Exercise Intolerance and Oxygen Desaturation in Patients with Parkinson’s Disease: Triggers for Respiratory Rehabilitation?
Abstract
1. Introduction
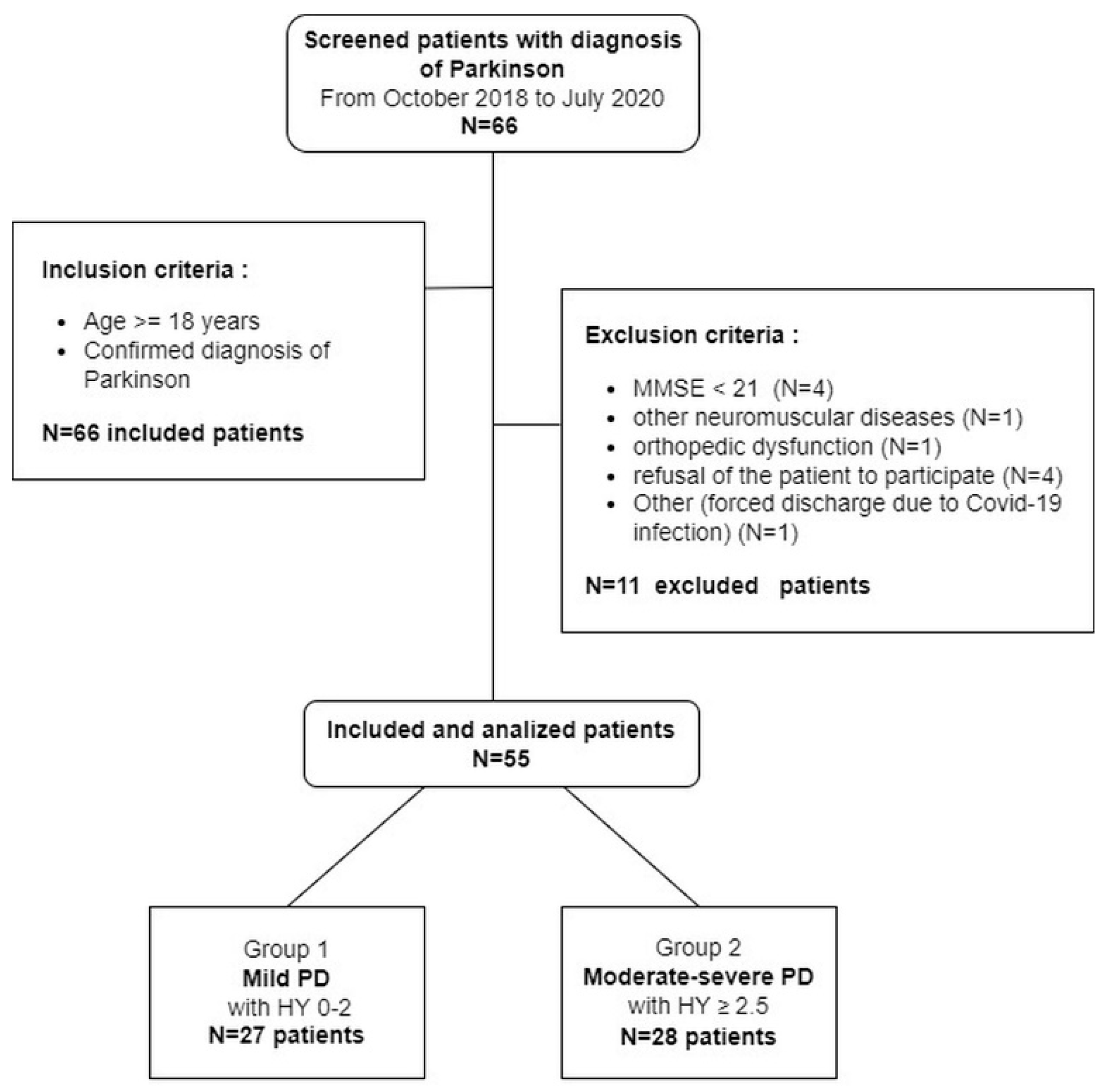
2. Materials and Methods
2.1. Patients
2.2. Measurements
2.3. Statistical Analysis
3. Results
Correlations
4. Discussion
4.1. Limitations and Strengths
4.2. Clinical Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schapira, A.H. Neurobiology and treatment of Parkinson’s disease. Trends Pharm. Sci. 2009, 30, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Baille, G.; De Jesus, A.M.; Perez, T.; Devos, D.; Dujardin, K.; Charley, C.M.; Defebvre, L.; Moreau, C. Ventilatory dysfunction in Parkinson’s disease. J. Parkinsons Dis. 2016, 6, 463–471. [Google Scholar] [CrossRef]
- López-López, L.; Rodríguez-Torres, J.R.; Cahalin, L.P.; Cabrera-Martos, I.; Torres Sánchez, I.; Valenza, M.C. Ventilatory impairments associated with Parkinson’s disease: A systematic review and meta-analysis. In Respiration; International Review of Thoracic Diseases; Karger: Basel, Switzerland, 2021; Volume 100, pp. 173–181. [Google Scholar]
- De Pandis, M.F.; Starace, A.; Stefanelli, F.; Marruzzo, P.; Meoli, I.; De Simone, G.; Prati, R.; Stocchi, F. Modification of respiratory function parameters in patients with severe Parkinson’s disease. Neurol. Sci. 2002, 23 (Suppl. S2), S69–S70. [Google Scholar] [CrossRef] [PubMed]
- Tandon, M.; Ahmad, F.M.H.; Narayanan, S.; Mohan, C.; Yadav, S. Impact of levodopa in lung functions in patients with Parkinson disease. Ann. Indian Acad. Neurol. 2020, 23, 338–341. [Google Scholar] [PubMed]
- Zhang, W.; Zhang, L.; Zhou, N.; Huang, E.; Li, Q.; Wang, T.; Ma, C.; Li, B.; Li, C.; Du, Y.; et al. Dysregulation of respiratory center drive (P0.1) and muscle strength in patients with early stage idiopathic Parkinson’s disease. Front. Neurol. 2019, 10, 724. [Google Scholar] [CrossRef]
- Santos, R.B.D.; Fraga, A.S.; Coriolano, M.; Tiburtino, B.F.; Lins, O.G.; Esteves, A.C.F.; Asano, N.M.J. Respiratory muscle strength and lung function in the stages of Parkinson’s disease. J. Bras. Pneumol. 2019, 45, e20180148. [Google Scholar] [CrossRef] [PubMed]
- Darling-White, M.; Huber, J.E. The impact of expiratory muscle strength training on speech breathing in individuals with parkinson’s disease: A preliminary study. Am. J. Speech Lang Pathol. 2017, 26, 1159–1166. [Google Scholar] [CrossRef]
- Silverman, E.P.; Sapienza, C.M.; Saleem, A.; Carmichael, C.; Davenport, P.W.; Hoffman-Ruddy, B.; Okun, M.S. Tutorial on maximum inspiratory and expiratory mouth pressures in individuals with idiopathic Parkinson disease (IPD) and the preliminary results of an expiratory muscle strength training program. NeuroRehabilitation 2006, 21, 71–79. [Google Scholar] [CrossRef]
- Huang, C.C.; Lai, Y.R.; Wu, F.A.; Kuo, N.Y.; Tsai, Y.C.; Cheng, B.C.; Tsai, N.W.; Lu, C.H. Simultaneously improved pulmonary and cardiovascular autonomic function and short-term functional outcomes in patients with Parkinson’s disease after respiratory muscle training. J. Clin. Med. 2020, 9, 316. [Google Scholar] [CrossRef]
- Montero Ferro, A.P.; Basso-Vanelli, R.; Moreira Mello, R.L.; Sanches Garcia-Araujo, A.; Gonçalves Mendes, R.; Costa, D.; Gianlorenço, A.C. Effects of inspiratory muscle training on respiratory muscle strength, lung function, functional capacity and cardiac autonomic function in Parkinson’s disease: Randomized controlled clinical trial protocol. Physiother. Res. Int. 2019, 24, e1777. [Google Scholar] [CrossRef]
- Riboldazzi, G.; Spinazza, G.; Beccarelli, L.; Prato, P.; Grecchi, B.; D’Abrosca, F.; Nicolini, A. Effectiveness of expiratory flow acceleration in patients with Parkinson’s disease and swallowing deficiency: A preliminary study. Clin. Neurol. Neurosurg. 2020, 199, 106249. [Google Scholar] [CrossRef]
- Sobreira-Neto, M.A.; Pena-Pereira, M.A.; Sobreira, E.S.T.; Chagas, M.H.N.; Almeida, C.M.O.; Fernandes, R.M.F.; Tumas, V.; Eckeli, A.L. Obstructive sleep apnea and Parkinson’s disease: Characteristics and associated factors. Arq. De Neuro Psiquiatr. 2019, 77, 609–616. [Google Scholar] [CrossRef]
- da Silva-Júnior, F.P., Jr.; do Prado, G.F.; Barbosa, E.R.; Tufik, S.; Togeiro, S.M. Sleep disordered breathing in Parkinson’s disease: A critical appraisal. Sleep Med. Rev. 2014, 18, 173–178. [Google Scholar] [CrossRef]
- Baille, G.; Perez, T.; Devos, D.; Machuron, F.; Dujardin, K.; Chenivesse, C.; Defebvre, L.; Moreau, C. Dyspnea is a specific symptom in Parkinson’s disease. J. Parkinsons Dis. 2019, 9, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Vijayan, S.; Singh, B.; Ghosh, S.; Stell, R.; Mastaglia, F.L. Dyspnea in Parkinson’s disease: An approach to diagnosis and management. Expert Rev. Neurother. 2020, 20, 619–626. [Google Scholar] [CrossRef]
- Weiner, P.; Inzelberg, R.; Davidovich, A.; Nisipeanu, P.; Magadle, R.; Berar-Yanay, N.; Carasso, R.L. Respiratory muscle performance and the Perception of dyspnea in Parkinson’s disease. Can. J. Neurol. Sci. 2002, 29, 68–72. [Google Scholar] [CrossRef]
- Bridges, K.W.; Goldberg, D.P. The validation of the GHQ-28 and the use of the MMSE in neurological in-patients. Br. J. Psychiatry 1986, 148, 548–553. [Google Scholar] [CrossRef]
- Vetra, A. White Book on Physical and rehabilitation medicine in Europe. Introductions, executive summary, and methodology. Eur. J. Phys. Rehabil. Med. 2018, 54, 125–155. [Google Scholar]
- Parmelee, P.A.; Thuras, P.D.; Katz, I.R.; Lawton, M.P. Validation of the cumulative illness rating scale in a geriatric residential population. J. Am. Geriatr. Soc. 1995, 43, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Martin, P.; Skorvanek, M.; Rojo-Abuin, J.M.; Gregova, Z.; Stebbins, G.T.; Goetz, C.G. Validation study of the hoehn and yahr scale included in the MDS-UPDRS. Mov. Disord. 2018, 33, 651–652. [Google Scholar] [CrossRef]
- Movement Disorder Society Task Force on Rating Scales for Parkinson’s Disease. The Unified Parkinson’s Disease Rating Scale (UPDRS): Status and recommendations. Mov. Disord. 2003, 18, 738–750. [Google Scholar] [CrossRef] [PubMed]
- Antonini, A.; Abbruzzese, G.; Ferini-Strambi, L.; Tilley, B.; Huang, J.; Stebbins, G.T.; Goetz, C.G.; Barone, P.; Bandettini di Poggio, M.; Fabbrini, G.; et al. Validation of the Italian version of the movement disorder society--Unified Parkinson’s disease rating scale. Neurol. Sci. 2013, 34, 683–687. [Google Scholar] [CrossRef] [PubMed]
- Morley, D.; Selai, C.; Thompson, A. The self-report Barthel Index: Preliminary validation in people with Parkinson’s disease. Eur. J. Neurol. 2012, 19, 927–929. [Google Scholar] [CrossRef]
- Dodds, T.A.; Martin, D.P.; Stolov, W.C.; Deyo, R.A. A validation of the functional independence measurement and its performance among rehabilitation inpatients. Arch. Phys. Med. Rehabil. 1993, 74, 531–536. [Google Scholar] [CrossRef]
- Berg, K.O.; Wood-Dauphinee, S.L.; Williams, J.I.; Maki, B. Measuring balance in the elderly: Validation of an instrument. Can. J. Public Health 1992, 83 (Suppl. S2), S7–S11. [Google Scholar]
- Arnaldi, D.; Cordano, C.; De Carli, F.; Accardo, J.; Ferrara, M.; Picco, A.; Tamburini, T.; Brugnolo, A.; Abbruzzese, G.; Nobili, F. Parkinson’s disease sleep scale 2: Application in an Italian population. Neurol. Sci. 2016, 37, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Campbell, A.J.; Neill, A.M.; Scott, D.A.R. Clinical reproducibility of the epworth sleepiness scale for patients with suspected sleep apnea. J. Clin. Sleep Med. JCSM Off. Publ. Am. Acad. Sleep Med. 2018, 14, 791–795. [Google Scholar] [CrossRef]
- Quanjer, P.H.; Tammeling, G.J.; Cotes, J.E.; Pedersen, O.F.; Peslin, R.; Yernault, J.C. Lung volumes and forced ventilatory flows. Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official Statement of the European Respiratory Society. Eur. Respir. J. Suppl. 1993, 16, 5–40. [Google Scholar] [CrossRef]
- Gibson, G.J. Standardized lung function testing. Report working party. Bull Eur. Physiopathol. Respir. 1983, 19 (Suppl. S5), 1–95. [Google Scholar]
- Kulnik, S.T.; MacBean, V.; Birring, S.S.; Moxham, J.; Rafferty, G.F.; Kalra, L. Accuracy of portable devices in measuring peak cough flow. Physiol. Meas. 2015, 36, 243–257. [Google Scholar] [CrossRef]
- ATS statement: Guidelines for the six-minute walk test. Am. J. Respir. Crit. Care Med. 2002, 166, 111–117. [CrossRef] [PubMed]
- Chang, C.H.; Lin, H.C.; Yang, C.H.; Gan, S.T.; Huang, C.H.; Chung, F.T.; Hu, H.C.; Lin, S.M. Factors associated with exercise-induced desaturation in patients with chronic obstructive pulmonary disease. Int. J. Chro.n Obstruct. Pulmon. Dis. 2020, 15, 2643–2652. [Google Scholar] [CrossRef]
- A Language and Environment for Statistical Computing; Version 4.0.5; R Foundation for Statistical Computing: Vienna, Austria, 2020.
- Hoehn, M.M.; Yahr, M.D. Parkinsonism: Onset, progression, and mortality. Neurology 2001, 57 (Suppl. S3), S11–S26. [Google Scholar] [PubMed]
- Martínez-Martín, P.; Rodríguez-Blázquez, C.; Mario, A.; Arakaki, T.; Arillo, V.C.; Chaná, P.; Fernández, W.; Garretto, N.; Martínez-Castrillo, J.C.; Rodríguez-Violante, M.; et al. Parkinson’s disease severity levels and MDS-unified Parkinson’s Disease rating scale. Parkinsonism Relat. Disord. 2015, 21, 50–54. [Google Scholar] [CrossRef]
- Kalia, L.V.; Lang, A.E. Parkinson’s disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef]
- Allen, N.E.; Sherrington, C.; Canning, C.G.; Fung, V.S. Reduced muscle power is associated with slower walking velocity and falls in people with Parkinson’s disease. Parkinsonism Relat. Disord. 2010, 16, 261–264. [Google Scholar] [CrossRef]
- Inkster, L.M.; Eng, J.J.; MacIntyre, D.L.; Stoessl, A.J. Leg muscle strength is reduced in Parkinson’s disease and relates to the ability to rise from a chair. Mov. Disord. 2003, 18, 157–162. [Google Scholar] [CrossRef]
- Schenkman, M.; Ellis, T.; Christiansen, C.; Barón, A.E.; Tickle-Degnen, L.; Hall, D.A.; Wagenaar, R. Profile of functional limitations and task performance among people with early- and middle-stage Parkinson disease. Phys. Ther. 2011, 91, 1339–1354. [Google Scholar] [CrossRef]
- Sauerbier, A.; Jenner, P.; Todorova, A.; Chaudhuri, K.R. Non motor subtypes and Parkinson’s disease. Parkinsonism Relat. Disord. 2016, 22 (Suppl. S1), S41–S46. [Google Scholar] [CrossRef] [PubMed]
- Ziemssen, T.; Reichmann, H. Non-motor dysfunction in Parkinson’s disease. Parkinsonism Relat. Disord. 2007, 13, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Radder, D.L.M.; Sturkenboom, I.H.; van Nimwegen, M.; Keus, S.H.; Bloem, B.R.; de Vries, N.M. Physical therapy and occupational therapy in Parkinson’s disease. Int. J. Neurosci. 2017, 127, 930–943. [Google Scholar] [CrossRef] [PubMed]
- Katzel, L.I.; Sorkin, J.D.; Macko, R.F.; Smith, B.; Ivey, F.M.; Shulman, L.M. Repeatability of aerobic capacity measurements in Parkinson disease. Med. Sci. Sports Exerc. 2011, 43, 2381–2387. [Google Scholar] [CrossRef]
- Demonceau, M.; Maquet, D.; Jidovtseff, B.; Donneau, A.F.; Bury, T.; Croisier, J.L.; Crielaard, J.M.; Rodriguez de la Cruz, C.; Delvaux, V.; Garraux, G. Effects of twelve weeks of aerobic or strength training in addition to standard care in Parkinson’s disease: A controlled study. Eur. J. Phys. Rehabil. Med. 2017, 53, 184–200. [Google Scholar] [CrossRef] [PubMed]
- Martignon, C.; Pedrinolla, A.; Ruzzante, F.; Giuriato, G.; Laginestra, F.G.; Bouça-Machado, R.; Ferreira, J.J.; Tinazzi, M.; Schena, F.; Venturelli, M. Guidelines on exercise testing and prescription for patients at different stages of Parkinson’s disease. Aging Clin. Exp. Res. 2021, 33, 221–246. [Google Scholar] [CrossRef]
- Canning, C.G.; Alison, J.A.; Allen, N.E.; Groeller, H. Parkinson’s disease: An investigation of exercise capacity, respiratory function, and gait. Arch. Phys. Med. Rehabil. 1997, 78, 199–207. [Google Scholar] [CrossRef]
- Rosen, M.J.; Sorkin, J.D.; Goldberg, A.P.; Hagberg, J.M.; Katzel, L.I. Predictors of age-associated decline in maximal aerobic capacity: A comparison of four statistical models. J. Appl. Physiol. 1998, 84, 2163–2170. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Atan, T.; Özyemişci Taşkıran, Ö.; Bora Tokçaer, A.; Kaymak Karataş, G.; Karakuş Çalışkan, A.; Karaoğlan, B. Effects of different percentages of body weight-supported treadmill training in Parkinson’s disease: A double-blind randomized controlled trial. Turk. J. Med. Sci. 2019, 49, 999–1007. [Google Scholar] [CrossRef]
- Sabaté, M.; González, I.; Ruperez, F.; Rodríguez, M. Obstructive and restrictive pulmonary dysfunctions in Parkinson’s disease. J. Neurol. Sci. 1996, 138, 114–119. [Google Scholar] [CrossRef]
- Joy, S.P.; Sinha, S.; Pal, P.K.; Panda, S.; Philip, M.; Taly, A.B. Serial macro-architectural alterations with levodopa in Parkinson’s disease: Polysomnography (PSG)-based analysis. Ann. Indian Acad. Neurol. 2015, 18, 309–313. [Google Scholar]
- Chaudhuri, K.R.; Odin, P.; Antonini, A.; Martinez-Martin, P. Parkinson’s disease: The non-motor issues. Parkinsonism Relat. Disord. 2011, 17, 717–723. [Google Scholar] [CrossRef]
- Wilczyński, J.; Habik, N. The effect of L-dopa on postural stability in Parkinson’s disease patients. Appl. Sci. 2019, 9, 409. [Google Scholar] [CrossRef]


| Overall n = 55 | Group 1: HY 0–2 n = 27 | Group 2: HY ≥ 2.5 n = 28 | p | Delta | 95% CI | |
|---|---|---|---|---|---|---|
| Age, years | 73 (68–79) | 72 (67–76) | 75 (70–79) | 0.1878 | ||
| Male, % | 53 | 59 | 46 | 0.4948 | ||
| BMI, Kg/m2 | 25.2 (22.9–29.9) | 24.3 (22.8–27.1) | 26.7 (23.4–31.3) | 0.0875 | ||
| Smokers, yes% | 3.7 | 3.7 | 3.7 | 1.0000 | ||
| Ex-smokers, yes% | 18.9 | 19.2 | 18.5 | 1.0000 | ||
| CIRS1, score | 1.5 (1.3–1.8) | 1.4 (1.2–1.5) | 1.6 (1.4–1.9) | 0.0022 | 0.3 | 0.1; 0.4 |
| CIRS2, score | 2.0 (2.0–4.0) | 2.0 (1.0–3.0) | 3.0 (2.0–4.2) | 0.0005 | 1.0 | 1.0; 2.0 |
| Comorbidities, yes% | 64.2 | 65.4 | 63.0 | 1.0000 | ||
| COPD, yes% | 7.5 | 3.8 | 11.1 | 0.6105 | ||
| CHF, yes% | 1.9 | 3.8 | 0 | 0.4906 | ||
| Diabetes, yes% | 9.4 | 3.8 | 14.8 | 0.3507 | ||
| Hypertension, yes% | 34.0 | 26.9 | 40.7 | 0.3869 | ||
| Others, yes% | 43.4 | 34.6 | 51.8 | 0.2709 | ||
| MMSE, score | 27.4 (25.3–3.0) | 28.0 (25.3–30.0) | 27.0 (25.6–28.0) | 0.2263 | ||
| UPDRSII, score | 9 (6–18) | 7 (3–9) | 15 (9–22) | 0.0004 | 7 | 3; 12 |
| UPDRSIII, score | 17 (11–28) | 13 (10–17) | 26 (16–44) | 0.0006 | 14 | 5; 25 |
| Barthel Index, score | 95 (80–100) | 100 (97–100) | 80 (60–95) | <0.0001 | −20 | −25; −10 |
| FIM, score | 113 (90–126) | 126 (104–126) | 91 (84–121) | 0.0006 | −18 | −32; −6 |
| Berg balance, score | 44 (35–50) | 47 (42–50) | 38 (33–47) | 0.0218 | −6 | −12; −1 |
| FEV1, % predicted | 95 (84–109) | 103 (90–113) | 93 (77–106) | 0.1947 | ||
| FVC, % predicted | 94 (81–109) | 98 (88–111) | 91 (77–104) | 0.0338 | −12 | −24; −1 |
| FEV1/FVC, % | 79 (75–83) | 77 (74–81) | 81 (76–86) | 0.1424 | ||
| PEF, % predicted | 84 (65–104) | 93 (69–108) | 78 (63–98) | 0.1572 | ||
| MIP, % predicted | 43 (34–51) | 45 (37–51) | 41 (33–50) | 0.3773 | ||
| MEP, % predicted | 40 (30–50) | 42 (29–56) | 36 (31–48) | 0.5275 | ||
| PCEF, L/min | 300 (218–405) | 366 (247–448) | 282 (208–308) | 0.0168 | −95 | −148; −18 |
| Night SatO2 ODI, n/h | 8.3 (2.9–15.3) | 4.3 (0.8–10.2) | 13.5 (6.6–22.8) | 0.0032 | 7.3 | 2.8; 12.7 |
| Night SatO2 T90, % | 1.40 (0.15–8.30) | 0.40 (0.05–1.19) | 3.95 (1.78–16.50) | 0.0002 | 2.60 | 1.34; 9.30 |
| Night SatO2 mean, % | 93.8 (92.3–95.5) | 94.9 (93.7–96.1) | 92.6 (91.9–94.6) | 0.0016 | −2.1 | −3.3; −0.8 |
| Night SatO2 nadir, % | 83.0 (75.5–86.2) | 84.0 (76.0–88.0) | 82.0 (75.0–85.0) | 0.3684 | ||
| Nighttime Desaturators, n (%) | 7 (12.7) | 2 (7.4) | 5 (17.6) | 0.4216 | ||
| 6MWT, % predicted | 56 (39–65) | 63 (57–68) | 41 (35–49) | <0.0001 | −20 | −26; −12 |
| 6MWT SatO2 bas, % | 96.0 (94.7–97.0) | 96.5 (95.4–97.1) | 95.7 (93.8–96.5) | 0.0410 | −1.0 | −2.0, 0.0 |
| 6MWT SatO2 mean, % | 94.5 (91.4–95.59) | 94.7 (92.5–96.3) | 94.0 (91.0–95.0) | 0.2894 | ||
| 6MWT SatO2 nadir, % | 91 (86–93) | 92 (87–94) | 91 (85–93) | 0.3219 | ||
| 6MWT Borg pre, score | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0.0950 | ||
| 6MWT Borg post, score | 3 (2–3) | 3 (2–3) | 3 (2–4) | 0.3261 | ||
| Exercise Desaturators, n (%) | 12 (21.8) | 7 (25.9) | 5 (17.9) | 1.0000 | ||
| PDSS, score | 102 (85–123) | 110 (95–126) | 92 (79–115) | 0.1112 | ||
| Epworth, score | 5 (3–9) | 5 (3–8) | 5 (3–12) | 0.3804 |
| Dependent Variables | Independent Variables | Estimated Beta | Standardized Beta | Adjusted R2 | p-Value |
|---|---|---|---|---|---|
| 6MWT% of pred. | 0.465 | <0.0001 *** | |||
| (Intercept) | 25.948 | 0.1904 | |||
| CIRS1 | −11.918 | −0.258 | 0.0560 ° | ||
| Barthel Index | 0.244 | 0.294 | 0.0246 * | ||
| FVC% pred. | 0.159 | 0.262 | 0.0683 ° | ||
| PCEF (L/min) | 0.029 | 0.229 | 0.0450 * | ||
| Night mean SpO2 | 0.515 | <0.0001 *** | |||
| (Intercept) | 80.101 | <0.0001 *** | |||
| BMI | −0.138 | −0.290 | 0.0056 ** | ||
| CIRS1 | −3.2541 | −0.438 | 0.0002 *** | ||
| 6MWT mean SpO2 | 0.240 | 0.326 | 0.0031 ** | ||
| 6MWT mean SpO2 | 0.430 | <0.0001 *** | |||
| (Intercept) | 9.734 | 0.5930 | |||
| Sex M | 1.791 | ------ | 0.0228 * | ||
| Age | 0.109 | 0.236 | 0.0431 * | ||
| BMI | 0.190 | 0.292 | 0.0150 * | ||
| Barthel Index | 0.049 | 0.268 | 0.0265 * | ||
| FEV1% pred. | 0.065 | 0.363 | 0.0023 ** | ||
| Night mean SpO2 | 0.651 | 0.479 | 0.0006 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vitacca, M.; Olivares, A.; Comini, L.; Vezzadini, G.; Langella, A.; Luisa, A.; Petrolati, A.; Frigo, G.; Paneroni, M. Exercise Intolerance and Oxygen Desaturation in Patients with Parkinson’s Disease: Triggers for Respiratory Rehabilitation? Int. J. Environ. Res. Public Health 2021, 18, 12298. https://doi.org/10.3390/ijerph182312298
Vitacca M, Olivares A, Comini L, Vezzadini G, Langella A, Luisa A, Petrolati A, Frigo G, Paneroni M. Exercise Intolerance and Oxygen Desaturation in Patients with Parkinson’s Disease: Triggers for Respiratory Rehabilitation? International Journal of Environmental Research and Public Health. 2021; 18(23):12298. https://doi.org/10.3390/ijerph182312298
Chicago/Turabian StyleVitacca, Michele, Adriana Olivares, Laura Comini, Giuliana Vezzadini, Annamaria Langella, Alberto Luisa, Anna Petrolati, Gianluigi Frigo, and Mara Paneroni. 2021. "Exercise Intolerance and Oxygen Desaturation in Patients with Parkinson’s Disease: Triggers for Respiratory Rehabilitation?" International Journal of Environmental Research and Public Health 18, no. 23: 12298. https://doi.org/10.3390/ijerph182312298
APA StyleVitacca, M., Olivares, A., Comini, L., Vezzadini, G., Langella, A., Luisa, A., Petrolati, A., Frigo, G., & Paneroni, M. (2021). Exercise Intolerance and Oxygen Desaturation in Patients with Parkinson’s Disease: Triggers for Respiratory Rehabilitation? International Journal of Environmental Research and Public Health, 18(23), 12298. https://doi.org/10.3390/ijerph182312298







