. [Google Scholar] [CrossRef]
- Raju, N.J. Arsenic in the geo-environment: A review of sources, geochemical processes, toxicity and removal technologies. Environ. Res. 2021, 203, 111782. [Google Scholar] [CrossRef]
- Atker, K.F.; Owens, G.; Davey, D.E.; Naidu, R. Arsenic Speciation and Toxicity in Biological Systems. Rev. Environ. Contam. Toxicol. 2005, 184, 97–149. [Google Scholar] [CrossRef]
- Nurchi, V.M.; Djordjevic, A.B.; Crisponi, G.; Alexander, J.; Bjørklund, G.; Aaseth, J. Arsenic Toxicity: Molecular Targets and Therapeutic Agents. Biomolecules 2020, 10, 235. [Google Scholar] [CrossRef]
- Cullen, W.; Reimer, K.J. Arsenic speciation in the environment. Chem. Rev. 1989, 89, 713–764. [Google Scholar] [CrossRef]
- Majumder, S.; Banik, P. Geographical variation of arsenic distribution in paddy soil, rice and rice-based products: A meta-analytic approach and implications to human health. J. Environ. Manage. 2019, 233, 184–199. [Google Scholar] [CrossRef] [PubMed]
- International Agency of Researh of Cancer. Some Drinking-Water Disinfectants and Contaminants, Including Arsenic; WHO: Lyon, France, 2004; Volume 84, p. 526. [Google Scholar]
- Garza-Lombó, C.; Pappa, A.; Panayiotidis, M.I.; Gonsebatt, M.E.; Franco, R. Arsenic-induced neurotoxicity: A mechanistic appraisal. J. Biol. Inorg. Chem. 2019, 24, 1305–1316. [Google Scholar] [CrossRef]
- Rahamanab, M.S.; Rahman, M.; Mise, N.; Sikderd, T.; Ichihara, G.; Uddin, K.; Kurasaki, M.; Ichihara, S. Environmental arsenic exposure and its contribution to human diseases, toxicity mechanism and management. Environ. Pollut. 2021, 289, 117940. [Google Scholar] [CrossRef]
- Cohen, J.M.; Beck, B.D.; Rhomberg, L.R. Historical perspective on the role of cell proliferation in carcinogenesis for DNA-reactive and non-DNA-reactive carcinogens: Arsenic as an example. Toxicology 2021, 456, 152783. [Google Scholar] [CrossRef]
- Pullella, K.; Kotsopoulos, J. Arsenic Exposure and Breast Cancer Risk: A Re-Evaluation of the Literature. Nutrients 2020, 12, 3305. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Drinking-Water Quality: Volume 1: Recommendations, 2nd ed.; World Health Organization: Geneva, Switzerland, 1993; p. 189. ISBN 964-7445-88-1. [Google Scholar]
- Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile of Arsenic; U.S. Department of Health and Human Services: Washington, DC, USA, 2007; p. 599. [Google Scholar]
- Zhao, F.J.; McGrath, S.P.; Meharg, A.A. Arsenic as a food chain contaminant: Mechanisms of plant uptake and metabolism and mitigation strategies. Annu. Rev. Plant. Biol. 2010, 61, 535–559. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Zhou, Y.; Zhang, L. The potential of arsenic biomagnification in marine ecosystems: A systematic investigation in Daya Bay in China. Sci. Total Environ. 2021, 773, 145068. [Google Scholar] [CrossRef]
- Rehman, M.U.; Khan, R.; Khan, A.; Qamar, W.; Arafah, A.; Ahmad, A.; Ahmad, A.; Akhter, R.; Rinklebe, J.; Ahmad, P. Fate of arsenic in living systems: Implications for sustainable and safe food chains. J. Hazard. Mater. 2021, 417, 126050. [Google Scholar] [CrossRef]
- Shaji, S.; Santosh, M.; Sarath, K.V.; Prakash, P.; Deepchand, V.; Divya, B.V. Arsenic contamination of groundwater: A global synopsis with focus on the Indian Peninsula. Geosci. Front. 2021, 12, 101079. [Google Scholar] [CrossRef]
- Rahman, M.A.; Hasegawa, H. Arsenic in freshwater systems: Influence of eutrophication on occurrence, distribution, speciation, and bioaccumulation. Appl. Geochem. 2012, 27, 304–314. [Google Scholar] [CrossRef]
- Husain, A.; Kannan, K.; Chan, H.M.; Laird, B.; Al-Amiri, H.; Dashti, B.; Sultan, A.; Al-Othman, A.; Mandekar, B. A Comparative Assessment of Arsenic Risks and the Nutritional Benefits of Fish Consumption in Kuwait: Arsenic Versus Omega 3-Fatty Acids. Arch. Environ. Contam. Toxicol. 2017, 72, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Kato, L.S.; Gomes Ferraria, R.; Meirelles Leiteb, J.V.; Conte-Junior, C.A. Arsenic in shellfish: A systematic review of its dynamics and potential health risk. Mar. Pollut. Bull. 2020, 161, 111693. [Google Scholar] [CrossRef]
- Li, C.; Zhong, H.; Zhang, W. A Scientometric Analysis of Recent Literature on Arsenic Bioaccumulation and Biotransformation in Marine Ecosystem. Bull. Environ. Contam. Toxicol. 2020, 104, 551–558. [Google Scholar] [CrossRef]
- Barrett, P.M.; Hull, E.A.; King, C.E.; Burkart, K.; Ott, K.A.; Ryan, J.N.; Gawel, J.E.; Neumanna, R.B. Increased exposure of plankton to arsenic in contaminated weakly-stratified lakes. Sci. Total Environ. 2018, 625, 1606–1614. [Google Scholar] [CrossRef]
- Mawi, A.M.; Hui, S.; Zhou, L.; Li, H.; Tabassum, J.; Lai, C.; Wang, J.; Shao, G.; Wei, X.; Tang, S.; et al. Inorganic arsenic toxicity and alleviation strategies in rice. J. Hazard. Mater. 2021, 408, 124751. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.M.; Bibi, I.; Niazi, N.K.; Shahid, M.; Iqbal, J.; Shakoor, M.B.; Ahmadf, A.; Shahc, N.S.; Bhattacharya, J.; Mao, K.; et al. Arsenic biogeochemical cycling in paddy soil-rice system: Interaction with various factors, amendments and mineral nutrients. Sci. Total Environ. 2021, 773, 145140. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Hasegawa, H.; Rahman, M.M.; Rahman, M.A.; Miah, M.A.M. Accumulation of arsenic in tissues of rice plant (Oryza sativa L.) and its distribution in fractions of rice grain. Chemosphere 2007, 69, 942–948. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Hasegawa, H.; Rahman, M.M.; Miah, M.A.M.; Tasmin, A. Arsenic accumulation in rice (Oryza sativa L.): Human exposure through food chain. Ecotox. Environ. Saf. 2008, 69, 317–324. [Google Scholar] [CrossRef]
- Biswas, A. A Systematic Review on Arsenic Bio-Availability in Human and Animals: Special Focus on the Rice–Human System. Rev. Environ. Contam. Toxicol. 2019. [Google Scholar] [CrossRef]
- Moulick, M.; Moulick, D.; Samanta, S.; Sarkar, S.; Mukherjee, A.; Pattnaik, B.K.; Saha, S.; Awasthi, P.; Bhowmick, S.; Ghosh, D. Arsenic contamination, impact and mitigation strategies in rice agroenvironment: An inclusive insight. Sci. Total Environ. 2021, 800, 149477. [Google Scholar] [CrossRef]
- Meharg, A.A.; Williams, P.N.; Adomako, E.; Lawgali, Y.Y.; Deacon, D.; Villada, A.; Cambell, R.C.J.; Sun, G.; Zhu, J.-G.; Feldmann, J.; et al. Geographical variation in total and inorganic arsenic content of polished (white) rice. Environ. Sci. Technol. 2009, 43, 1612–1617. [Google Scholar] [CrossRef]
- Karagas, M.R.; Punshon, T.; Davis, M.; Bulka, C.M.; Slaughter, F.; Karalis, D.; Argos, M.; Ahsan, H. Rice intake and emerging concerns on arsenic in rice: A review of the human evidence and methodologic challenges. Curr. Environ. Health Rep. 2019, 6, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-T.; Liang, Z.Z.; Ding, J.; Xue, X.-M.; Li, G.; Fu, X.-L.; Zhu, D. Arsenic bioaccumulation in the soil fauna alters its gut microbiome and microbial arsenic biotransformation capacity. J. Hazard. Mater. 2021, 417, 126018. [Google Scholar] [CrossRef]
- Li, Y.P.; Fekih, I.B.; Fru, E.C.; Moraleda-Muñoz, A.; Li, X.; Rosen, B.P.; Yoshinaga, M.; Rensing, C. Antimicrobial Activity of Metals and Metalloids. Annu. Rev. Microbiol. 2021, 75, 175–197. [Google Scholar] [CrossRef] [PubMed]
- Lyons, T.M.; Reinhard, C.T.; Planavsky, N.J. The rise of oxygen in earth’s early ocean and atmosphere. Nature 2014, 506, 307–314. [Google Scholar] [CrossRef]
- Páez-Espino, D.; Tamames, J.; de Lorenzo, V.; Cánovas, D. Microbial responses to environmental arsenic. BioMetals 2009, 22, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Dhuldhaj, U.P.; Yadav, I.C.; Singh, S.; Sharma, N.K. Microbial interactions in the arsenic cycle: Adoptive strategies and applications in environmental management. Rev. Environ. Contam. Toxicol. 2013, 224, 1–38. [Google Scholar] [CrossRef]
- Mazumder, P.; Sharma, S.K.; Taki, K.; Kalamdhad, A.S.; Kumar, M. Microbes involved in arsenic mobilization and respiration: A review on isolation, identification, isolates and implications. Environ. Geochem. Health 2020, 42, 3443–3469. [Google Scholar] [CrossRef]
- Shi, K.; Wang, Q.; Wang, G. Microbial Oxidation of Arsenite: Regulation, Chemotaxis, Phosphate Metabolism and Energy Generation. Front. Microbiol. 2020, 11, 569282. [Google Scholar] [CrossRef]
- Wang, Q.; Han, Y.; Shi, K.; Fan, X.; Wang, L.; Li, M.; Wang, G. An Oxidoreductase AioE is Responsible for Bacterial Arsenite Oxidation and Resistance. Sci. Rep. 2017, 7, 41536. [Google Scholar] [CrossRef]
- Huber, R.; Sacher, M.; Vollmann, A.; Huber, H.; Rose, D. Respiration of arsenate and selenate by hyperthermophilic archaea. Syst. Appl. Microbiol. 2000, 23, 305–314. [Google Scholar] [CrossRef]
- Anderson, G.L.; Williams, J.; Hille, R. The purification and characterization of arsenite oxidase from Alcaligenes faecalis, a molybdenum-containing hydroxylase. J. Biol. Chem. 1992, 267, 23674–23682. [Google Scholar] [CrossRef]
- Silver, S.; Phung, L.T. Genes and Enzymes Involved in Bacterial Oxidation and Reduction of Inorganic Arsenic. Appl. Environ. Microbiol. 2005, 71, 599–608. [Google Scholar] [CrossRef]
- Zhu, Y.-G.; Yoshinaga, M.; Zhao, F.-J.; Rosen, B.P. Earth Abides Arsenic Biotransformations. Annu. Rev. Earth Planet. Sci. 2014, 42, 443–467. [Google Scholar] [CrossRef]
- Fekih, I.B.; Zhang, C.; Li, Y.P.; Zhao, Y.; Alwathnani, H.A.; Saquib, Q.; Rensing, C.; Cervantes, C. Distribution of Arsenic Resistance Genes in Prokaryotes. Front. Microbiol. 2018, 9, 2473. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, R.; Rosen, B.P. Arsenate Reductases in Prokaryotes and Eukaryotes. Environ. Heal. Perspect. 2002, 110, 745–748. [Google Scholar] [CrossRef]
- Yüksel, B.; Şen, N.; Türksoy, V.A.; Tutkun, E.; Söylemezoğlu, T. Effect of exposure time and smoking habit on arsenic levels in biological samples of metal workers in comparison with controls. Marmara Pharm. J. 2018, 22, 218–226. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Ghosh, U.C. Environmental, economic and health perspectives of arsenic toxicity in Bengal Delta. World Sci. News 2015, 4, 111–139. [Google Scholar]
- Tseng, C.H. A review on environmental factors regulating arsenic methylation in humans. Toxicol. Appl. Phamacol. 2009, 235, 338–350. [Google Scholar] [CrossRef]
- Cullen, W.R. The toxicity of trimethylarsine: An urban myth. J. Environ. Monit. 2005, 7, 11–15. [Google Scholar] [CrossRef]
- Nadar, S.V.; Chen, J.; Dheeman, D.S.; Galván, A.E.; Sakurai, K.Y.; Kandavelu, P.; Sankaran, P.; Kuramata, M.; Ishikawa, S.; Rosen, B.P.; et al. Arsinothricin, an arsenic-containing non-proteinogenic amino acid analog of glutamate, is a broad-spectrum antibiotic. Commun. Biol. 2019, 2, 131. [Google Scholar] [CrossRef] [PubMed]
- Bentley, R.; Chasteen, T.G. Microbial Methylation of Metalloids: Arsenic, Antimony, and Bismuth. Microbiol. Mol. Biol. Revs. 2002, 66, 250–271. [Google Scholar] [CrossRef]
- Qin, J.; Rosen, B.P.; Zhang, Y.; Wang, G.; Franke, S.; Rensing, C. Arsenic detoxification and evolution of trimethylarsine gas by a microbial arsenite S-adenosylmethionine methyltransferase. Proc. Natl. Acad. Sci. USA 2006, 103, 2075–2080. [Google Scholar] [CrossRef] [PubMed]
- Ajees, A.A.; Rosen, B.P. As(III) S-adenosylmethionine methyltransferases and other arsenic binding proteins. Geomicrobiol. J. 2015, 32, 570–576. [Google Scholar] [CrossRef]
- Yan, G.; Chen, X.; Du, S.; Deng, Z.; Wang, L.; Chen, S. Genetic mechanisms of arsenic detoxification and metabolism in bacteria. Curr. Genet. 2019, 65, 329–338. [Google Scholar] [CrossRef]
- Chen, J.; Rosen, B.P. The Arsenic Methylation Cycle: How Microbial Communities Adapted Methylarsenicals for Use as Weapons in the Continuing War for Dominance. Front. Environ. Sci. 2020, 8, 43. [Google Scholar] [CrossRef]
- Lomax, C.; Liu, W.-J.; Wu, L.; Xue, K.; Xiong, J.; Zhou, J.; McGrath, S.P.; Meharg, M.A.; Miller, A.J.; Zhao, F.J. Methylated arsenic species in plants originate from soil microorganisms. New Phytol. 2012, 193, 665–672. [Google Scholar] [CrossRef]
- Viacava, K.; Leberballe Meibon, K.; Ortega, D.; Dyer, S.; Gelb, A.; Falquet, L.; Minton, N.P.; Merstrot, A.; Bernier-Latmani, R. Variability in Arsenic Methylation Efficiency across Aerobic and Anaerobic Microorganisms. Environ. Sci. Technol. 2020, 54, 14343–14351. [Google Scholar] [CrossRef] [PubMed]
- Rosen, B.P.; Tamás, M.J. Arsenic transport in prokaryotes and eukaryotic microbes. Adv. Exp. Med. Biol. 2010, 679, 47–55. [Google Scholar] [CrossRef]
- Mukhopadhyay, R.; Bhattacharjee, H.; Rosen, B.P. Aquaglyceroporins: Generalized metalloid channels. Biochim. Biophys. Acta 2014, 1840, 1583–1591. [Google Scholar] [CrossRef]
- Maciaszczyk-Dziubinska, E.; Wawrzycka, D.; Wysocki, R. Arsenic and antimony transporters in eukaryotes. Int. J. Mol. Sci. 2012, 13, 3527–3548. [Google Scholar] [CrossRef]
- Garbinski, L.D.; Rosen, B.P.; Chen, J. Pathways of arsenic uptake and efflux. Environ. Int. 2019, 126, 585–597. [Google Scholar] [CrossRef]
- Zhang, S.-Y.; Sun, G.-X.; Yin, X.-X.; Rensing, C.; Zhu, Y.-G. Biomethylation and volatilization of arsenic by the marine microalgae Ostreococcus tauri. Chemosphere 2015, 63, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ding, T.; Zhang, C. Biosorption and toxicity responses to arsenite (As[III]) in Scenedesmus quadricauda. Chemosphere 2013, 92, 1077–1084. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Xu, P.; Liu, C.; Liu, M.; Wang, C.; Wang, Y.; Zhang, C.; Ge, Y. Review of arsenic speciation, toxicity and metabolism in microalgae. Rev. Environ. Sci. Biotechnol. 2015, 14, 427–451. [Google Scholar] [CrossRef]
- Papry, R.I.; Fujisawaa, S.; Yinghana, Z.; Akhyara, O.; Mamuna, A.M.A.; Mashiod, A.S.; Hasegawa, H. Integrated effects of important environmental factors on arsenic biotransformation and photosynthetic efficiency by marine microalgae. Ecotox. Environ. Saf. 2020, 201, 110797. [Google Scholar] [CrossRef]
- Wu, L.; Yi, H.; Zhang, H. Reactive oxygen species and Ca2+ are involved in sodium arsenite-induced cell killing in yeast cells. FEMS Microbiol. Lett. 2013, 343, 57–63. [Google Scholar] [CrossRef]
- Du, L.; Yu, Y.; Chen, J.; Liu, Y.; Xia, Y.; Chen, Q.; Liu, X. Arsenic induces caspase- and mitochondria-mediated apoptosis in Saccharomyces cerevisiae. FEMS Yeast Res. 2007, 7, 860–865. [Google Scholar] [CrossRef]
- Ralph, S.J. Arsenic-Based Antineoplastic Drugs and Their Mechanisms of Action. Met. Based Drugs 2008, 1–13. [Google Scholar] [CrossRef]
- Thorsen, M.; Perrone, G.G.; Kristiansson, E.; Traini, M.; Ye, T.; Dawes, I.W.; Nerman, O.; Tamás, M.J. Genetic basis of arsenite and cadmium tolerance in Saccharomyces cerevisiae. BMC Genom. 2009, 10, 105. [Google Scholar] [CrossRef]
- Techo, T.; Charoenpuntaweesin, S.; Auesukareea, C. Involvement of the Cell Wall Integrity Pathway of Saccharomyces cerevisiae in Protection against Cadmium and Arsenate Stresses. Appl. Environ. Microbiol. 2020, 86, e01339-20. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.J.; Veljanoskia, F.; O’Dohertya, P.J.; Zamana, M.S.; Petersinghama, G.; Baileya, T.D.; Münch, G.; Kersaitis, G.C.; Wu, M.J. Molecular insight into arsenic toxicity via the genome-wide deletion mutant screening of Saccharomyces cerevisiae. Metallomics 2016, 8, 228–235. [Google Scholar] [CrossRef]
- Hosiner, D.; Lempiäinen, H.; Reiter, W.; Urban, J.; Loewith, R.; Ammerer, G.; Schweyen, R.; Shore, D.; Schüller, C. Arsenic toxicity to Saccharomyces cerevisiae is a consequence of inhibition of the TORC1 kinase combined with a chronic stress response. Mol. Biol. Cell. 2009, 20, 1048–1057. [Google Scholar] [CrossRef][Green Version]
- Batista-Nascimento, L.; Toledano, M.B.; Thiele, D.J.; Rodrigues-Pousada, C. Yeast protective response to arsenate involves the repression of the high affinity iron uptake system. Biochim. Biophys. Acta 2013, 1833, 997–1005. [Google Scholar] [CrossRef]
- Panagaki, D.; Crofta, J.T.; Keuenhof, K.; Berglund, L.L.; Andersson, S.; Kohlerb, V.; Büttnerb, S.; Tamás, M.J.; Nyströmc, T.; Neutzea, R.; et al. Nuclear envelope budding is a response to cellular stress. Proc. Natl. Acad. Sci. USA 2021, 118, e2020997118. [Google Scholar] [CrossRef]
- Karadjova, I.B.; Slaveykova, V.I.; Tsalevb, D.L. The biouptake and toxicity of arsenic species on the green microalga Chlorella salina in seawater. Aquat. Toxicol. 2008, 87, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Levy, J.L.; Stauber, J.L.; Adams, M.S.; Maher, W.A.; Kirby, J.K.; Jolley, D.F. Toxicity, biotransformation, and mode of action of arsenic in two freshwater microalgae (Chlorella sp. and Monoraphidium arcuatum). Environ. Toxicol. Chem. 2005, 24, 2630–2639. [Google Scholar] [CrossRef]
- Díaz, S.; De Francisco, P.; Olsson, S.; Aguilera, A.; González-Toril, E.; Martín-González, A. Toxicity, Physiological, and Ultrastructural Effects ofArsenic and Cadmium on the Extremophilic Microalga Chlamydomonas acidophila. Int. J. Environ. Res. Public Health 2020, 17, 1650. [Google Scholar] [CrossRef] [PubMed]
- Szivák, I.; Behra, R.; Sigg, L. Metal-induced Reactive Oxygen Species production in Chlamydomonas reinhardtii. J. Phycol. 2009, 45, 427–435. [Google Scholar] [CrossRef]
- Arora, N.; Gulati, K.; Patel, A.; Pruthi, P.A.; Poluri, K.M.; Pruthi, V. A hybrid approach integrating arsenic detoxification with biodiesel production using oleaginous microalgae. Algal Res. 2017, 24, 29–39. [Google Scholar] [CrossRef]
- Arora, A.; Dubey, D.; Sharma, M.; Patel, A.; Guleria, A.; Pruthi, P.A.; Kumar, D.; Pruthi, V.; Poluri, K.M. NMR-Based Metabolomic Approach To Elucidate the Differential Cellular Responses during Mitigation of Arsenic(III, V) in a Green Microalga. ACS Omega 2018, 3, 11847–11856. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.; Poluri, K.M. Heavy metal detoxification mechanisms by microalgae: Insights from transcriptomics analysis. Environ. Pollut. 2021, 285, 117443. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, H.P.; Hana, B.; Yua, X. Coupling of abiotic stresses and phytohormones for the production of lipids and high-value by-products by microalgae: A review. Biores. Tech. 2019, 274, 549–566. [Google Scholar] [CrossRef] [PubMed]
- Kruger, M.C.; Bertin, P.N.; Heipieper, H.J.; Arsène-Ploetze, F. Bacterial metabolism of environmental arsenic--mechanisms and biotechnological applications. Appl. Microbiol. Biotechnol. 2013, 97, 3827–3841. [Google Scholar] [CrossRef]
- Tsai, S.-L.; Singh, S.; Chen, W. Arsenic metabolism by microbes in nature and the impact on arsenic remediation. Curr. Opin. Biotechnol. 2009, 20, 659–667. [Google Scholar] [CrossRef]
- Maciaszczyk-Dziubinska, E.; Migocka, M.; Wysocki, R. Acr3p is a plasma membrane antiporter that catalyzes As(III)/H(+) and Sb(III)/H(+) exchange in Saccharomyces cerevisiae. Biochim. Biophys. Acta 2011, 1808, 1855–1859. [Google Scholar] [CrossRef]
- Wysocki, R.; Chéry, C.C.; Wawrzycka, D.; Van Hulle, M.; Cornelis, R.; Thevelein, J.M.; Tamás, M.J. The glycerol channel Fps1p mediates the uptake of arsenite and antimonite in Saccharomyces cerevisiae. Mol. Microbiol. 2001, 40, 1391–1401. [Google Scholar] [CrossRef]
- Ghosh, M.; Shen, J.; Rosen, B.P. Pathways of As(III) detoxification in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 1999, 96, 5001–5006. [Google Scholar] [CrossRef]
- Wysocki, R.; Tamás, M.J. Saccharomyces cerevisiae as a Model Organism for Elucidating Arsenic Tolerance Mechanisms. In Cellular Effects of Heavy Metals; Bánfalvi, G., Ed.; Springer: Berlín, Germany, 2011; pp. 87–112. [Google Scholar] [CrossRef]
- Wawrzycka, D.; Markowska, K.; Maciaszczyk-Dziubinska, E.; Migocka, M.; Wysocki, R. Transmembrane topology of the arsenite permease Acr3 from Saccharomyces cerevisiae. Biochim. Biophys. Acta Biomembr. 2017, 1859, 117–125. [Google Scholar] [CrossRef]
- Maciaszczyk-Dziubinska, E.; Wawrzycka, D.; Sloma, E.; Migocka, M.; Wysocki, R. The yeast permease Acr3p is a dual arsenite and antimonite plasma membrane transporter. Biochim. Biophys. Acta 2010, 1798, 2170–2175. [Google Scholar] [CrossRef] [PubMed]
- Paumi, C.M.; Chuk, M.; Snider, J.; Stagljar, I.; Michaelis, S. ABC transporters in Saccharomyces cerevisiae and their interactors: New technology advances the biology of the ABCC (MRP) subfamily. Microbiol. Mol. Biol. Rev. 2009, 73, 577–593. [Google Scholar] [CrossRef]
- Mukhopadhyay, R.; Shi, J.; Rosen, B.P. Purification and characterization of ACR2p, the Saccharomyces cerevisiae arsenate reductase. J. Biol. Chem. 2000, 275, 21149–21157. [Google Scholar] [CrossRef]
- Li, L.; Zeng, X.; Williams, P.N.; Gao, X.; Zhang, L.; Zhang, J.; Shan, H.; Su, S. Arsenic resistance in fungi conferred by extracellular bonding and vacuole-septa compartmentalization. J. Hazard. Mater. 2021, 401, 123370. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, F.; Liu, Y.; Huang, F.; Zhang, C. Effect of extracellular polymeric substances on arsenic accumulation in Chlorella pyrenoidosa. Sci. Total Environ. 2021, 704, 135368. [Google Scholar] [CrossRef] [PubMed]
- Danouche, M.; El Ghachtouli, N.; El Arroussi, H. Phycoremediation mechanisms of heavy metals using living Green microalgae: Physicochemical and molecular approaches for enhancing selectivity and removal capacity. Helyon 2021, 7, e07609. [Google Scholar] [CrossRef]
- Duncan, E.G.; Maher, W.A.; Foster, S.D. Contribution of arsenic species in unicellular algae to the cycling of arsenic in marine ecosystems. Environ. Sci. Technol. 2015, 49, 33–50. [Google Scholar] [CrossRef]
- Kumari, N.; Jagadevan, S. Genetic identification of arsenate reductase and arsenite oxidase in redox transformations carried out by arsenic metabolising prokaryotes—A comprehensive review. Chemosphere 2016, 163, 400–412. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, C.; Zheng, Y.; Ge, Y. Bioaccumulation kinetics of arsenite and arsenate in Dunaliella salina under different phosphate regimes. Environ. Sci. Pollut. Res. 2017, 24, 21213–21221. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, C.; Yu, X.; Ge, Y. Arsenite Oxidation by Dunaliella salina is Affected by External Phosphate Concentration. Bull. Environ. Cont. Toxicol. 2020, 105, 868–873. [Google Scholar] [CrossRef]
- Qin, J.; Lehr, C.R.; Yuan, C.; Le, X.C.; McDermott, T.R.; Rosen, B.P. Biotransformation of arsenic by a Yellowstone thermoacidophilic eukaryotic alga. Proc. Natl. Acad. Sci. USA 2009, 106, 5213–5217. [Google Scholar] [CrossRef]
- Wang, N.X.; Huang, B.; Xu, S.; Wei, Z.-B.; Miao, A.J.; Ji, R.; Yang, L.Y. Effects of nitrogen and phosphorus on arsenite accumulation, oxidation, and toxicity in Chlamydomonas reinhardtii. Aquat. Toxicol. 2014, 157, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Wang, L.; Duan, G.; Sun, G. Characterization of arsenate transformation and identification of arsenate reductase in a green alga Chlamydomonas reinhardtii. J. Environ. Sci. 2011, 23, 1186–1193. [Google Scholar] [CrossRef]
- Bahar, M.M.; Megharaj, M.; Naidu, N. Influence of phosphate on toxicity and bioaccumulation of arsenic in a soil isolate of microalga Chlorella sp. Environ. Sci. Pollut. Res. Int. 2016, 23, 2663–2668. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.C.; Amaro, F.; Díaz, S.; de Francisco, P.; Cubas, L.L.; Martín-González, A. Ciliate metallothioneins: Unique microbial eukaryotic heavy-metal-binder molecules. J. Biol. Inorg. Chem. 2011, 16, 1025–1034. [Google Scholar] [CrossRef]
- Balzano, S.; Sardo, A.; Blasio, M.; Bou Chahine, T.; Dell’Anno, F.; Sansone, C.; Brunet, C. Microalgal Metallothioneins and Phytochelatins and Their Potential Use in Bioremediation. Front. Microbiol. 2020, 11, 517. [Google Scholar] [CrossRef]
- Qi, Q.; Wang, Q.; Wang, H.; Tan, M. Metallothionein Attenuated Arsenic-Induced Cytotoxicity: The Underlying Mechanism Reflected by Metabolomics and Lipidomics. J. Agric. Food Chem. 2021, 69, 5372–5380. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.T.; De Ley, M. Arsenic Induction of Metallothionein and Metallothionein Induction Against Arsenic Cytotoxicity. Rev. Environ. Cont. Toxicol. 2017, 240, 151–168. [Google Scholar] [CrossRef]
- Pawlik-Skowrońska, B.; Pirszel, J.; Kalinowska, R.; Skowroński, T. Arsenic availability, toxicity and direct role of GSH and phytochelatins in As detoxification in the green alga Stichococcus bacillaris. Aquat. Toxicol. 2004, 70, 201–212. [Google Scholar] [CrossRef]
- Morelli, E.; Mascherpa, M.C.; Scarano, G. Biosynthesis of phytochelatins and arsenic accumulation in the marine microalga Phaeodactylum tricornutum in response to arsenate exposure. Biometals 2005, 18, 587–593. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, C.; Zhenga, Y.; Gea, Y. Phytochelatin synthesis in Dunaliella salina induced by arsenite and arsenate under various phosphate regimes. Ecotox. Environ. Saf. 2017, 136, 150–160. [Google Scholar] [CrossRef]
- De Francisco, P.; Melgar, L.M.; Díaz, S.; Martín-González, A.; Gutiérrez, J.C. The Tetrahymena metallothionein gene family: Twenty-one new cDNAs, molecular characterization, phylogenetic study and comparative analysis of the gene expression under different abiotic stressors. BMC Genom. 2016, 17, 346. [Google Scholar] [CrossRef] [PubMed]
- Amaro, F.; Turkewitz, A.P.; Martín-González, A.; Gutiérrez, J.C. Whole-cell biosensors for detection of heavy metal ions in environmental samples based on metallothionein promoters from Tetrahymena thermophila. Microb. Biotechnol. 2011, 4, 513–522. [Google Scholar] [CrossRef]
- Pratush, A.; Kumar, A.; Hu, Z. Adverse effect of heavy metals (As, Pb, Hg, and Cr) on health and their bioremediation strategies: A review. Int. Microbiol. 2018, 21, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Sher, S.; Rehman, A. Use of heavy metals resistant bacteria—a strategy for arsenic bioremediation. Appl. Microbiol. Biotech. 2019, 103, 6007–6021. [Google Scholar] [CrossRef] [PubMed]
- Rahman, Z.; Singh, V.P. Bioremediation of toxic heavy metals (THMs) contaminated sites: Concepts, applications and challenges. Environ. Sci. Pollut. Res. 2020, 27, 27563–27581. [Google Scholar] [CrossRef]
- Irshad, S.; Xie1, Z.; Mehmood, S.; Nawaz, A.; Ditta, A.; Mahmood, Q. Insights into conventional and recent technologies for arsenic bioremediation: A systematic review. Environ. Sci. Pollut. Res. 2021, 28, 18870–18892. [Google Scholar] [CrossRef]
- Leong, L.K.; Chang, J.S. Bioremediation of heavy metals using microalgae: Recent advances and mechanisms. Biores. Tech. 2021, 303, 122886. [Google Scholar] [CrossRef] [PubMed]
- Altowayti, H.A.H.; Almoalemi, A.; Shahir, S.; Othman, N. Comparison of culture-independent and dependent approaches for identification of native arsenic-resistant bacteria and their potential use for arsenic bioremediation. Ecotox. Environ. Saf. 2020, 205, 111267. [Google Scholar] [CrossRef]
- Gustave, W.; Yuan, Z.; Liu, F.; Chen, Z. Mechanisms and challenges of microbial fuel cells for soil heavy metal (loid)s remediation. Sci. Total Environ. 2021, 756, 143865. [Google Scholar] [CrossRef]
- Giachino, A.; Focarelli, F.; Wright, J.-M.; Waldron, K.J. Synthetic biology approaches to copper remediation: Bioleaching, accumulation and recycling. FEMS Microb. Ecol. 2021, 97, fiaa249. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Francisco, P.; Martín-González, A.; Rodriguez-Martín, D.; Díaz, S. Interactions with Arsenic: Mechanisms of Toxicity and Cellular Resistance in Eukaryotic Microorganisms. Int. J. Environ. Res. Public Health 2021, 18, 12226. https://doi.org/10.3390/ijerph182212226
De Francisco P, Martín-González A, Rodriguez-Martín D, Díaz S. Interactions with Arsenic: Mechanisms of Toxicity and Cellular Resistance in Eukaryotic Microorganisms. International Journal of Environmental Research and Public Health. 2021; 18(22):12226. https://doi.org/10.3390/ijerph182212226
Chicago/Turabian StyleDe Francisco, Patricia, Ana Martín-González, Daniel Rodriguez-Martín, and Silvia Díaz. 2021. "Interactions with Arsenic: Mechanisms of Toxicity and Cellular Resistance in Eukaryotic Microorganisms" International Journal of Environmental Research and Public Health 18, no. 22: 12226. https://doi.org/10.3390/ijerph182212226
APA StyleDe Francisco, P., Martín-González, A., Rodriguez-Martín, D., & Díaz, S. (2021). Interactions with Arsenic: Mechanisms of Toxicity and Cellular Resistance in Eukaryotic Microorganisms. International Journal of Environmental Research and Public Health, 18(22), 12226. https://doi.org/10.3390/ijerph182212226





