Sublethal Effects of Arsenic on Oxygen Consumption, Hematological and Gill Histopathological Indices in Chanos chanos
Abstract
1. Introduction
2. Materials and Methods
2.1. Determination of LC50 Values
2.2. Estimation of Oxygen Consumption
2.3. Preparation of Blood Smear
2.4. Estimation of Hemoglobin and Red Blood Corpuscular (RBC) Count
2.5. Histology
2.6. Scanning Electron Microscope (SEM) Studies of Gill
2.7. Statistical Analysis
3. Results
3.1. LC50 Values of Arsenic
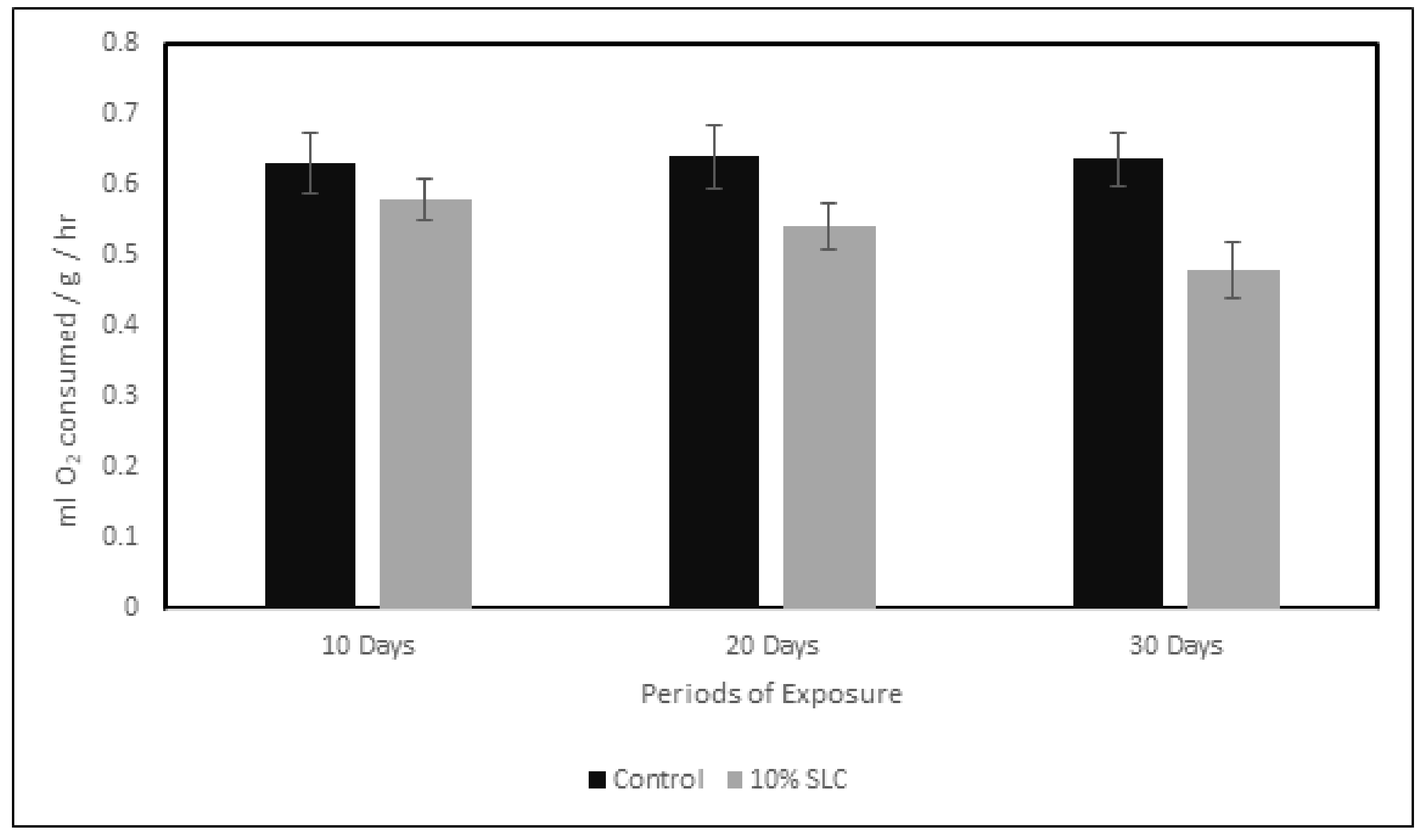
3.2. Rate of Oxygen Consumption
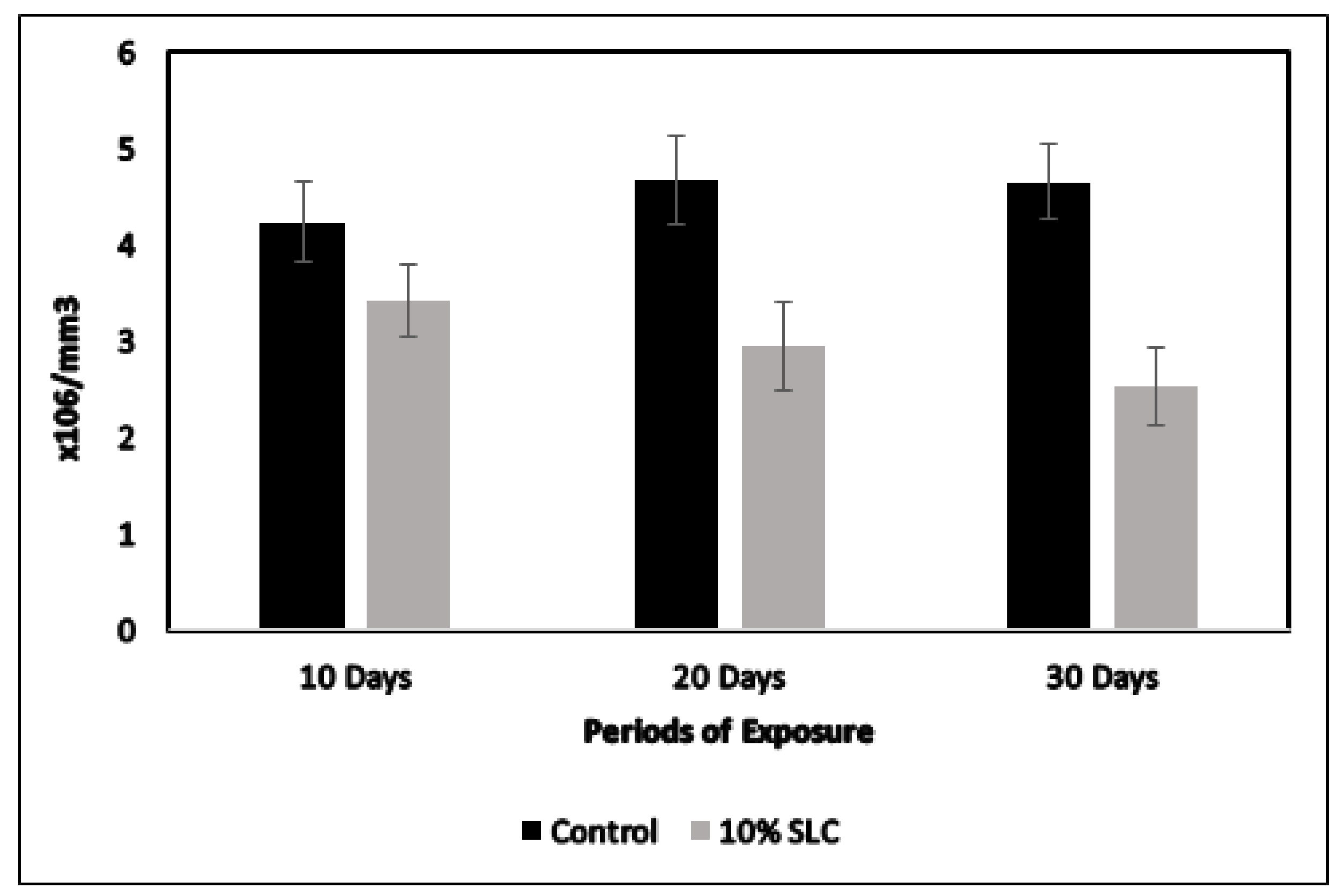
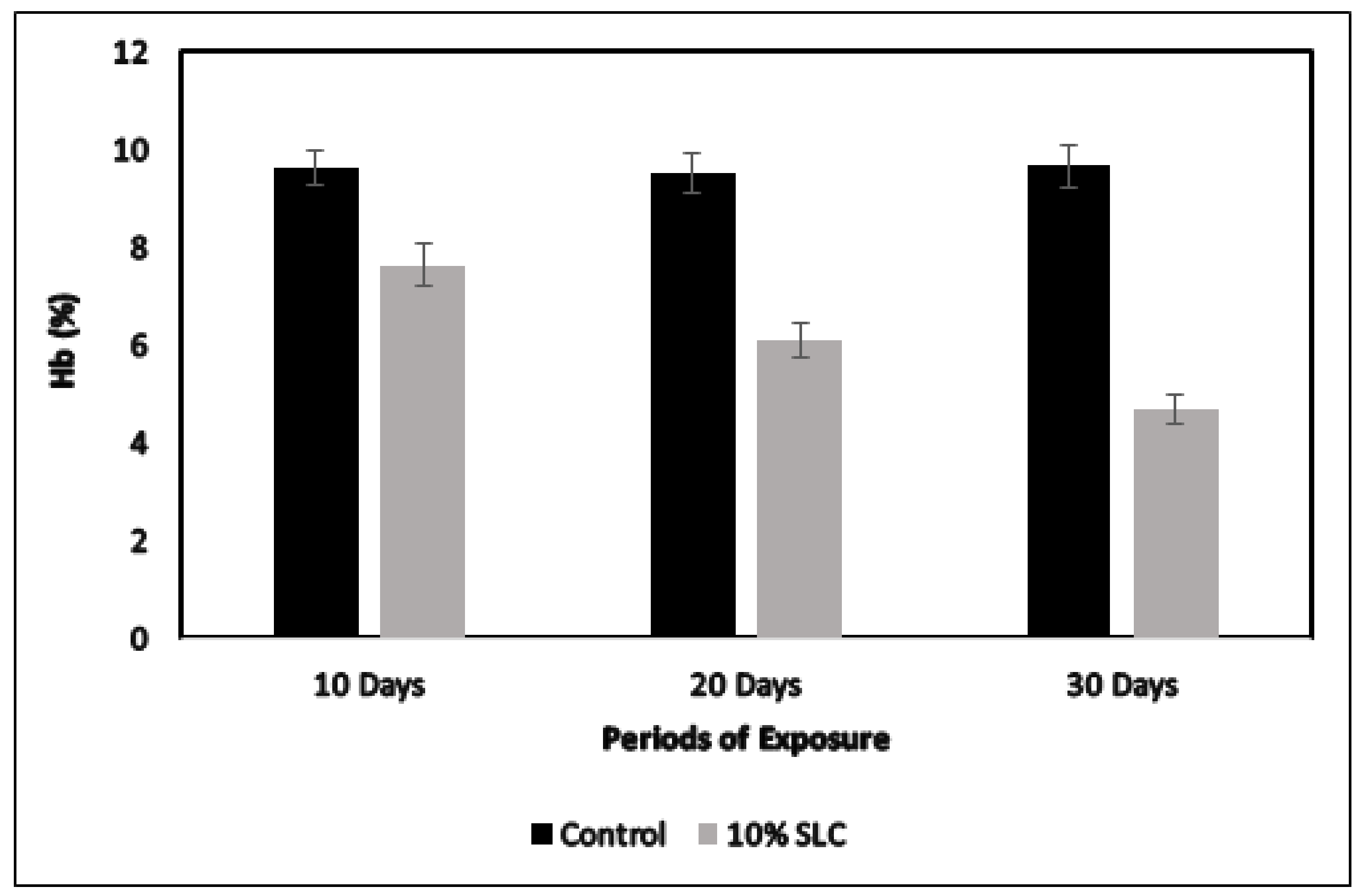
3.3. Changes in the RBC and Hb Content of Chanos Chanos to Sublethal Concentration of Arsenic
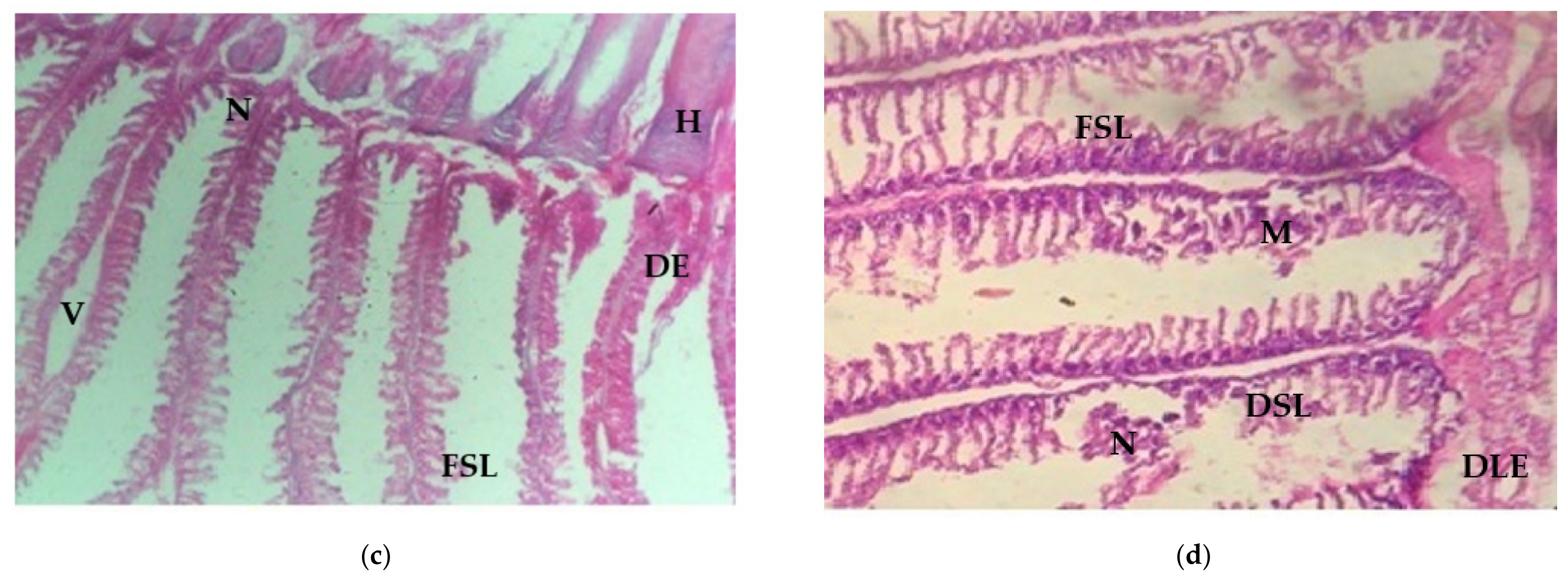
3.4. Histological Study
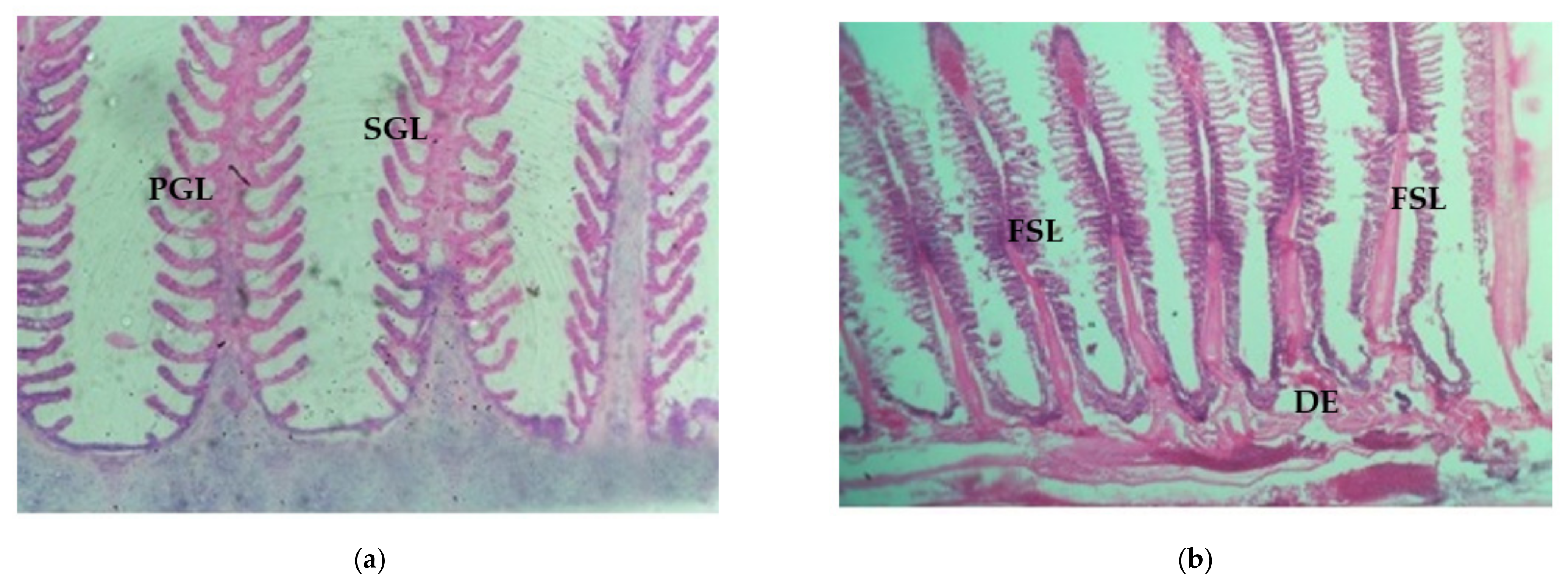
3.4.1. Control Gill, Light and SEM Microscopic Observation
3.4.2. Histological Changes of Gill Tissue Induced by Arsenic under Light Microscopic Observation
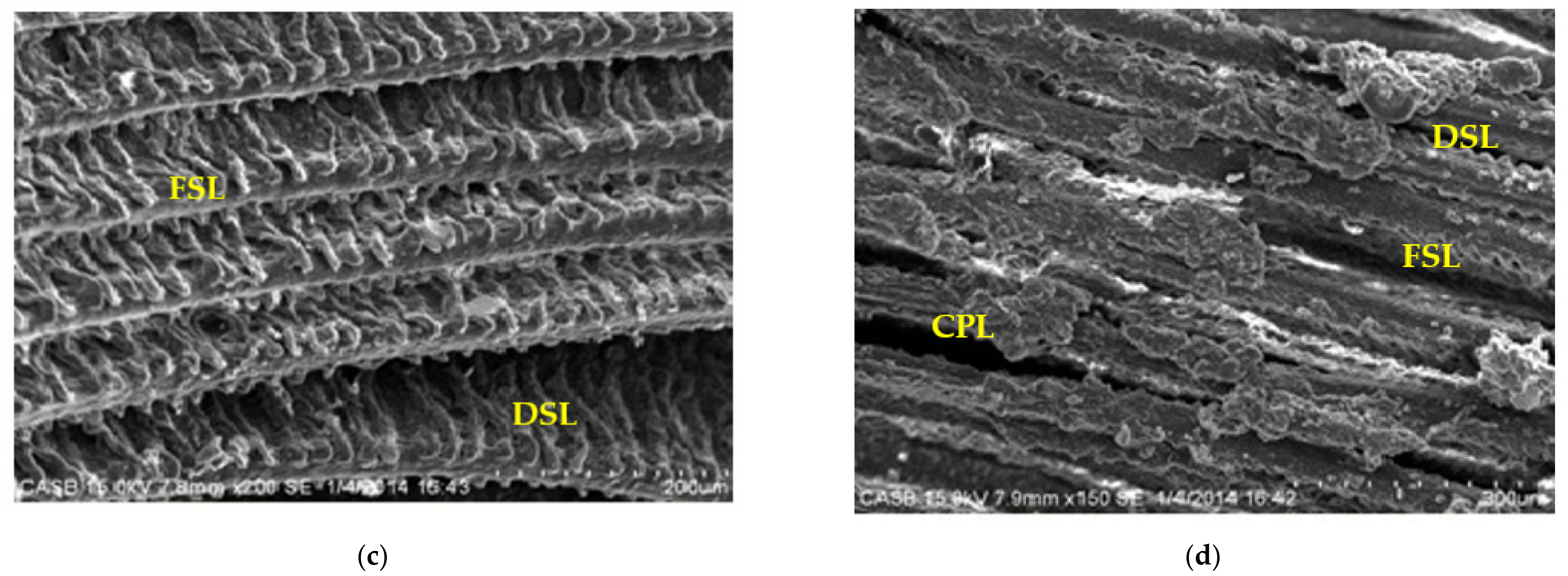
3.4.3. Histological Alterations of Gill in Arsenic-Treated Fish under SEM Observation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Muley, D.V.; Karanjkar, D.M.; Maske, S.V. Impact of industrial effluents on the biochemical composition of fresh water fish Labeo rohita. J. Environ. Biol. 2007, 28, 245–249. [Google Scholar]
- Grande, M. Use of fish for toxicity studies fauna (Blindern) (Nor.), 31:73. Aquat. Sci. Fish. Abs 1978, 8, 10869. [Google Scholar]
- Hale, J.G. Toxicity of metal mining wastes. Bull. Environ. Contam. Toxicol. 1977, 17, 66–73. [Google Scholar] [CrossRef]
- Sprague, J.B. The ABC’s of pollutant bioassay using fish. In Biological Methods for the Assessment of Water Quality; ASTM International: West Conshohocken, PA, USA, 1973. [Google Scholar]
- Sprague, J.B. This Week’s Citation Classic 1971; Fisheries Research Board of Canada: St. Andrews, NB, Canada, 1979. [Google Scholar]
- Gupta, D.K.; Tiwari, S.; Razafindrabe, B.H.N.; Chatterjee, S. Arsenic Contamination from Historical Aspects to the Present. In Arsenic Contamination in the Environment; Springer: Singapore, 2017; pp. 1–12. [Google Scholar]
- Han, J.-M.; Park, H.-J.; Kim, J.-H.; Jeong, D.-S.; Kang, J.-C. Toxic effects of arsenic on growth, hematological parameters, and plasma components of starry flounder, Platichthys stellatus, at two water temperature conditions. Fish. Aquat. Sci. 2019, 22, 3. [Google Scholar] [CrossRef]
- Saxena, A.; Rao, D.S.; Khan, Z. Studies on the acute toxicities of copper, mercury, and cadmium to Danio malabaricus and Puntius ticto. J. Environ. Sci. Health Part A Environ. Sci. Eng. 1982, 17, 657–665. [Google Scholar] [CrossRef]
- Ventura-Lima, J.; de Castro, M.; Acosta, D.; Fattorini, D.; Regoli, F.; de Carvalho, L.M.; Bohrer, D.; Geracitano, L.A.; Barros, D.M.; Marins, L.; et al. Effects of arsenic (As) exposure on the antioxidant status of gills of the zebrafish Danio rerio (Cyprinidae). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2009, 149, 538–543. [Google Scholar] [CrossRef]
- Magesh, S.; Kumaraguru, A.K. Acute Toxicity of Endosulfan to the Milkfish, Chanos chanos, of the Southeast Coast of India. Bull. Environ. Contam. Toxicol. 2006, 76, 622–628. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Chou, H.-N. Ichthyotoxicity studies of milkfish Chanos chanos fingerlings exposed to a harmful dinoflagellate Alexandrium minutum. J. Exp. Mar. Biol. Ecol. 2001, 262, 211–219. [Google Scholar] [CrossRef]
- Cruz, E.R.; Tamse, C.T. Acute toxicity of potassium permanganate to milkfish fingerlings, Chanos chanos. Bull. Environ. Contam. Toxicol. 1989, 43, 785–788. [Google Scholar] [CrossRef]
- Dinesan, K.C. Histopathological Studies on Zinc Toxicity in Milkfish Chanos chanos Forsskal. Ph.D. Thesis, Central Marine Fisheries Research Institute, Kochi, India, 1988. [Google Scholar]
- Jumalon, N.A. Acute toxicity of unionized ammonia to milkfish (Chanos chanos Forsskal) fry. SEAFDEC Aquac. Dep. Q. Res. Rep. 1979, 3, 10–14. [Google Scholar]
- Hidayati, D.; Soegianto, A. Toxicity of Cd and Cu to milk fish (Chanos chanos): Considerations of osmoregulation and histological changes in gills. Pollut. Res 2020, 39, 1116–1121. [Google Scholar]
- Pei, J.; Zuo, J.; Wang, X.; Yin, J.; Liu, L.; Fan, W. The Bioaccumulation and Tissue Distribution of Arsenic Species in Tilapia. Int. J. Environ. Res. Public Health 2019, 16, 757. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Banerjee, T.K. Analysis of Arsenic Bioaccumulation in Different Organs of the Nutritionally Important Catfish, Clarias batrachus (L.) Exposed to the Trivalent Arsenic Salt, Sodium Arsenite. Bull. Environ. Contam. Toxicol. 2012, 89, 445–449. [Google Scholar] [CrossRef]
- Sohn, I.D.; Henry, I.B. Todd-Sunford Clnica Diagnosis by Laboratory Methods, 14th ed.; W.B. Saunders Company: Philadelphia, PA, USA; London, UK; Toronta, BC, Canada, 1969; pp. 139–143. [Google Scholar]
- Sahil, T. Text Book of Clinical Pathology; Ward, S.C., Williams, E., Eds.; Williams and Co.: Baltimore, MD, USA, 1962. [Google Scholar]
- Gurr, E. Methods of Analytical Histology and Histo-Chemistry; Leonard Hill: Westwood, CA, USA, 1958. [Google Scholar]
- Roy, P.K.; Munshi, J.D. Oxygen consumption and ventilation rate of a freshwater major carp, Cirrhinus mrigala (Ham.) in fresh and malathion treated waters. J. Environ. Biol. 1988, 9, 5–13. [Google Scholar]
- Sarkar, S.K. Effects of two heavy metals (Copper sulphate and Cadmium sulphate) on the oxygen consumption of the fish Cyprinus carpio (Linn.). Uttar Pradesh J. Zool. 1969, 19, 13–16. [Google Scholar]
- Adeyemo, O.K. Haematological Profile of Clarias gariepinus (Burchell, 1822) Exposed to Lead. Turk. J. Fish. Aquat. Sci. 2007, 7, 163–169. [Google Scholar]
- Muthukumaravel, K.; Vasanthi, N.; Stalin, A.; Alam, L.; Santhanabharathi, B.; Musthafa, M.S. Sublethal effects of phenol on histology of selected organs of freshwater fish Mystus vittatus. Environ. Sci. Pollut. Res. 2021, 28, 13752–13760. [Google Scholar] [CrossRef]
- Singh, S.R. Changes in O2 consumption of a siluroid fish (Mystus vittatus) put to different concentrations of some heavy metal salts. Indian J. Exp. Biol. 1979, 5, 622–624. [Google Scholar]
- Jezierska, B.; Sarnowski, P. The effect of mercury, copper and cadmium during single and combined exposure on oxygen consumption of Oncorhynchus mykiss Wal. and Cyprinus carpio L. larvae. Arch. Polish Fish. Rybactwa Pol. 2002, 10, 15–22. [Google Scholar]
- Mitrasinovic-Brulic, M.; Suljević, D. Impacts of copper sulfate on hematological parameters of rainbow trout (Oncorhynchus mykiss, Walbaum, 1792). Surv. Fish. Sci. 2019, 6, 88–98. [Google Scholar] [CrossRef]
- Vutukuru, S.S. Acute Effects of Hexavalent Chromium on Survival, Oxygen Consumption, Hematological Parameters and Some Biochemical Profiles of the Indian Major Carp, Labeo rohita. Int. J. Environ. Res. Public Health 2005, 2, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Hassan, B.K. The effect of copper and cadmium on oxygen consumption of the juvenile common carp, Cyprinus carpio (L.). Mesopot. J. Mar. Sci. 2011, 26, 25–34. [Google Scholar]
- Sruthisree, C.; Gowda, G.; Nayak, H.; Harish, B.D.; Amin, A. Effect of Heavy Metal (Lead) on Oxygen Consumption and Ammonia Excretion of Iridescent Shark (Pangusius Hypohthalmus). J. Exp. Zool. India 2015, 18, 637–640. [Google Scholar]
- Natarajan, G. Effect of sublethal concentration of metasystox on selected oxidative enzymes, tissue respiration, and hematology of the freshwater air-breathing fish, Channa striatus (Bleeker). Pestic. Biochem. Physiol. 1984, 21, 194–198. [Google Scholar] [CrossRef]
- Amin, A.; Naik, A.T.R.; Priyadarshini, N.; Nayak, H.; Sree, C.S. Toxic effect of heavy metal lead on oxygen consumption of rohu (Labeo rohita) fingerlings. Biochem. Cell. Arch. 2017, 17, 225–228. [Google Scholar]
- Mastan, S.; Priya, G.I.; Babu, E. Haematological Profile of Clarias batrachus (Linn.) Exposed to Sub-Lethal Doses of Lead Nitrate. Int. J. Hematol. 2009, 6, 4–7. [Google Scholar] [CrossRef]
- Senthamilselvan, D.; Chezhian, A.; Suresh, E.; Ezhilmathy, R. Toxic effects of heavy metals (cadmium plus mercury) on haematological parameters and DNA damage in Lates calcarifer. J. Toxicol. Environ. Health Sci. 2012, 4, 156–161. [Google Scholar] [CrossRef][Green Version]
- Bujjamma, P.; Padmavathi, P. Effect of cadmium on Haematological changes in a freshwater catfish, Heteropneustes fossilis. Int. J. Zool. Stud. 2018, 3, 132–141. [Google Scholar]
- Khalesi, K.; Abedi, Z.; Behrouzi, S.; Eskandari, S.K. Haematological, blood biochemical and histopathological effects of sublethal cadmium and lead concentrations in common carp. Bulg. J. Vet. Med. 2017, 20, 141–150. [Google Scholar] [CrossRef]
- Vasanthi, K.N.; Muthukumaravel, O.; Sathick, J.S. Toxic effect of mercury on the freshwater fish Oreochromis mossambicus. Res. J. Life Sci. 2019, 364–376. [Google Scholar] [CrossRef]
- Bose, M.J.; Ilavazhahan, M.; Tamilselvi, R.; Viswanathan, M. Effect of Heavy Metals on the Histopathology of Gills and Brain of Fresh Water Fish Catla catla. Biomed. Pharmacol. J. 2013, 6, 99–105. [Google Scholar] [CrossRef]
- Muthukumaravel, K.; Rajaraman, P. A study on the toxicity of chromium on the histology of gill and liver of freshwater fish Labeo rohita. Int. J. Pure Appl. Zool 2013, 1, 122–126. [Google Scholar]
- Choudhary, L.; Vyas, T.; Chauhan, N.R.S.; Madhavi, B.; Yadav, G.K.; Bharadwaj, S. Histopathological changes due to lead toxicity in gills of P. ticto (hem). Int. Res. J. Sci. Eng. 2019, 7, 92–95. [Google Scholar]
- Anandhan, R.; Kavitha, V. Effect of aluminium chloride toxicity against histopathology of gill of fresh water fish Labeo rohita (Ham). Int. J. Curr. Adv. Res. 2019, 8, 6–8. [Google Scholar]
- Agnihotri, U.; Bahadure, R.; Akarte, S. Gill lamellar changes in fresh water fish Channa punctatus due to influence of arsenic trioxide. Biochem. Biophys Res. Commun. 2010, 3, 61–65. [Google Scholar]


| Hours of Exposure | LC50 | L.C.L | U.C.L | Regression Equation | Calculated X2 Value | Table X2 Value |
|---|---|---|---|---|---|---|
| 24 | 3.649099 | 4.0547773 | 0.284008 | Y = 1.491007 + 6.241699X | 13.9002 | 9.49 |
| 48 | 3.185816 | 3.661036 | 2.772282 | Y = 2.731714 + 4.507538X | 19.97842 | 11.07 |
| 72 | 2.890422 | 3.139665 | 2.660965 | Y = 3.6252 + 2.982463X | 1.457794 | 11.07 |
| 96 | 2.247149 | 2.464002 | 2.04938 | Y = 4.172045 + 2.354607X | 8.465149 | 12.59 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muthukumaravel, K.; Pradhoshini, K.P.; Vasanthi, N.; Kanagavalli, V.; Ahadu Shareef, M.; Musthafa, M.S.; Rajagopal, R.; Alfarhan, A.; Thirupathi, A.; Ravindran, B. Sublethal Effects of Arsenic on Oxygen Consumption, Hematological and Gill Histopathological Indices in Chanos chanos. Int. J. Environ. Res. Public Health 2021, 18, 12967. https://doi.org/10.3390/ijerph182412967
Muthukumaravel K, Pradhoshini KP, Vasanthi N, Kanagavalli V, Ahadu Shareef M, Musthafa MS, Rajagopal R, Alfarhan A, Thirupathi A, Ravindran B. Sublethal Effects of Arsenic on Oxygen Consumption, Hematological and Gill Histopathological Indices in Chanos chanos. International Journal of Environmental Research and Public Health. 2021; 18(24):12967. https://doi.org/10.3390/ijerph182412967
Chicago/Turabian StyleMuthukumaravel, Kannayiram, Kumara Perumal Pradhoshini, Natarajan Vasanthi, Venkatachalam Kanagavalli, Mohamed Ahadu Shareef, Mohamed Saiyad Musthafa, Rajakrishnan Rajagopal, Ahmed Alfarhan, Anand Thirupathi, and Balasubramani Ravindran. 2021. "Sublethal Effects of Arsenic on Oxygen Consumption, Hematological and Gill Histopathological Indices in Chanos chanos" International Journal of Environmental Research and Public Health 18, no. 24: 12967. https://doi.org/10.3390/ijerph182412967
APA StyleMuthukumaravel, K., Pradhoshini, K. P., Vasanthi, N., Kanagavalli, V., Ahadu Shareef, M., Musthafa, M. S., Rajagopal, R., Alfarhan, A., Thirupathi, A., & Ravindran, B. (2021). Sublethal Effects of Arsenic on Oxygen Consumption, Hematological and Gill Histopathological Indices in Chanos chanos. International Journal of Environmental Research and Public Health, 18(24), 12967. https://doi.org/10.3390/ijerph182412967








