Development of Colorimetric Whole-Cell Biosensor for Detection of Heavy Metals in Environment for Public Health
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strain, Plasmid, Medium, and Heavy Metals
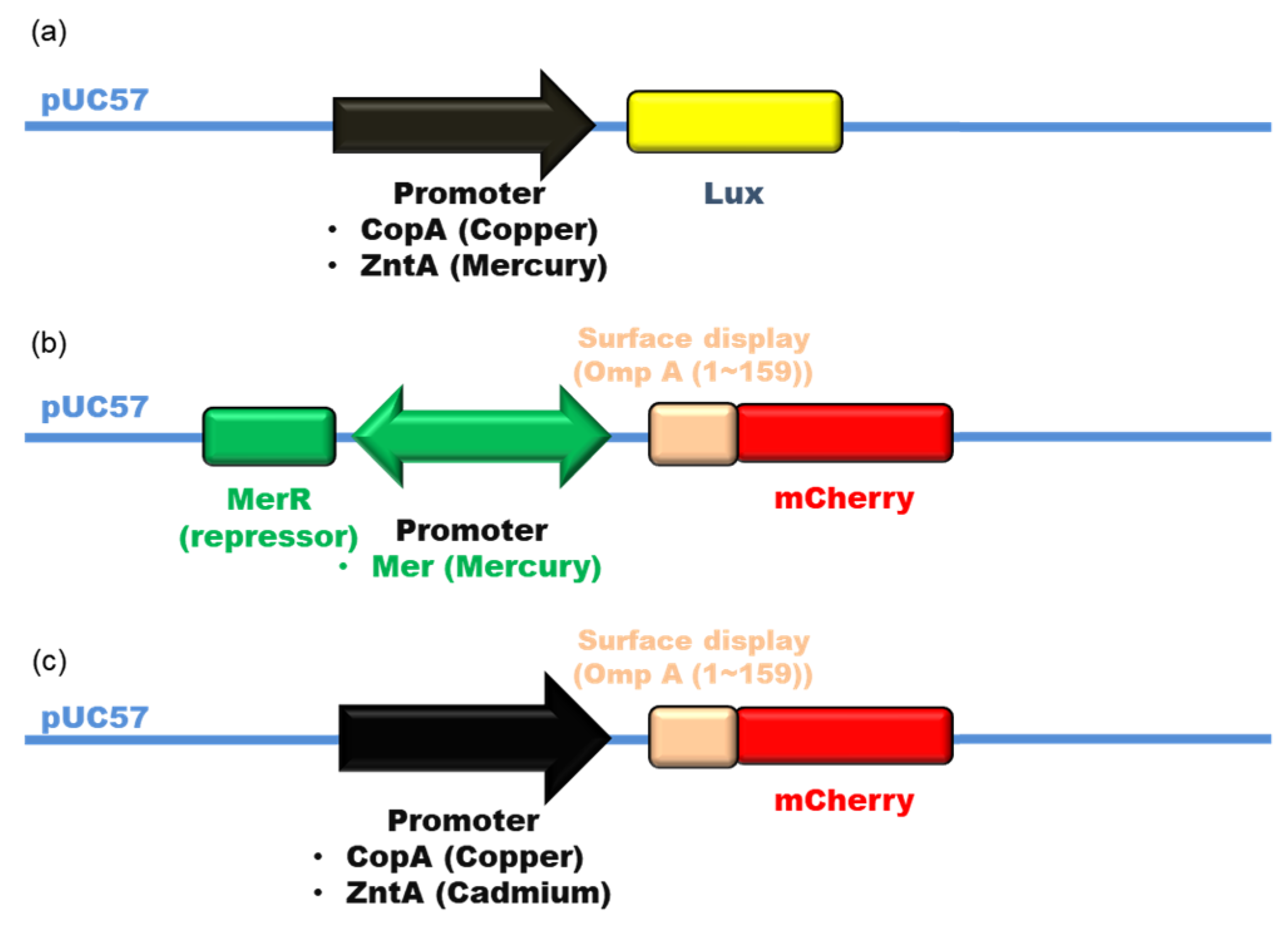
2.2. Construction of Heavy Metal-Sensing Plasmids
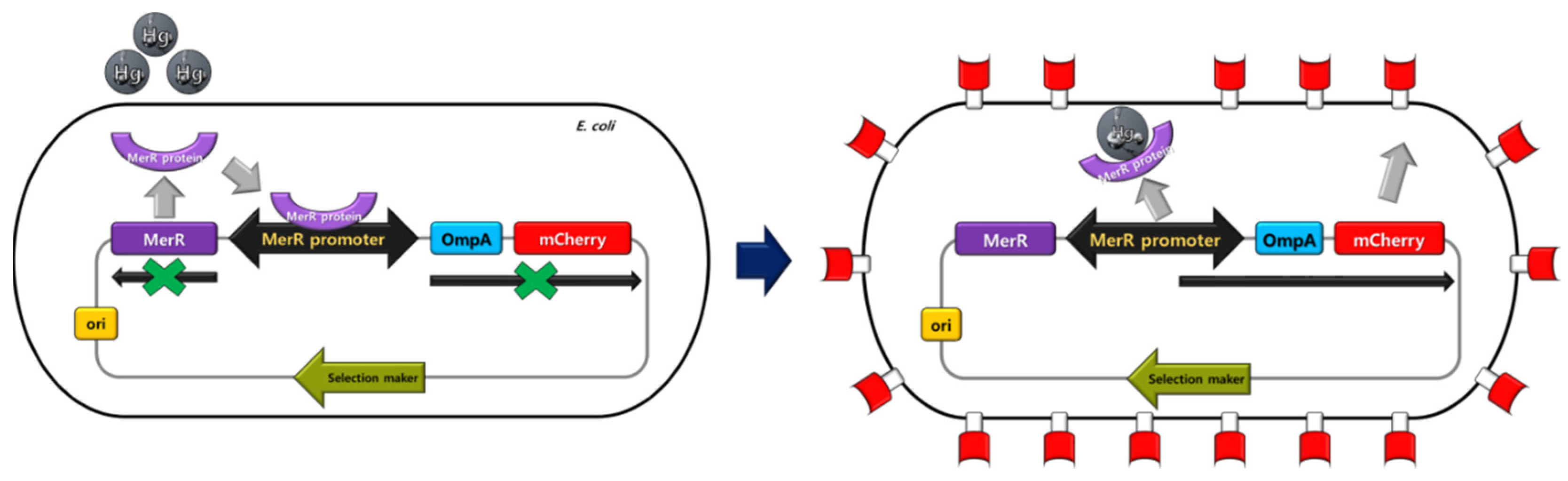
2.3. Engineering of Metal-Sensing E. coli
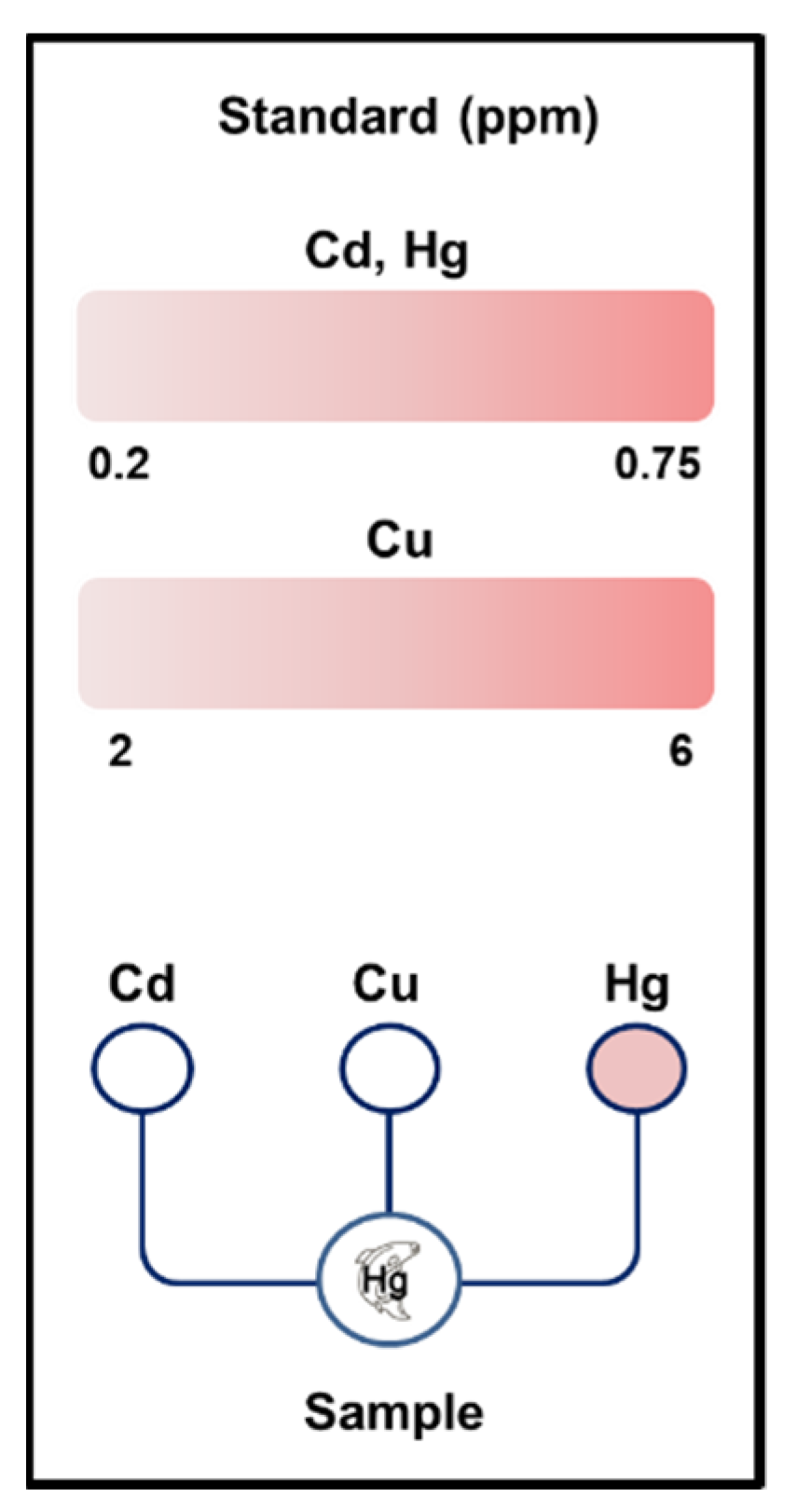
2.4. Detection of the Three Metals
2.5. Immobilization of the Metal-Sensing Strain
3. Results and Discussion
3.1. Construction of Metal-Sensing Strains
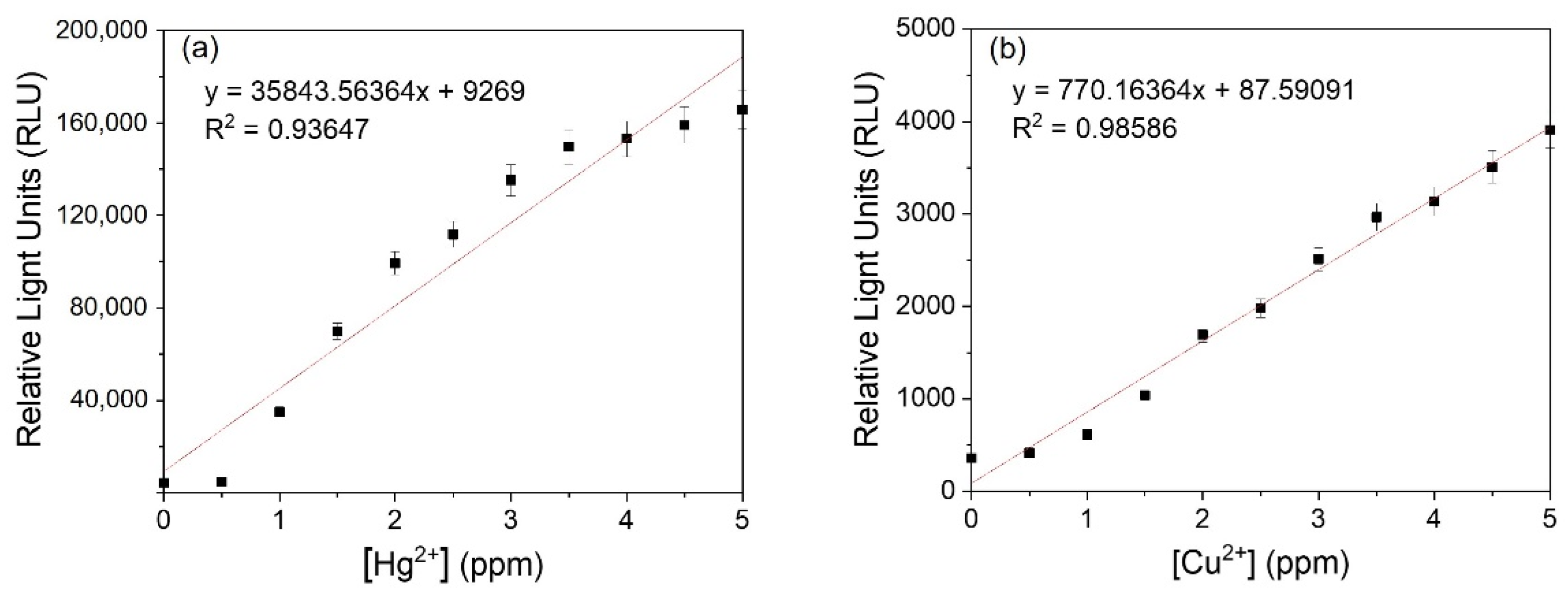
3.2. Detection of Heavy Metals by Luminescent Metal-Sensing Strains
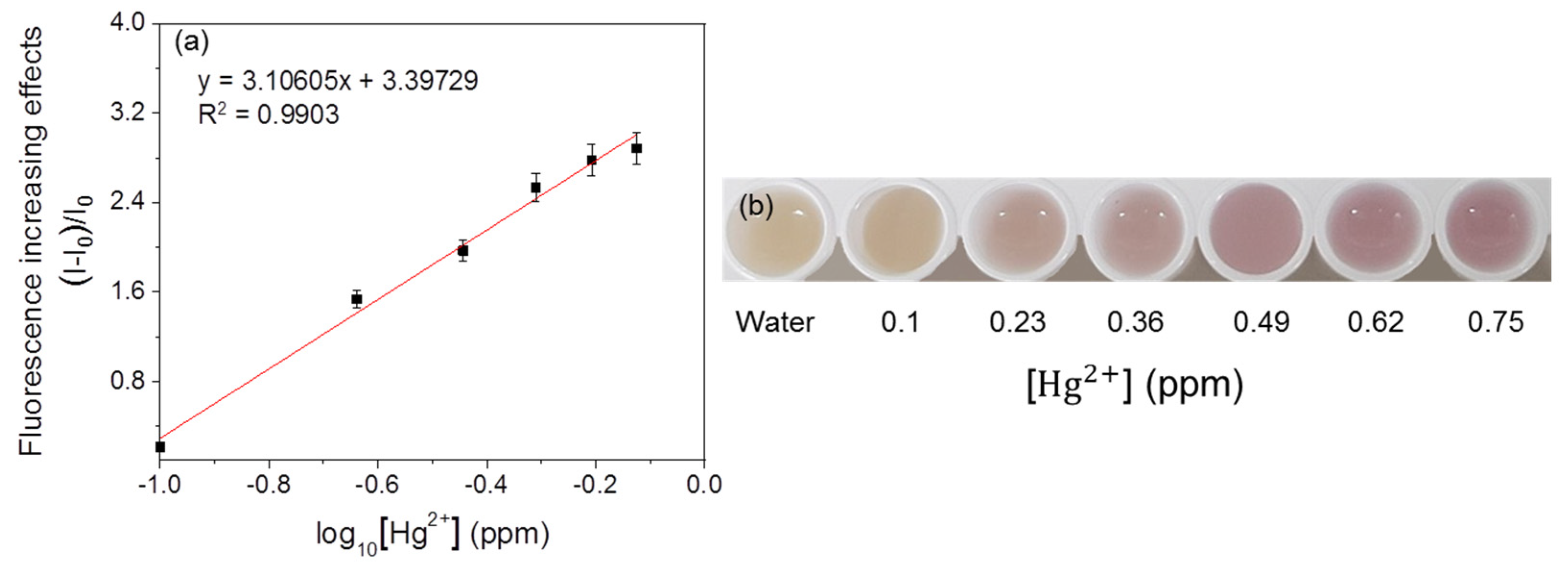
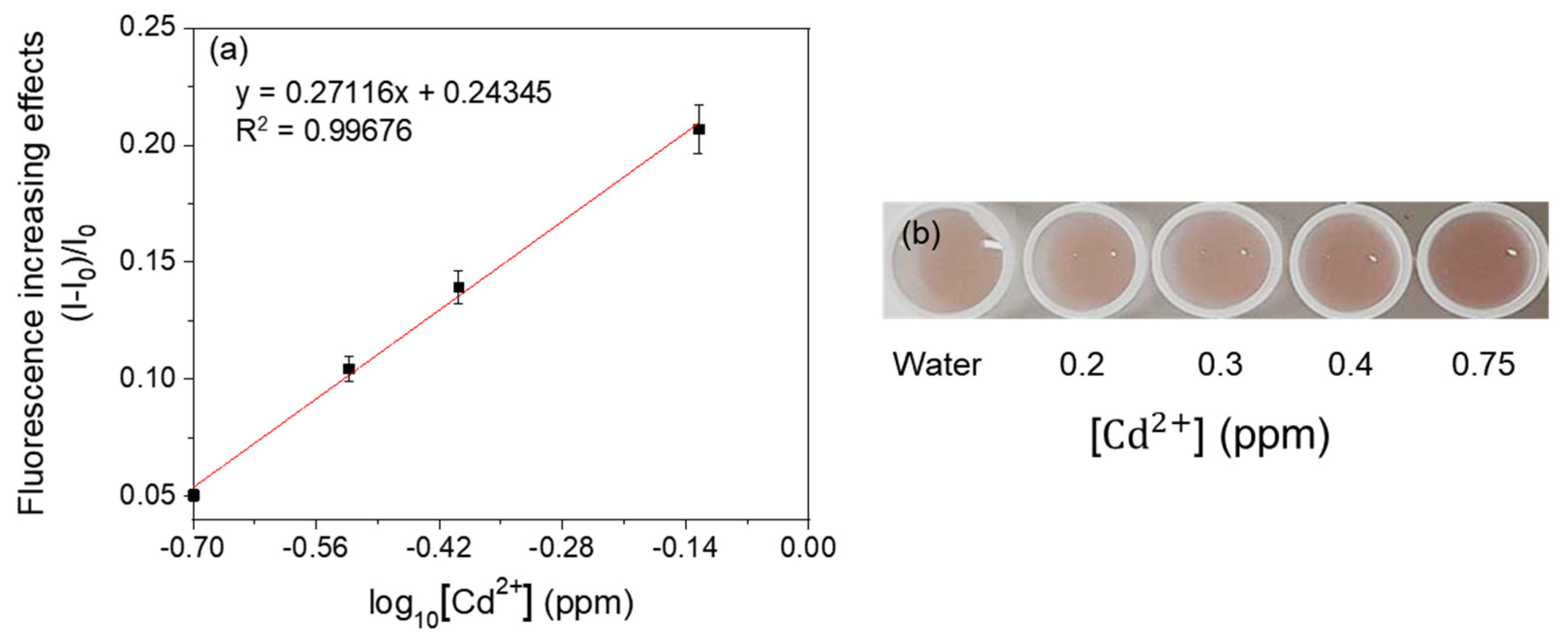
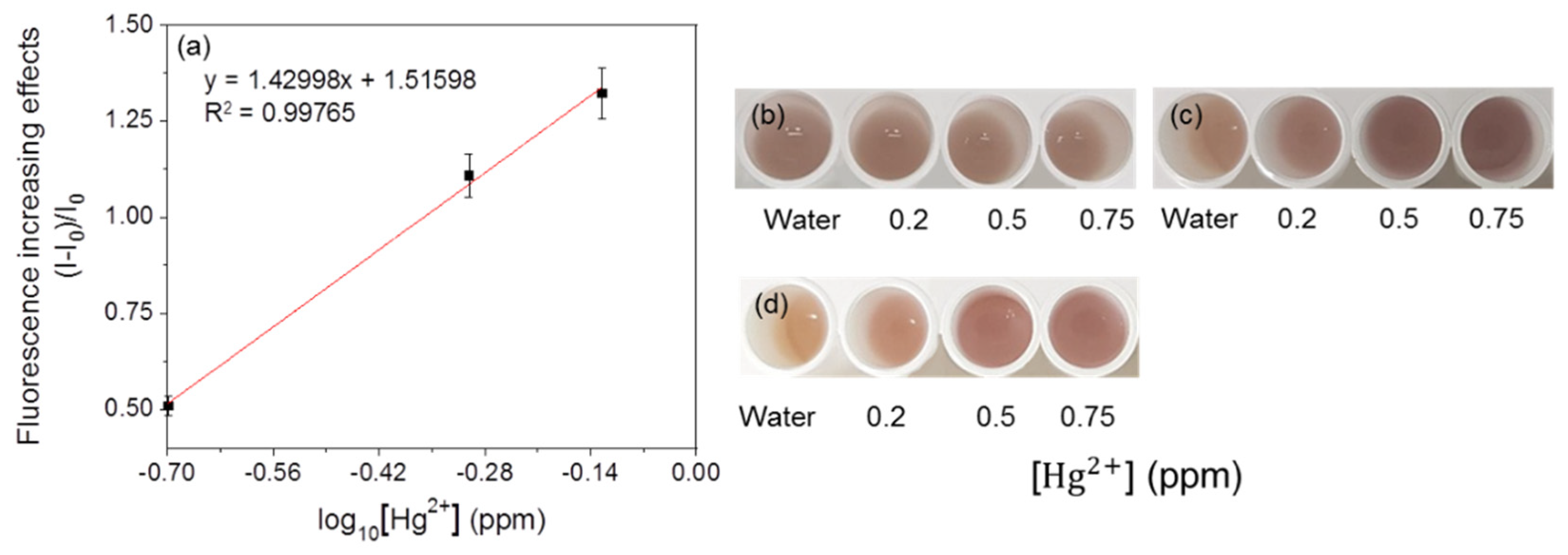
3.3. Detection of Heavy Metals by Fluorescent Metal-Sensing Strains
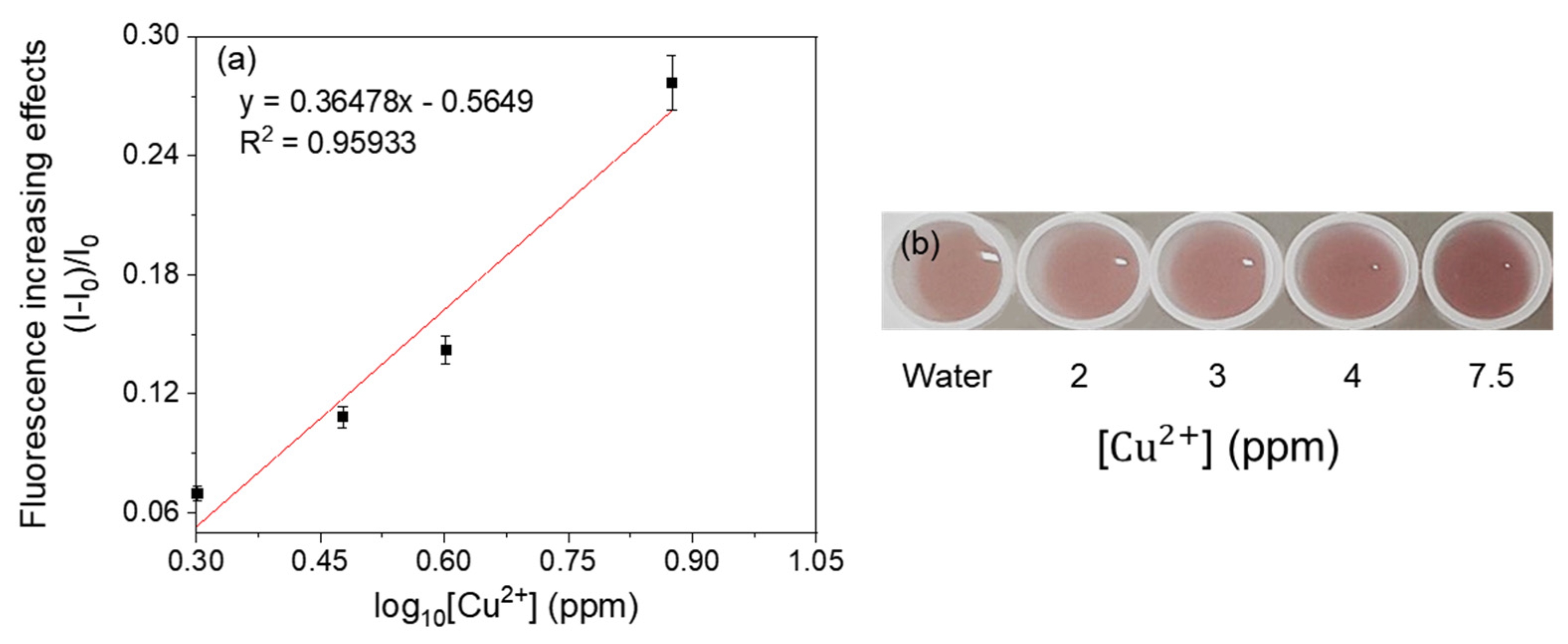
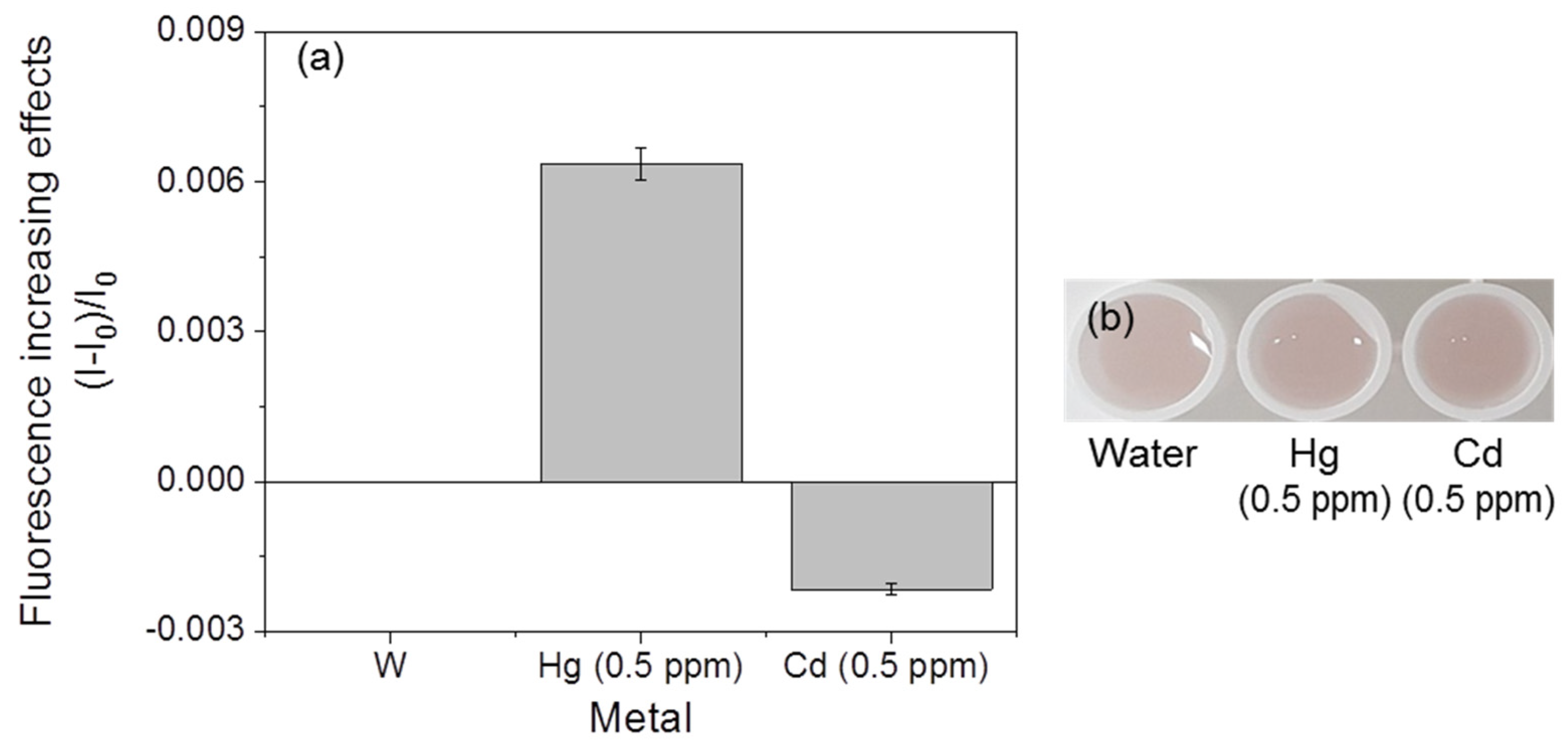
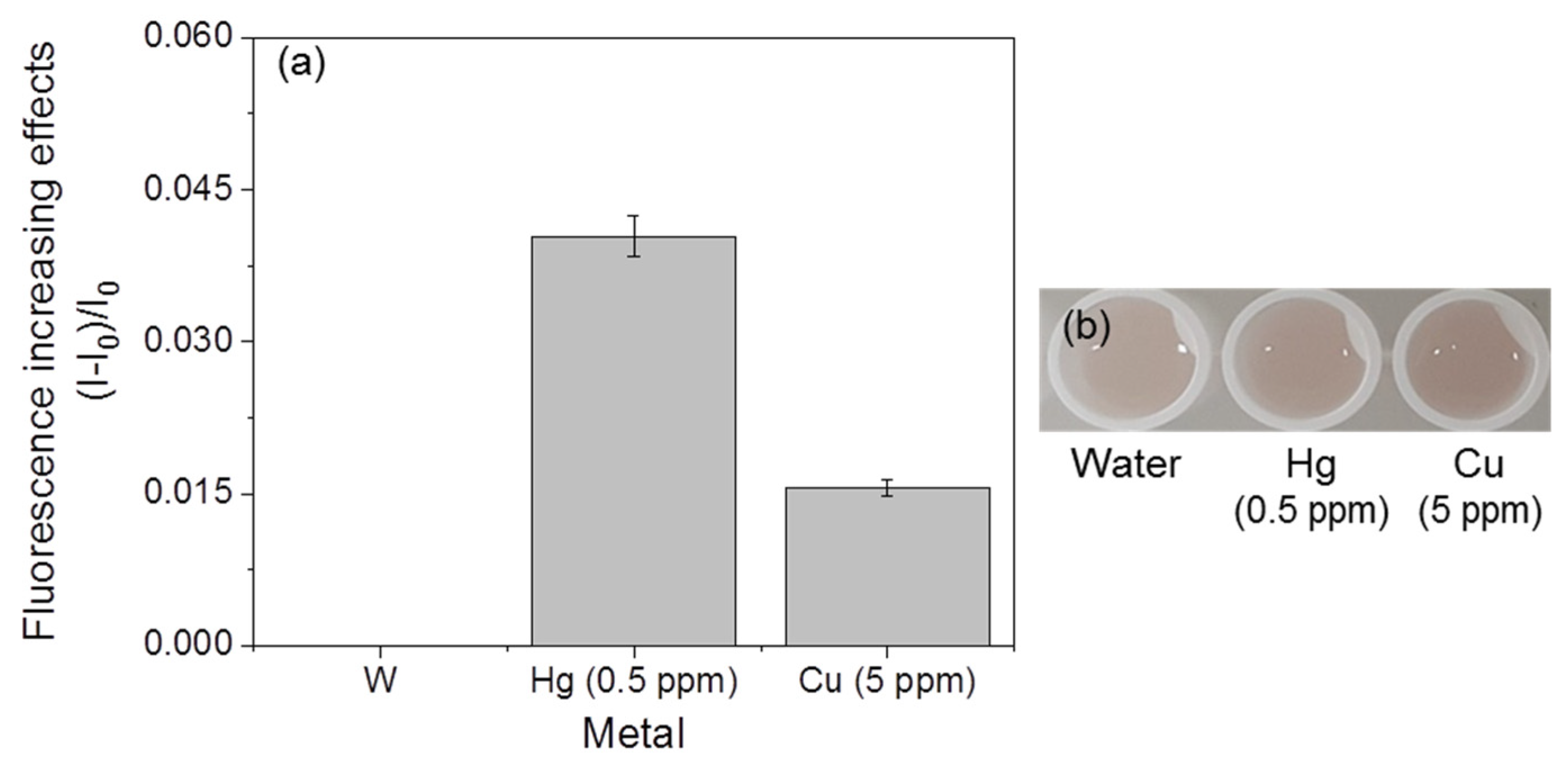
3.4. Selectivity of Fluorescent Metal-Sensing Strains
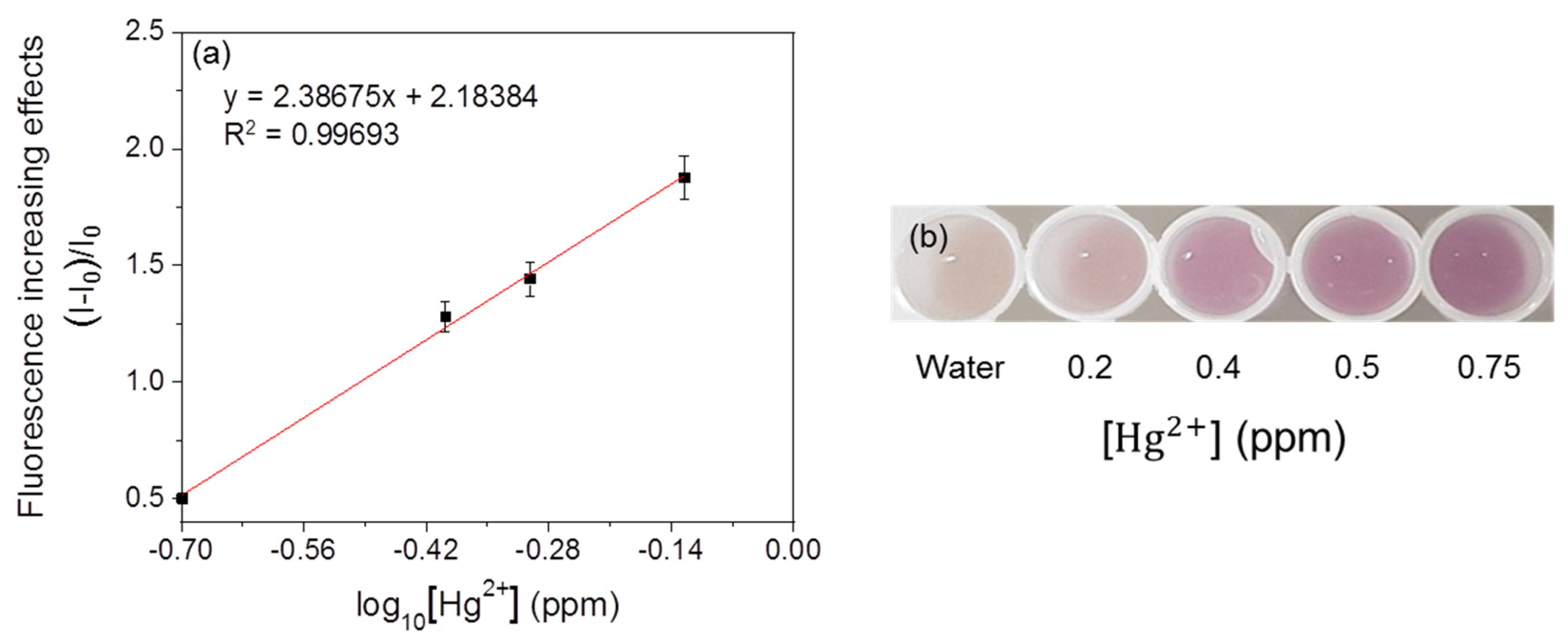
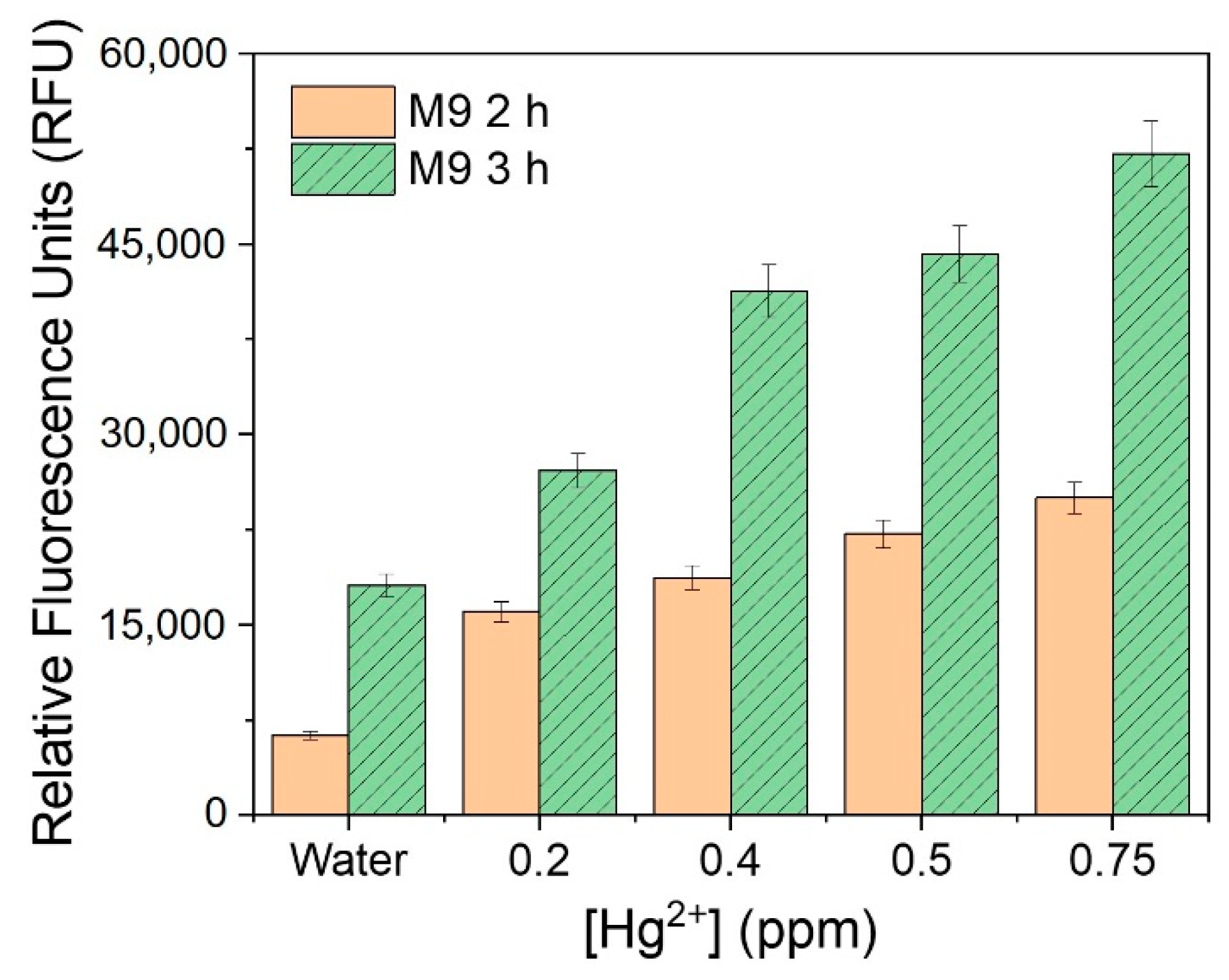
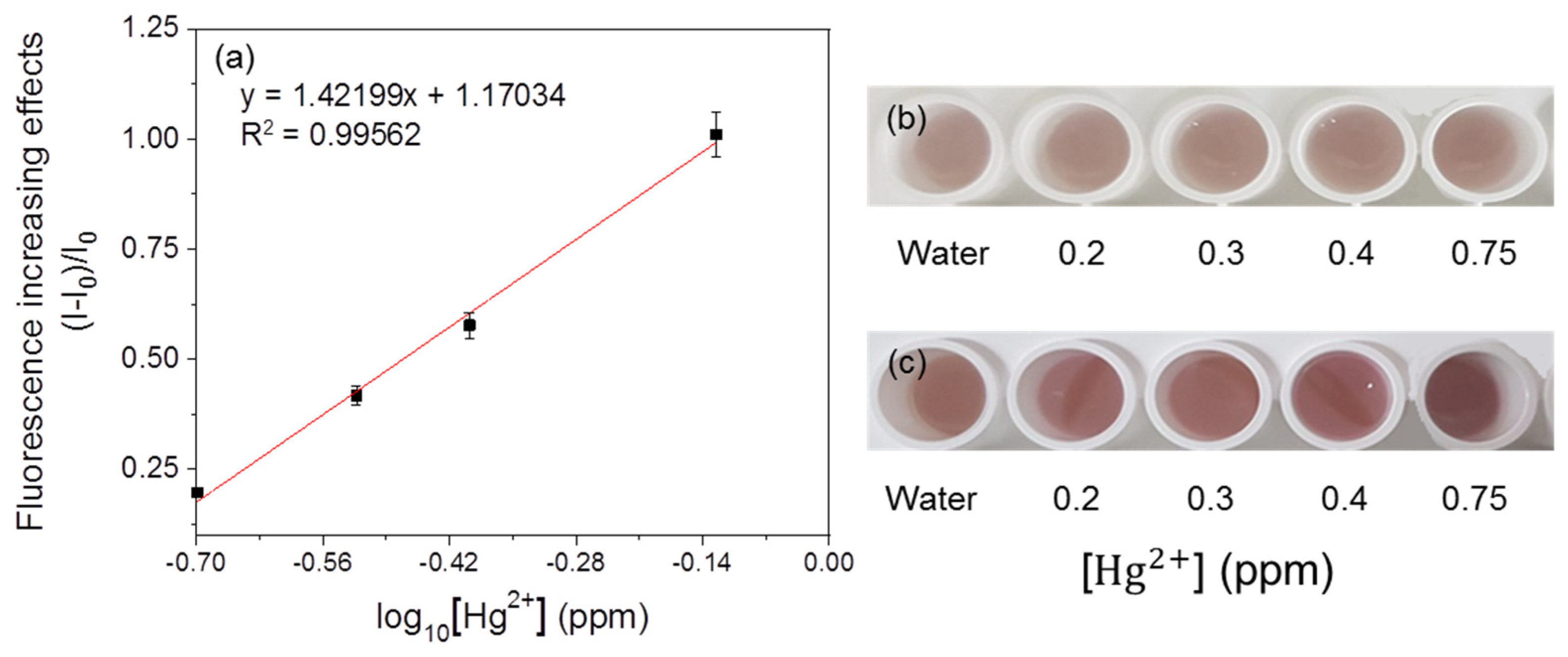
3.5. Immobilization of the Fluorescent Metal-Sensing Strain
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Duan, L.; Hu, X.; Sun, D.; Liu, Y.; Guo, Q.; Zhang, T.; Zhang, B. Rapid removal of low concentrations of mercury from wastewater using coal gasification slag. Korean J. Chem. Eng. 2020, 37, 1166–1173. [Google Scholar] [CrossRef]
- Sun, C.; Zhu, S.; Zhao, B.; Li, W.; Gao, X.; Wang, X. Effect of Land Use Conversion on Surface Soil Heavy Metal Contamination in a Typical Karst Plateau Lakeshore Wetland of Southwest China. Int. J. Environ. Res. Public Health 2020, 17, 84. [Google Scholar] [CrossRef] [Green Version]
- Chashchin, V.; Kovshov, A.A.; Thomassen, Y.; Sorokina, T.; Gorbanev, S.A.; Morgunov, B.; Gudkov, A.B.; Chashchin, M.; Sturlis, N.V.; Trofimova, A.; et al. Health Risk Modifiers of Exposure to Persistent Pollutants among Indigenous Peoples of Chukotka. Int. J. Environ. Res. Public Health 2020, 17, 128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bosch, A.C.; O’Neill, B.; Sigge, G.O.; Kerwath, S.E.; Hoffman, L.C. Heavy metals in marine fish meat and consumer health: A review. J. Sci. Food Agric. 2016, 96, 32–48. [Google Scholar] [CrossRef] [PubMed]
- Llobet, J.M.; Falcoä, G.; Casas, C.; Teixidoä, A.; Domingo, J.L. Concentrations of arsenic, cadmium, mercury, and lead in common foods and estimated daily intake by children, adolescents, adults, and seniors of Catalonia, Spain. J. Agric. Food Chem. 2003, 51, 838–842. [Google Scholar] [CrossRef]
- Lim, J.H.; Bae, D.; Fong, A. Titanium dioxide in food products: Quantitative analysis using ICP-MS and Raman spectroscopy. J. Agric. Food Chem. 2018, 66, 13533–13540. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zheng, Y.; Xu, L.; Fan, X.; Wei, N.; Jin, N.; Huang, S.; Xiao, Q.; Wua, Z. Engineered cells for selective detection and remediation of Hg2+ based on transcription factor MerR regulated cell surface displayed systems. Biochem. Eng. J. 2019, 150, 107289. [Google Scholar] [CrossRef]
- Ciotta, E.; Prosposito, P.; Tagliatesta, P.; Lorecchio, C.; Stella, L.; Kaciulis, S.; Soltani, P.; Placidi, E.; Pizzoferrato, R. Discriminating between Different Heavy Metal Ions with Fullerene-Derived Nanoparticles. Sensors 2018, 18, 1496. [Google Scholar] [CrossRef] [Green Version]
- Gui, Q.; Lawson, T.; Shan, S.; Yan, L.; Liu, Y. The Application of Whole Cell-Based Biosensors for Use in Environmental Analysis and in Medical Diagnostics. Sensors 2017, 17, 1623. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Son, J.; Lee, J.; Yoo, H.Y.; Lee, T.; Jang, M.; Oh, J.-M.; Park, C. Improved production of bacterial cellulose through investigation of effects of inhibitory compounds from lignocellulosic hydrolysates. GCB Bioenergy 2021, 13, 436–444. [Google Scholar] [CrossRef]
- Yang, H.J.; Lee, T.; Kim, J.R.; Choi, Y.-E.; Park, C. Improved production of bacterial cellulose from waste glycerol through investigation of inhibitory effects of crude glycerol-derived compounds by Gluconacetobacter xylinus. J. Ind. Eng. Chem. 2019, 75, 158–163. [Google Scholar] [CrossRef]
- Kim, H.Y.; Yoo, H.Y.; Park, N.; Kim, H.; Lee, J.; Baek, Y.; Lee, T.; Oh, J.M.; Cho, J.; Park, C. Enhanced l-Lysine into 1,5-Diaminopentane Conversion via Statistical Optimization of Whole-Cell Decarboxylation System. Polymers 2019, 11, 1372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahbub, K.R.; Krishnan, K.; Naidu, R.; Megharaj, M. Development of a whole cell biosensor for the detection of inorganic mercury. Environ. Technol. Innov. 2017, 8, 64–70. [Google Scholar] [CrossRef]
- Kang, Y.; Lee, W.; Kim, S.; Jang, G.; Kim, B.-G.; Yoon, Y. Enhancing the copper-sensing capability of Escherichia coli-based whole-cell bioreporters by genetic engineering. Appl. Microbiol. Biotechnol. 2018, 102, 1513–1521. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.; Lim, J.-W.; Kim, T. Reusable and storable whole-cell microbial biosensors with a microchemostat platform for in situ on-demand heavy metal detection. Sens. Actuators B Chem. 2018, 264, 372–381. [Google Scholar] [CrossRef]
- Kang, Y.; Lee, W.; Jang, G.; Kim, B.-G.; Yoon, Y. Modulating the sensing properties of Escherichia coli-based bioreporters for cadmium and mercury. Appl. Microbiol. Biotechnol. 2018, 102, 4863–4872. [Google Scholar] [CrossRef]
- Brown, N.L.; Stoyanov, J.V.; Kidd, S.P.; Hobman, J.L. The MerR family of transcriptional regulators. FEMS Microbiol. Rev. 2003, 27, 145–163. [Google Scholar] [CrossRef] [Green Version]
- Rademacher, C.; Masepohl, B. Copper-responsive gene regulation in bacteria. Microbiology 2012, 158, 2451–2464. [Google Scholar] [CrossRef] [PubMed]
- Sagi, E.; Hever, N.; Rosen, R.; Bartolome, A.J.; Premkumar, J.R.; Ulber, R.; Lev, O.; Scheper, T.; Belkin, S. Fluorescence and bioluminescence reporter functions in genetically modified bacterial sensor strains. Sens. Actuators B Chem. 2003, 90, 2–8. [Google Scholar] [CrossRef]
- Han, H.M.; Kim, I.J.; Yun, E.J.; Lee, J.W.; Cho, Y.; Jin, Y.S.; Kim, K.H. Overproduction of Exopolysaccharide Colanic Acid by Escherichia coli by Strain Engineering and Media Optimization. Appl. Biochem. Biotechnol. 2021, 193, 111–127. [Google Scholar] [CrossRef]
- Sciuto, E.L.; Petralia, S.; van der Meer, J.R.; Conoci, S. Miniaturized electrochemical biosensor based on whole-cell for heavy metal ions detection in water. Biotechnol. Bioeng. 2021, 118, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.H.; Kim, Y.G.; Kim, J.-S.; Jeong, Y.; Park, H.M.; Kim, J.W.; Kim, J.-E.; Kim, H.; Paek, N.-S.; Kang, C.-H. Evaluating the Cryoprotective Encapsulation of the Lactic Acid Bacteria in Simulated Gastrointestinal Conditions. Biotechnol. Bioprocess. Eng. 2020, 25, 287–292. [Google Scholar] [CrossRef]
- Junter, G.-A.; Jouenne, T. Immobilized viable microbial cells: From the process to the proteome… or the cart before the horse. Biotechnol. Adv. 2004, 22, 633–658. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Guo, Y.; Liu, M.; Song, X.; Duan, J. Porous nano-hydroxyapatites doped into substrate for thin film composite forward osmosis membrane to show high performance. Korean J. Chem. Eng. 2020, 37, 1573–1584. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.; Choi, H.; Shin, W.H.; Oh, J.-M.; Koo, S.-M.; Kim, Y.; Lee, T.; Yu, B.J.; Park, C. Development of Colorimetric Whole-Cell Biosensor for Detection of Heavy Metals in Environment for Public Health. Int. J. Environ. Res. Public Health 2021, 18, 12721. https://doi.org/10.3390/ijerph182312721
Kim Y, Choi H, Shin WH, Oh J-M, Koo S-M, Kim Y, Lee T, Yu BJ, Park C. Development of Colorimetric Whole-Cell Biosensor for Detection of Heavy Metals in Environment for Public Health. International Journal of Environmental Research and Public Health. 2021; 18(23):12721. https://doi.org/10.3390/ijerph182312721
Chicago/Turabian StyleKim, Yihyang, Hyeunseok Choi, Weon Ho Shin, Jong-Min Oh, Sang-Mo Koo, Younghun Kim, Taek Lee, Byung Jo Yu, and Chulhwan Park. 2021. "Development of Colorimetric Whole-Cell Biosensor for Detection of Heavy Metals in Environment for Public Health" International Journal of Environmental Research and Public Health 18, no. 23: 12721. https://doi.org/10.3390/ijerph182312721






