Adjusted Morbidity Groups and Intracerebral Haemorrhage: A Retrospective Primary Care Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
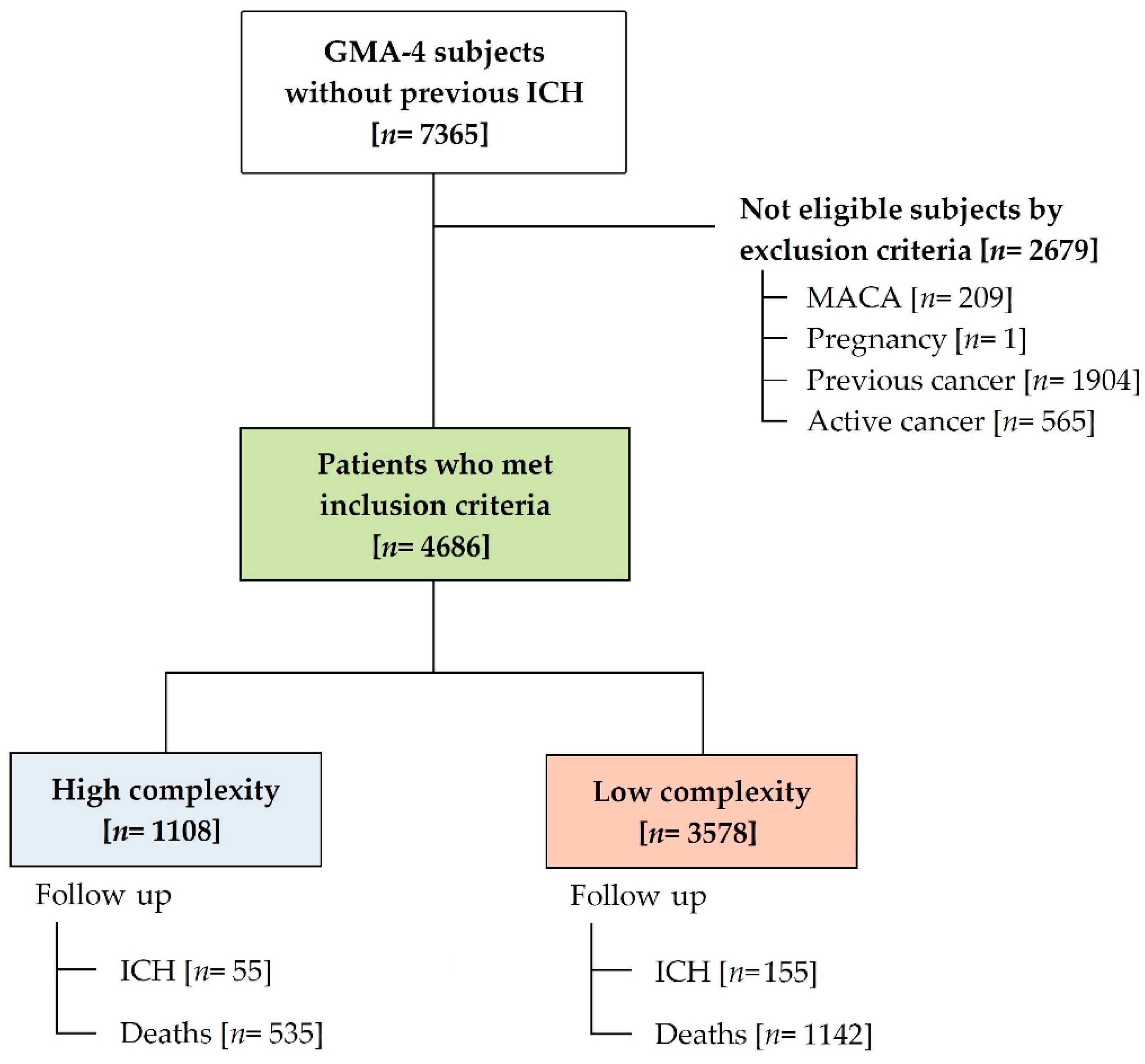
2.2. Patients
2.3. Outcomes
2.4. Covariates
2.4.1. Categorical Variables
2.4.2. Continuous Variables
2.5. Data Source
2.6. Statistical Analysis
3. Results
3.1. Characteristics of the Study Population
3.2. ICH Incidence Rates
3.3. ICH Predictive Factors
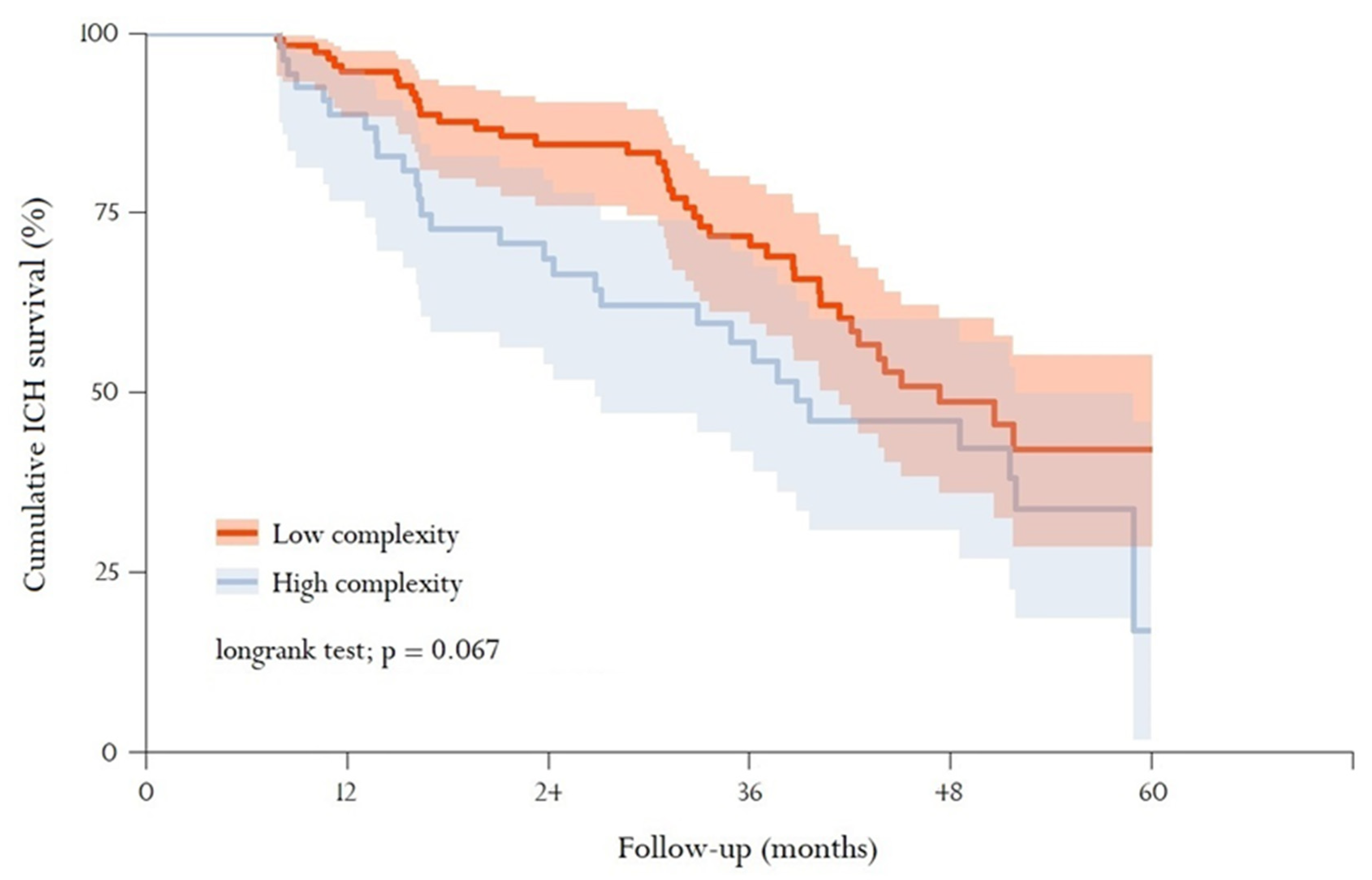
3.4. Mortality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Krishnamurthi, R.V.; Ikeda, T.; Feigin, V.L. Global, Regional and Country-Specific Burden of Ischaemic Stroke, Intracerebral Haemorrhage and Subarachnoid Haemorrhage: A Systematic Analysis of the Global Burden of Disease Study 2017. Neuroepidemiology 2020, 54, 171–179. [Google Scholar] [CrossRef]
- Lioutas, V.A.; Beiser, A.S.; Aparicio, H.J.; Himali, J.J.; Selim, M.H.; Romero, J.R.; Seshadri, S. Assessment of Incidence and Risk Factors of Intracerebral Haemorrhage Among Participants in the Framingham Heart Study Between 1948 and 2016. JAMA Neurol. 2020, 77, 1252–1260. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Multimorbidity. 2016. Available online: https://apps.who.int/iris/bitstream/handle/10665/252275/9789241511650-eng.pdf?sequence=1 (accessed on 14 May 2021).
- World Health Organization. 2018. Available online: https://www.euro.who.int/__data/assets/pdf_file/0006/364191/gpb-population-stratification-spain.pdf?ua=1 (accessed on 14 May 2021).
- Norrving, B.; Barrick, J.; Davalos, A.; Dichgans, M.; Cordonnier, C.; Guekht, A.; Kutluk, K.; Mikulik, R.; Wardlaw, J.; Richard, E.; et al. Action Plan for Stroke in Europe 2018–2030. Eur. Stroke J. 2018, 3, 309–336. [Google Scholar] [CrossRef] [Green Version]
- Stroke Alliance for Europe (SAFE). 2018. Available online: http://www.strokeeurope.eu/downloads/TheBurdenOfStrokeInEuropeReport.pdf (accessed on 11 September 2021).
- Fang, M.C.; Go, A.S.; Chang, Y.; Borowsky, L.H.; Pomernacki, N.K.; Udaltsova, N.; Singer, D.E. A new risk scheme to predict warfarin-associated haemorrhage: The ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation) Study. J. Am. Coll. Cardiol. 2011, 58, 395–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Brien, E.C.; Simon, D.N.; Thomas, L.E.; Hylek, E.M.; Gersh, B.J.; Ansell, J.E.; Kowey, P.R.; Mahaffey, K.W.; Chang, P.; Fonarow, G.C.; et al. The ORBIT bleeding score: A simple bedside score to assess bleeding risk in atrial fibrillation. Eur. Heart J. 2015, 36, 3258–3264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hijazi, Z.; Oldgren, J.; Lindbäck, J.; Alexander, J.H.; Connolly, S.J.; Eikelboom, J.W.; Ezekowitz, M.D.; Held, C.; Hylek, E.M.; Lopes, R.D.; et al. The novel biomarker-based ABC (age, biomarkers, clinical history)-bleeding risk score for patients with atrial fibrillation: A derivation and validation study. Lancet 2016, 387, 2302–2311. [Google Scholar] [CrossRef]
- Kirchhof, P.; Benussi, S.; Kotecha, D.; Ahlsson, A.; Atar, D.; Casadei, B.; Castella, M.; Diener, H.-C.; Heidbuchel, H.; Hendriks, J.; et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur. J. Cardiothorac. Surg. 2016, 50, e1–e88. [Google Scholar] [CrossRef]
- January, C.T.; Wann, L.S.; Calkins, H.; Chen, L.Y.; Cigarroa, J.E.; Cleveland, J.C., Jr.; Ellinor, P.T.; Ezekowitz, M.D.; Field, M.E.; Furie, K.L.; et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients with Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J. Am. Coll. Cardiol. 2019, 74, 104–132. [Google Scholar] [CrossRef] [PubMed]
- Pisters, R.; Lane, D.A.; Nieuwlaat, R.; de Vos, C.B.; Crijns, H.J.; Lip, G.Y. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: The Euro Heart Survey. Chest 2010, 138, 1093–1100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lip, G.Y.; Lane, D.A. Bleeding risk assessment in atrial fibrillation: Observations on the use and misuse of bleeding risk scores. J. Thromb. Haemost. 2016, 14, 1711–1714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-Henares, M.A.; Clua-Espuny, J.L.; Lorman-Carbo, B.; Fernández-Sáez, J.; Tomas, L.Q.; Muria-Subirats, E.; Ballesta-Ors, J.; Gil-Guillén, V.F. Risk of Long-Term Mortality for Complex Chronic Patients with Intracerebral Haemorrhage: A Population-Based e-Cohort Observational Study. Adv. Ther. 2020, 37, 833–846. [Google Scholar] [CrossRef] [PubMed]
- Lorman-Carbó, B.; Clua-Espuny, J.L.; Muria-Subirats, E.; Ballesta-Ors, J.; González-Henares, M.A.; Fernández-Sáez, J.; Martín-Luján, F.M.; on behalf Ebrictus Research Group. Complex chronic patients as an emergent group with high risk of intracerebral haemorrhage: An observational cohort study. BMC Geriatr. 2021, 21, 106. [Google Scholar] [CrossRef] [PubMed]
- Bases conceptuals i model d’atenció per a les persones fràgils, amb cronicitat complexa (PCC) o avançada (MACA). 2020. Generalitat de Catalunya. Departament de Salut. Available online: https://salutweb.gencat.cat/web/.content/_ambits-actuacio/Linies-dactuacio/Estrategies-de-salut/Cronicitat/Documentacio-cronicitat/arxius/Model-de-Bases-de-Cronicitat.pdf (accessed on 20 June 2021).
- White Paper on Deployment of Stratification Methods. 2016. Available online: http://assehs.eu/upload/docpublicos/20/assehs_executive-summary_en.pdf (accessed on 11 May 2021).
- Monterde, D.; Vela, E.; Clèries, M.; Grupo Colaborativo GMA. Los grupos de morbilidad ajustados: Nuevo agrupador de morbilidad poblacional de utilidad en el ámbito de la atención primaria [Adjusted morbidity groups: A new multiple morbidity measurement of use in Primary Care]. Aten. Primaria 2016, 48, 674–682. [Google Scholar] [CrossRef] [Green Version]
- Dueñas-Espín, I.; Vela, E.; Pauws, S.; Bescos, C.; Cano, I.; Cleries, M.; Contel, J.C.; Keenoy, E.D.M.; Garcia-Aymerich, J.; Gomez-Cabrero, D.; et al. Proposals for enhanced health risk assessment and stratification in an integrated care scenario. BMJ Open 2016, 6, e010301. [Google Scholar] [CrossRef] [Green Version]
- Estupiñán-Ramírez, M.; Tristancho-Ajamil, R.; Company-Sancho, M.C.; Sánchez-Janáriz, H. Comparación de modelos predictivos para la selección de pacientes de alta complejidad [Comparison of predictive models for the selection of high-complexity patients]. Gac. Sanit. 2019, 33, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Informe del proyecto de Estratificación de la Población por Grupos de Morbilidad Ajustados (GMA) en el Sistema Nacional de Salud (2014–2016). Available online: https://www.mscbs.gob.es/organizacion/sns/planCalidadSNS/pdf/informeEstratificacionGMASNS_2014-2016.pdf (accessed on 11 May 2021).
- O’Donnell, M.J.; Xavier, D.; Liu, L.; Zhang, H.; Chin, S.L.; Rao-Melacini, P.; Rangarajan, S.; Islam, S.; Pais, P.; McQueen, M.J.; et al. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): A case-control study. Lancet 2010, 376, 112–123. [Google Scholar] [CrossRef]
- Diener, H.-C.; Hankey, G.J. Primary and Secondary Prevention of Ischemic Stroke and Cerebral Haemorrhage. J. Am. Coll. Cardiol. 2020, 75, 1804–1818. [Google Scholar] [CrossRef]
- Carlsson, M.; Wilsgaard, T.; Johnsen, S.H.; Vangen-Lønne, A.M.; Løchen, M.-L.; Njølstad, I.; Mathiesen, E.B. Temporal trends in incidence and case fatality of intracerebral haemorrhage: The Tromso Study 1995–2012. Cerebrovasc. Dis. Extra. 2016, 6, 40–49. [Google Scholar] [CrossRef]
- Ögren, J.; Irewall, A.L.; Söderström, L.; Mooe, T. Serious haemorrhages after ischemic stroke or TIA—Incidence, mortality, and predictors. PLoS ONE 2018, 13, e0195324. [Google Scholar] [CrossRef] [PubMed]
- An, S.J.; Kim, T.J.; Yoon, B.W. Epidemiology, Risk Factors, and Clinical Features of Intracerebral Haemorrhage: An Update. J. Stroke 2017, 19, 3–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camacho, E.; LoPresti, M.A.; Bruce, S.; Lin, D.; Abraham, M.; Appelboom, G.; Taylor, B.; McDowell, M.; DuBois, B.; Sathe, M.; et al. The role of age in intracerebral haemorrhages. J. Clin. Neurosci. 2015, 22, 1867–1870. [Google Scholar] [CrossRef]
- Stein, M.; Misselwitz, B.; Hamann, G.F.; Scharbrodt, W.; Schummer, D.I.; Oertel, M.F. Intracerebral haemorrhage in the very old: Future demographic trends of an aging population. Stroke 2012, 43, 1126–1128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steiner, T.; Salman, R.A.-S.; Beer, R.; Christensen, H.; Cordonnier, C.; Csiba, L.; Forsting, M.; Harnof, S.; Klijn, C.; Krieger, D.; et al. European Stroke Organisation (ESO) guidelines for the management of spontaneous intracerebral haemorrhage. Int. J. Stroke 2014, 9, 840–855. [Google Scholar] [CrossRef] [PubMed]
- ASCEND Study Collaborative Group; Bowman, L.; Mafham, M.; Wallendszus, K.; Stevens, W.; Buck, G.; Barton, J.; Murphy, K.; Aung, T.; Haynes, R.; et al. Effects of Aspirin for Primary Prevention in Persons with Diabetes Mellitus. N. Eng. J. Med. 2018, 379, 1529–1539. [Google Scholar] [CrossRef]
- Gaziano, J.M.; Brotons, C.; Coppolecchia, R.; Cricelli, C.; Darius, H.; Gorelick, P.B.; Howard, G.; Pearson, T.A.; Rothwell, P.M.; Ruilope, L.M.; et al. Use of aspirin to reduce risk of initial vascular events in patients at moderate risk of cardiovascular disease (ARRIVE): A randomised, double-blind, placebo-controlled trial. Lancet 2018, 392, 1036–1046. [Google Scholar] [CrossRef]
- Piepoli, M.F.; Hoes, A.W.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A.L.; Cooney, M.T.; Corrà, U.; Cosyns, B.; Deaton, C.; et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur. Heart J. 2016, 37, 2315–2381. [Google Scholar] [CrossRef] [PubMed]
- Halvorsen, S.; Andreotti, F.; Ten Berg, J.M.; Cattaneo, M.; Coccheri, S.; Marchioli, R.; Morais, J.; Verheugt, F.W.; De Caterina, R. Aspirin therapy in primary cardiovascular disease prevention: A position paper of the European Society of Cardiology working group on thrombosis. J. Am. Coll. Cardiol. 2014, 64, 319–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meschia, J.F.; Bushnell, C.; Boden-Albala, B.; Braun, L.T.; Bravata, D.M.; Chaturvedi, S.; Creager, M.A.; Eckel, R.H.; Elkind, M.S.; Fornage, M.; et al. Guidelines for the primary prevention of stroke: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014, 45, 3754–3832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 140, e596–e646. [Google Scholar] [CrossRef]
- McNeil, J.J.; Wolfe, R.; Woods, R.L.; Tonkin, A.M.; Donnan, G.A.; Nelson, M.R.; Reid, C.M.; Lockery, J.E.; Kirpach, B.; Storey, E.; et al. Effect of Aspirin on Cardiovascular Events and Bleeding in the Healthy Elderly. N. Eng. J. Med. 2018, 379, 1509–1518. [Google Scholar] [CrossRef] [PubMed]
- Gurbel, P.; Tantry, U.; Weisman, S. A narrative review of the cardiovascular risks associated with concomitant aspirin and NSAID use. J. Thromb. Thrombolysis. 2019, 47, 16–30. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.M.; Poly, T.N.; Walther, B.A.; Yang, H.C.; Lin, M.C.; Li, Y.C. Risk of Hemorrhagic Stroke in Patients Exposed to Nonsteroidal Anti-Inflammatory Drugs: A Meta-Analysis of Observational Studies. Neuroepidemiology 2018, 51, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Poon, M.T.; Bell, S.M.; Al-Shahi Salman, R. Epidemiology of Intracerebral Haemorrhage. Front. Neurol. Neurosci. 2015, 37, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Morriss, R. Antidepressants are associated with hospital admitted intracranial bleeds in people taking other medication associated with bleeding. Evid. Based Ment. Health 2016, 19, 24. [Google Scholar] [CrossRef]
- Shin, J.Y.; Park, M.J.; Lee, S.H.; Choi, S.H.; Kim, M.H.; Choi, N.K.; Lee, J.; Park, B.J. Risk of intracranial haemorrhage in antidepressant users with concurrent use of non-steroidal anti-inflammatory drugs: Nationwide propensity score matched study. BMJ 2015, 351, h3517. [Google Scholar] [CrossRef] [Green Version]
- Muria-Subirats, E.; Clua-Espuny, J.L.; Ballesta-Ors, J.; Lorman-Carbo, B.; Lechuga-Duran, I.; Fernández-Saez, J.; Pla-Farnos, R. Incidence and Risk Assessment for Atrial Fibrillation at 5 Years: Hypertensive Diabetic Retrospective Cohort. Int. J. Environ. Res. Public Health 2020, 17, 3491. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, D. STOPP/START criteria for potentially inappropriate medications/potential prescribing omissions in older people: Origin and progress. Expert Rev. Clin. Pharmacol. 2020, 13, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Health Information Systems. 3M™ Clinical Risk Groups: Measuring Risk, Managing Care. 2012. Available online: https://multimedia.3m.com/mws/media/765833O/3m-crgs-measuring-risk-managing-care-white-paper.pdf (accessed on 20 June 2021).
- González, A.I.; Gómez, A.M.; Morales, D.R.; Pascual, M.H.; Perruca, L.S.; Herrera, I.M.; Grupo de Trabajo, G.M.; del Servicio Madrileño, G.D. Concordancia y utilidad de un sistema de estratificación para la toma de decisiones clínicas [Concordance and usefulness of a stratification system for clinical decision making]. Aten Primaria 2017, 49, 240–247. [Google Scholar] [CrossRef] [Green Version]
- Weinstock, M.J.; Uhlmann, E.J.; Zwicker, J.I. Intracranial hemorrhage in cancer patients treated with anticoagulation. Thromb. Res. 2016, 140 Suppl. 1, S60–S65. [Google Scholar] [CrossRef]
- Velander, A.J.; DeAngelis, L.M.; Navi, B.B. Intracranial hemorrhage in patients with cancer. Curr. Atheroscler. Rep. 2012, 14, 373–381. [Google Scholar] [CrossRef]

| Total (N = 4686) | Without ICH (N = 4516) | With ICH (N = 170) | p * | |
|---|---|---|---|---|
| Socio-demographic | ||||
| Age (years) | 84 (75–90) | 84 (75–90) | 84.5 (79–90) | 0.082 |
| ≥65 years | 4358 (93.0) | 4195 (92.9) | 163 (95.9) | 0.178 |
| ≥80 years | 3013 (64.3) | 2889 (64.0) | 124 (72.9) | 0.021 |
| Sex (female) | 2680 (57.2) | 2589 (57.3) | 91 (53.5) | 0.366 |
| Institutionalised | 219 (4.7) | 216 (4.8) | 3 (1.8) | 0.100 |
| Cardiovascular risk factors | ||||
| Hypertension | 3828 (81.7) | 3685 (81.6) | 143 (84.1) | 0.464 |
| Diabetes mellitus | 2048 (43.7) | 1974 (43.7) | 74 (43.5) | 1.000 |
| Hypercholesterolemia | 2823 (60.2) | 2728 (60.4) | 95 (55.9) | 0.270 |
| Comorbidities | ||||
| Cardiovascular disease | 1439 (30.7) | 1372 (30.4) | 67 (39.4) | 0.015 |
| Coronary artery disease | 932 (19.9) | 891 (19.7) | 41 (24.1) | 0.190 |
| Stroke or transient ischemic attack | 325 (6.9) | 305 (6.8) | 20 (11.8) | 0.018 |
| Peripheral artery disease | 379 (8.1) | 362 (8.1) | 17 (10.0) | 0.431 |
| Atrial fibrillation | 1053 (22.5) | 1008 (22.3) | 45 (26.5) | 0.238 |
| Heart failure | 910 (19.4) | 875 (19.4) | 35 (20.6) | 0.769 |
| Thromboembolism | 371 (7.9) | 360 (8) | 11 (6.5) | 0.571 |
| Chronic kidney disease | 1072 (22.9) | 1030 (22.8) | 42 (24.7) | 0.627 |
| Chronic liver disease | 370 (7.9) | 357 (7.9) | 13 (7.7) | 1.000 |
| Record of previous falls | 395 (8.4) | 377 (8.4) | 18 (10.6) | 0.373 |
| Cognitive impairment/dementia | 478 (10.2) | 457 (10.1) | 21 (12.4) | 0.415 |
| Toxics | ||||
| High-risk alcohol consumption | 863 (18.4) | 833 (18.4) | 30 (17.6) | 0.871 |
| Smoking | 838 (17.9) | 809 (17.9) | 29 (17.1) | 0.854 |
| Clinical data | ||||
| Systolic blood pressure (mmHg) | 134 (125–140) | 134 (125–140) | 135 (130–142) | 0.016 |
| Systolic blood pressure ≥ 160 mmHg | 121 (3.5) | 117 (3.5) | 4 (3.2) | 1.000 |
| Glycosylated haemoglobin A1c (%) | 6.2 (5.6–7.1) | 6.2 (5.6–7.0) | 6.4 (5.5–7.4) | 0.212 |
| HAS-BLED score | 0.149 | |||
| 0 | 7 (0.2) | 7 (0.2) | 0 (0.0) | |
| 1 | 251 (7.3) | 248 (7.5) | 3 (2.4) | |
| 2 | 1653 (48.2) | 1592 (48.2) | 61 (48.8) | |
| 3 | 1182 (34.5) | 1139 (34.5) | 43 (34.4) | |
| 4 | 304 (8.9) | 287 (8.7) | 17 (13.6) | |
| ≥5 | 29 (0.9) | 28 (0.9) | 1 (0.8) | |
| Medication | ||||
| Oral anticoagulant (VKA/NOACs) | 1117 (23.8) | 1069 (23.7) | 48 (28.2) | 0.201 |
| VKA | 989 (21.2) | 951 (21.1) | 38 (22.4) | 0.756 |
| NOACs | 128 (2.7) | 118 (2.6) | 10 (5.9) | 0.025 |
| Antiplatelet agents | 2593 (55.3) | 2480 (54.9) | 113 (66.5) | 0.004 |
| NSAIDs | 3814 (81.4) | 3675 (81.4) | 139 (81.8) | 0.978 |
| Statins | 3110 (66.4) | 2993 (66.3) | 117 (68.8) | 0.543 |
| SSRIs | 1702 (36.3) | 1631 (36.1) | 71 (41.8) | 0.115 |
| PPI | 4232 (90.3) | 4080 (90.3) | 152 (89.4) | 0.786 |
| GMA Multimorbidity | ||||
| 3 chronic diseases | 179 (3.8) | 173 (3.8) | 6 (3.5) | 1.000 |
| ≥4 chronic diseases | 4507 (96.2) | 4343 (96.2) | 164 (96.5) | |
| GMA Complexity | ||||
| Low complexity (levels 2–3) | 3578 (76.4) | 3463 (76.7) | 115 (67.7) | 0.037 |
| High complexity (levels 4–5) | 1108 (23.6) | 1053 (23.4) | 55 (32.4) |
| Women (N = 91) | Men (N = 79) | p * | |
|---|---|---|---|
| Socio-demographic | |||
| Age (years) | 87 (82–92) | 82 (77–88) | <0.001 |
| ≥65 years | 89 (97.8) | 74 (93.7) | 0.252 |
| ≥80 years | 72 (79.1) | 52 (65.8) | 0.076 |
| Institutionalised | 1 (1.1) | 2 (2.5) | 0.598 |
| Cardiovascular risk factors | |||
| Hypertension | 74 (81.3) | 69 (87.3) | 0.389 |
| Diabetes mellitus | 32 (35.2) | 42 (53.2) | 0.027 |
| Hypercholesterolemia | 58 (63.7) | 37 (46.8) | 0.040 |
| Comorbidities | |||
| Cardiovascular disease | 29 (31.9) | 38 (48.1) | 0.045 |
| Coronary artery disease | 18 (19.8) | 23 (29.1) | 0.215 |
| Stroke or transient ischemic attack | 9 (9.9) | 11 (13.9) | 0.565 |
| Peripheral artery disease | 5 (5.5) | 12 (15.2) | 0.065 |
| Atrial fibrillation | 25 (27.2) | 20 (25.3) | 0.886 |
| Heart failure | 17 (18.7) | 875 (19.4) | 0.769 |
| Thromboembolism | 6 (6.6) | 5 (6.3) | 1.000 |
| Chronic kidney disease | 20 (22.0) | 22 (27.8) | 0.480 |
| Chronic liver disease | 4 (4.4) | 9 (11.4) | 0.155 |
| Record of previous falls | 12 (13.2) | 6 (7.6) | 0.351 |
| Cognitive impairment/dementia | 13 (14.3) | 8 (10.1) | 0.556 |
| Toxics | |||
| High-risk alcohol consumption | 3 (3.3) | 27 (34.2) | <0.001 |
| Smoking | 4 (4.4) | 25 (31.6) | <0.001 |
| Clinical data | |||
| Systolic blood pressure (mmHg) | 135 (129–140) | 136 (130–144) | 0.140 |
| Systolic blood pressure ≥160 mmHg | 1 (1.6) | 3 (4.9) | 0.357 |
| Glycosylated haemoglobin A1c (%) | 6.2 (5.4–7.2) | 7 (5.6–7.8) | 0.032 |
| HAS-BLED score | 0.001 | ||
| 1 | 2 (3.1) | 1 (1.6) | |
| 2 | 41 (64.1) | 20 (32.8) | |
| 3 | 18 (28.1) | 25 (41.0) | |
| 4 | 3 (4.7) | 14 (23.0) | |
| ≥5 | 0 (0.0) | 1 (1.6) | |
| Medication | |||
| Oral anticoagulant (VKA/NOACs) | 22 (24.2) | 26 (32.9) | 0.275 |
| VKA | 19 (20.9) | 19 (24.1) | 0.756 |
| NOACs | 3 (3.3) | 7 (8.9) | 0.191 |
| Antiplatelet agents | 59 (64.8) | 54 (68.4) | 0.748 |
| NSAIDs | 74 (81.3) | 65 (82.3) | 1.000 |
| Statins | 66 (72.5) | 51 (64.6) | 0.341 |
| SSRIs | 45 (49.5) | 26 (32.9) | 0.043 |
| PPI | 80 (87.9) | 72 (91.1) | 0.666 |
| GMA Multimorbidity | |||
| 3 chronic diseases | 2 (2.2) | 4 (5.1) | 0.418 |
| ≥ 4 chronic diseases | 89 (97.8) | 75 (94.9) | |
| GMA Complexity | |||
| Low complexity (levels 2–3) | 64 (70.3) | 51 (64.6) | 0.291 |
| High complexity (levels 4–5) | 27 (29.7) | 28 (35.4) |
| High complexity (N = 1108) | Low complexity (N = 3578) | p * | |
|---|---|---|---|
| ICH | 55 (5.0) | 115 (3.2) | 0.009 |
| Socio-demographic | |||
| Age (years) | 86 (78–91) | 84 (75–90) | <0.001 |
| ≥65 years | 1055 (95.2) | 3303 (92.3) | 0.001 |
| ≥80 years | 785 (70.8) | 2228 (62.3) | <0.001 |
| Sex (female) | 571 (51.5) | 2109 (58.9) | <0.001 |
| Institutionalised | 67 (6.1) | 152 (4.3) | 0.017 |
| Cardiovascular risk factors | |||
| Hypertension | 936 (84.5) | 2892 (80.8) | 0.007 |
| Diabetes mellitus | 578 (52.2) | 1470 (41.1) | <0.001 |
| Hypercholesterolemia | 672 (60.6) | 2151 (60.1) | 0.778 |
| Comorbidities | |||
| Cardiovascular disease | 515 (46.5) | 924 (25.8) | <0.001 |
| Coronary artery disease | 372 (33.6) | 560 (15.7) | <0.001 |
| Stroke or transient ischemic attack | 111 (10.0) | 214 (6.0) | <0.001 |
| Peripheral artery disease | 145 (13.1) | 234 (6.5) | <0.001 |
| Atrial fibrillation | 382 (34.5) | 671 (18.8) | <0.001 |
| Heart failure | 418 (37.7) | 492 (13.8) | <0.001 |
| Thromboembolism | 112 (10.1) | 259 (7.2) | 0.002 |
| Chronic kidney disease | 95 (8.6) | 275 (7.7) | 0.371 |
| Chronic liver disease | 381 (34.4) | 691 (19.3) | <0.001 |
| Record of previous falls | 121 (10.9) | 274 (7.7) | 0.001 |
| Cognitive impairment/dementia | 133 (12.0) | 345 (9.6) | 0.027 |
| Toxics | |||
| High-risk alcohol consumption | 189 (17.1) | 674 (18.8) | 0.197 |
| Smoking | 204 (18.4) | 634 (17.7) | 0.631 |
| Clinical data | |||
| Systolic blood pressure (mmHg) | 133 (124–140) | 134 (126–140) | 0.467 |
| Systolic blood pressure ≥160 mmHg | 43 (5.1) | 78 (3.0) | 0.005 |
| Glycosylated haemoglobin A1c (%) | 6.2 (5.6–7.1) | 6.2 (5.6–7.0) | 0.098 |
| HAS-BLED score | <0.001 | ||
| 0 | 1 (0.1) | 6 (0.2) | |
| 1 | 41 (4.9) | 210 (8.1) | |
| 2 | 338 (40.3) | 1315 (50.8) | |
| 3 | 336 (40.1) | 846 (32.7) | |
| 4 | 110 (13.1) | 194 (7.5) | |
| ≥5 | 12 (1.4) | 17 (0.7) | |
| Medication | |||
| Oral anticoagulant (VKA/NOACs) | 412 (37.2) | 705 (19.7) | <0.001 |
| VKA | 369 (33.3) | 620 (17.3) | <0.001 |
| NOACs | 43 (3.9) | 85 (2.4) | 0.010 |
| Antiplatelet agents | 702 (63.4) | 1891 (52.9) | <0.001 |
| NSAIDs | 859 (77.5) | 2955 (82.6) | <0.001 |
| Statins | 807 (72.8) | 2303 (64.4) | <0.001 |
| SSRIs | 401 (36.2) | 1301 (36.4) | 0.947 |
| PPI | 1010 (91.2) | 3222 (90.1) | 0.304 |
| Population Considered | GMA-4 (n) | ICH Episodes (n) | ICH-ID (10,000 Person–Years) |
|---|---|---|---|
| Total | 4686 | 170 | 85.8 (85.4–86.2) |
| High complexity | 1108 | 55 | 129.0 (128.6–129.3) |
| <65 years | 53 | 3 | 125.8 (124.3–127.2) |
| 65–74 years | 140 | 7 | 109.9 (109.1–110.7) |
| 75–84 years | 308 | 16 | 123.8 (123.2–124.4) |
| ≥85 years | 607 | 29 | 138.3 (137.8–138.8) |
| HAS-BLED | |||
| <3 | 1681 | 64 | 78.1 (77.9–78.3) |
| ≥3 | 1515 | 61 | 95.1 (94.8–95.3) |
| OR | 95% CI | p-Value | |
|---|---|---|---|
| Sex (men) | 1.22 | (0.88–1.69) | 0.224 |
| Age (≥80 years) | 1.46 | (1.03–2.08) | 0.033 |
| Stroke or transient ischemic attack | 1.51 | (0.92–2.46) | 0.100 |
| Antiplatelet agents | 1.47 | (1.06–2.05) | 0.022 |
| SSRIs | 1.35 | (0.98–1.87) | 0.069 |
| High GMA-complexity | 1.42 | (1.02–1.99) | 0.037 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lorman-Carbó, B.; Clua-Espuny, J.L.; Muria-Subirats, E.; Ballesta-Ors, J.; González-Henares, M.A.; Pallejà-Millán, M.; Martín-Luján, F.M. Adjusted Morbidity Groups and Intracerebral Haemorrhage: A Retrospective Primary Care Cohort Study. Int. J. Environ. Res. Public Health 2021, 18, 13320. https://doi.org/10.3390/ijerph182413320
Lorman-Carbó B, Clua-Espuny JL, Muria-Subirats E, Ballesta-Ors J, González-Henares MA, Pallejà-Millán M, Martín-Luján FM. Adjusted Morbidity Groups and Intracerebral Haemorrhage: A Retrospective Primary Care Cohort Study. International Journal of Environmental Research and Public Health. 2021; 18(24):13320. https://doi.org/10.3390/ijerph182413320
Chicago/Turabian StyleLorman-Carbó, Blanca, Josep Lluis Clua-Espuny, Eulalia Muria-Subirats, Juan Ballesta-Ors, Maria Antònia González-Henares, Meritxell Pallejà-Millán, and Francisco M. Martín-Luján. 2021. "Adjusted Morbidity Groups and Intracerebral Haemorrhage: A Retrospective Primary Care Cohort Study" International Journal of Environmental Research and Public Health 18, no. 24: 13320. https://doi.org/10.3390/ijerph182413320






