Short- and Medium-Chain Chlorinated Paraffins in Polyvinylchloride and Rubber Consumer Products and Toys Purchased on the Belgian Market
Abstract
:1. Introduction
2. Materials and Methods
2.1. Standards and Reagents
2.2. Sampling and Sample Preparation
2.3. LC–MS/MS Analysis and Quantification
2.4. GC–MS Analysis and Identification of Other Plasticizers
2.5. Quality Assurance and Quality Control
2.6. Statistical Analyses
3. Results and Discussion
3.1. SCCP Concentrations in Consumer Goods
3.2. MCCPs Concentrations in Consumer Goods
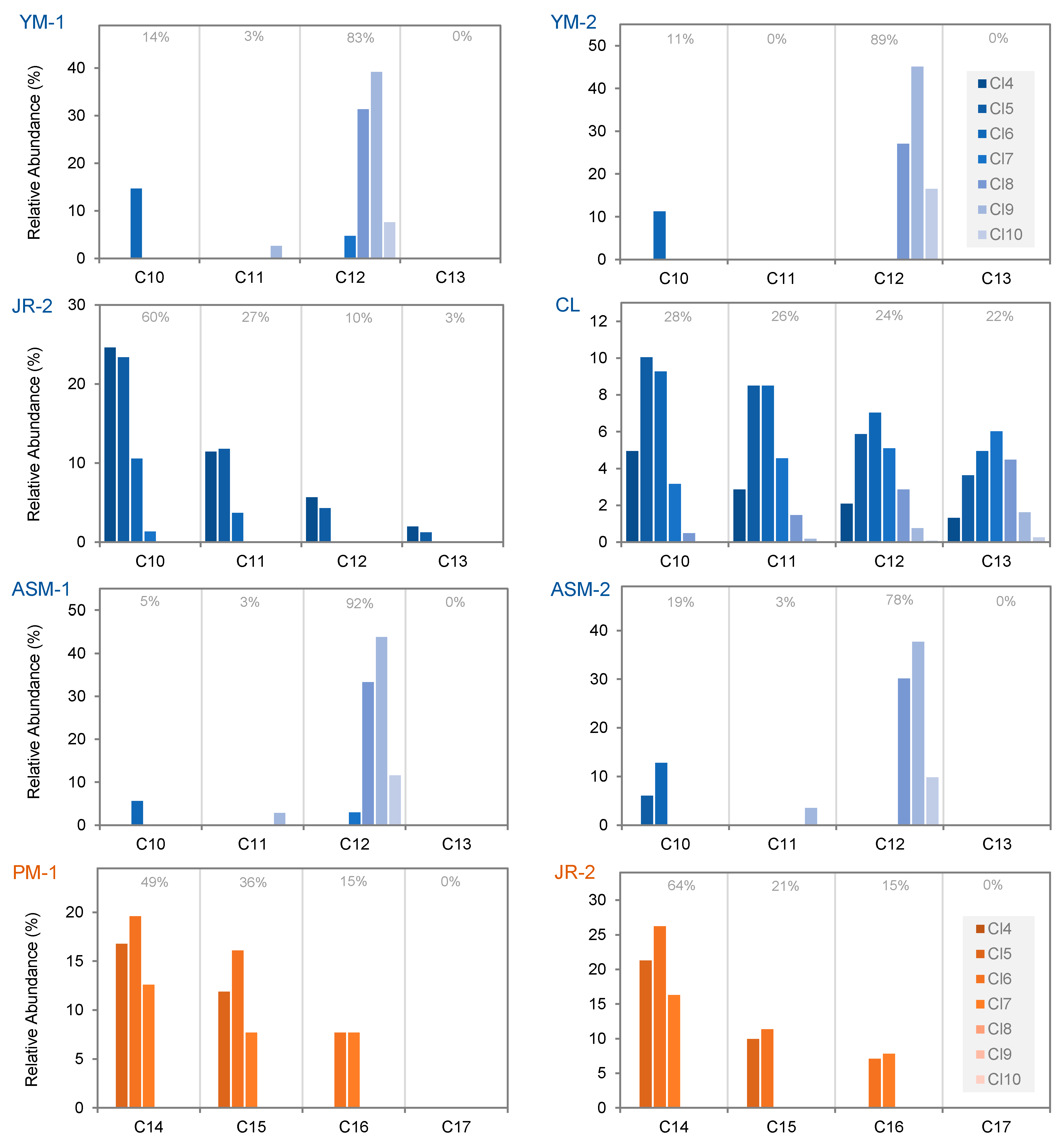
3.3. Congener Group Patterns
3.4. Presence of Other Plasticizers
3.5. Implications for Human Exposure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- USEPA. Short-Chain Chlorinated Paraffins (SCCPs) and Other Chlorinated Paraffins Action Plan. Available online: https://www.epa.gov/sites/production/files/2015-09/documents/sccps_ap_2009_1230_final.pdf (accessed on 19 October 2020).
- Van Mourik, L.M.; Gaus, C.; Leonards, P.E.G.; de Boer, J. Chlorinated paraffins in the environment: A review on their production, fate, levels and trends between 2010 and 2015. Chemosphere 2016, 155, 415–428. [Google Scholar] [CrossRef] [PubMed]
- UNEP. Draft Technical Guidelines on the Environmentally Sound Management of Wastes Consisting of, Containing or Contaminated with Short-Chain Chlorinated Paraffins; United Nations Environment Programme: Geneva, Switzerland, 2018. [Google Scholar]
- UNEP. Short-Chained Chlorinated Paraffins: Risk Profile UNEP/POPS/POPRC.11/10/Add.2., United Nations Environmental Programme Stockholm Convention on Persistent Organic Pollutants; UNEP: Geneva, Switzerland, 2015. [Google Scholar]
- UNEP. UNEP/POPS/COP.8/SC-8/11. SC-8/11, Listing of Short-Chain Chlorinated Paraffins. Available online: http://chm.pops.int/Portals/0/download.aspx?d=UNEP-POPS-COP.8-SC-8-11.English.pdf (accessed on 20 May 2020).
- Glüge, J.; Wang, Z.; Bogdal, C.; Scheringer, M.; Hungerbühler, K. Global production, use, and emission volumes of short-chain chlorinated paraffins—A minimum scenario. Sci. Total Environ. 2016, 573, 1132–1146. [Google Scholar] [CrossRef] [PubMed]
- Brandsma, S.H.; Brits, M.; Groenewoud, Q.R.; van Velzen, M.J.M.; Leonards, P.E.G.; de Boer, J. Chlorinated Paraffins in Car Tires Recycled to Rubber Granulates and Playground Tiles. Environ. Sci. Technol. 2019, 53, 7595–7603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, F.; Suzuki, G.; Michinaka, C.; Yuan, B.; Takigami, H.; de Wit, C.A. Dioxin-like activities, halogenated flame retardants, organophosphate esters and chlorinated paraffins in dust from Australia, the United Kingdom, Canada, Sweden and China. Chemosphere 2017, 168, 1248–1256. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Strid, A.; Darnerud, P.O.; de Wit, C.A.; Nyström, J.; Bergman, Å. Chlorinated paraffins leaking from hand blenders can lead to significant human exposures. Environ. Int. 2017, 109, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Glüge, J.; Schinkel, L.; Hungerbühler, K.; Cariou, R.; Bogdal, C. Environmental risks of medium-chain chlorinated paraffins (MCCPs): A review. Environ. Sci. Technol. 2018, 52, 6743–6760. [Google Scholar] [CrossRef]
- Zitko, V.; Arsenault, E. Chlorinated Paraffins: Properties, Uses and Pollution Potential; Technical Report No. 491; Environment Canada: Saint Andrews, NB, Canada, 1974.
- Potrykus, A.; Milunov, M.; Weißenbacher, J. Identification of Potentially POP-Containing Wastes and Recyclates—Derivation of Limit Values; German Federal Environment Agency: Dessau-Roßlau, Germany, 2015.
- Guida, Y.; Capella, R.; Weber, R. Chlorinated paraffins in the technosphere: A review of available information and data gaps demonstrating the need to support the Stockholm Convention implementation. Emerg. Contam. 2020, 6, 143–154. [Google Scholar] [CrossRef]
- Wang, C.; Gao, W.; Liang, Y.; Wang, Y.; Jiang, G. Concentrations and congener profiles of chlorinated paraffins in domestic polymeric products in China. Environ. Pollut. 2018, 238, 326–335. [Google Scholar] [CrossRef]
- Gallistl, C.; Sprengel, J.; Vetter, W. High levels of medium-chain chlorinated paraffins and polybrominated diphenyl ethers on the inside of several household baking oven doors. Sci. Total Environ. 2018, 615, 1019–1027. [Google Scholar] [CrossRef]
- Schinkel, L.; Bogdal, C.; Canonica, E.; Cariou, R.; Bleiner, D.; McNeill, K.; Heeb, N.V. Analysis of medium-chain and long-chain chlorinated paraffins: The urgent need for more specific analytical standards. Environ. Sci. Technol. Lett. 2018, 5, 708–717. [Google Scholar] [CrossRef]
- Zhan, F.; Zhang, H.; Wang, J.; Xu, J.; Yuan, H.; Gao, Y.; Su, F.; Chen, J. Release and Gas-Particle Partitioning Behaviors of Short-Chain Chlorinated Paraffins (SCCPs) during the Thermal Treatment of Polyvinyl Chloride Flooring. Environ. Sci. Technol. 2017, 51, 9005–9012. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Gao, W.; Liang, Y.; Jiang, Y.; Wang, Y.; Zhang, Q.; Jiang, G. Migration of chlorinated paraffins from plastic food packaging into food simulants: Concentrations and differences in congener profiles. Chemosphere 2019, 225, 557–564. [Google Scholar] [CrossRef]
- Poma, G.; McGrath, T.J.; Christia, C.; Malarvannan, G.; Covaci, A. Emerging halogenated flame retardants in the indoor environment. Compr. Anal. Chem. 2020, 88, 107–140. [Google Scholar]
- Van Mourik, L.M.; Leonards, P.E.G.; Gaus, C.; de Boer, J. Recent developments in capabilities for analysing chlorinated paraffins in environmental matrices: A review. Chemosphere 2015, 136, 259–272. [Google Scholar] [CrossRef]
- Gong, Y.; Zhang, H.; Geng, N.; Xing, L.; Fan, J.; Luo, Y.; Song, X.; Ren, X.; Wang, F.; Chen, J. Short-chain chlorinated paraffins (SCCPs) induced thyroid disruption by enhancement of hepatic thyroid hormone influx and degradation in male Sprague Dawley rats. Sci. Total Environ. 2018, 625, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhang, B.; Gao, Y.; Chen, Y.; Yin, D.; Xu, T. The chlorine contents and chain lengths influence the neurobehavioral effects of commercial chlorinated paraffins on zebrafish larvae. J. Hazard. Mater. 2019, 377, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, J.; Zhu, J.; Liu, J.; Zhang, J.; Zhao, M. Assessment of the endocrine-disrupting effects of short-chain chlorinated paraffins in in vitro models. Environ. Int. 2016, 94, 43–50. [Google Scholar] [CrossRef]
- EC. Regulation (EC) No 1272/2008 of the European Parliament and of the Council, on Classification, Labelling and Packaging of Substances and Mixtures, Amending and Repealing Directives 67/548/EEC and 1999/45/EC, and Amending Regulation (EC) No 1907/2006. Official Journal of the European Union, L 353/1. 2008. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32008R1272 (accessed on 1 December 2020).
- Ren, X.; Geng, N.; Zhang, H.; Wang, F.; Gong, Y.; Song, X.; Luo, Y.; Zhang, B.; Chen, J. Comparing the disrupting effects of short-, medium- and long-chain chlorinated Paraffins on cell viability and metabolism. Sci. Total Environ. 2019, 685, 297–307. [Google Scholar] [CrossRef]
- EC. Directive 2002/45/EC of the European Parliament and of the Council of 25 June 2002 Amending for the Twentieth Time Council Directive 76/769/EEC Relating to Restrictions on the Marketing and Use of Certain Dangerous Substances and Preparations (Short-Chain Chlorinated Paraffins). Official Journal of European Communities, L 177/21. 2002. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32002L0045&from=GA (accessed on 20 October 2020).
- EC. Regulation (EC) 2015/2030 Amending Regulation (EC) No 850/2004 of the European Parliament and of the Council on Persistent Organic Pollutants as Regards Annex I. Official Journal of the European Union, L298/1. 2015. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32015R2030&from=EN (accessed on 16 July 2020).
- UNEP. Preliminary Draft Guidance on Preparing Inventories of Short-Chain Chlorinated Paraffins (SCCPs); UNEP/POPS/COP.9/INF/19, Secretariat of the Basel, Rotterdam and Stockholm Conventions, United Nations Environment Programme: Geneva, Switzerland, 2019. [Google Scholar]
- Xu, C.; Gao, L.; Zheng, M.; Qiao, L.; Cui, L.; Wang, K.; Huang, D. Short- and medium-chain chlorinated paraffins in commercial rubber track products and raw materials. J. Hazard. Mater. 2019, 380, 120854. [Google Scholar] [CrossRef]
- Matsukami, H.; Takemori, H.; Takasuga, T.; Kuramochi, H.; Kajiwara, N. Liquid chromatography–electrospray ionization-tandem mass spectrometry for the determination of short-chain chlorinated paraffins in mixed plastic wastes. Chemosphere 2020, 244, 125531. [Google Scholar] [CrossRef]
- Shen, V.K.; Siderius, D.W.; Krekelberg, W.P.; Hatch, H.W. NIST Standard Reference Simulation Website; NIST Standard Reference Database Number 173 National Institute of Standards and Technology: Gaithersburg, MD, USA, 2020.
- Brooke, D.N.; Crookes, M.J.; Merckel, D. Environmental Risk Assessment: Long-Chain Chlorinated Paraffins; Environment Agency: Bristol, UK, 2009.
- Li, T.; Gao, S.; Ben, Y.; Zhang, H.; Kang, Q.; Wan, Y. Screening of Chlorinated Paraffins and Unsaturated Analogues in Commercial Mixtures: Confirmation of Their Occurrences in the Atmosphere. Environ. Sci. Technol. 2018, 52, 1862–1870. [Google Scholar] [CrossRef] [PubMed]
- Bogdal, C.; Alsberg, T.; Diefenbacher, P.S.; Macleod, M.; Berger, U. Fast quantification of chlorinated paraffins in environmental samples by direct injection high-resolution mass spectrometry with pattern deconvolution. Anal. Chem. 2015, 87, 2852–2860. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Bogdal, C.; Berger, U.; MacLeod, M.; Gebbink, W.A.; Alsberg, T.; de Wit, C.A. Quantifying Short-Chain Chlorinated Paraffin Congener Groups. Environ. Sci. Technol. 2017, 51, 10633–10641. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhang, H.; Su, F.; Tian, Y.; Chen, J. Environmental occurrence and distribution of short chain chlorinated paraffins in sediments and soils from the liaohe river basin, P. R. China. Environ. Sci. Technol. 2012, 46, 3771–3778. [Google Scholar] [CrossRef] [PubMed]
- Sprengel, J.; Vetter, W. NMR and GC/MS analysis of industrial chloroparaffin mixtures. Anal. Bioanal. Chem. 2020, 412, 4669–4679. [Google Scholar] [CrossRef] [PubMed]
- Tomy, G.T.; Stern, G.A.; Muir, D.C.G.; Fisk, A.T.; Cymbalisty, C.D.; Westmore, J.B. Quantifying C10-C13 polychloroalkanes in environmental samples by high-resolution gas chromatography/electron capture negative ion high-resolution mass spectrometry. Anal. Chem. 1997, 69, 2762–2771. [Google Scholar] [CrossRef]
- Reth, M.; Zencak, Z.; Oehme, M. New quantification procedure for the analysis of chlorinated paraffins using electron capture negative ionization mass spectrometry. J. Chromatogr. A 2005, 1081, 225–231. [Google Scholar] [CrossRef]
- Mézière, M.; Krätschmer, K.; Rkons, I.P.; Zacs, D.; Marchand, P.; Dervilly, G.; le Bizec, B.; Schächtele, A.; Cariou, R.; Vetter, W. Addressing Main Challenges Regarding Short- and Medium-Chain Chlorinated Paraffin Analysis Using GC/ECNI-MS and LC/ESI-MS Methods. J. Am. Soc. Mass Spectrom. 2020, 31, 1885–1895. [Google Scholar] [CrossRef]
- REACH. Annex XVII Restricted Substances List. Available online: https://echa.europa.eu/substances-restricted-under-reach/-/dislist/details/0b0236e1807e2d0d (accessed on 26 November 2020).
- Benjamin, S.; Masai, E.; Kamimura, N.; Takahashi, K.; Anderson, R.C.; Faisal, P.A. Phthalates impact human health: Epidemiological evidences and plausible mechanism of action. J. Hazard. Mater. 2017, 340, 360–383. [Google Scholar] [CrossRef]
- Wirnitzer, U.; Rickenbacher, U.; Katerkamp, A.; Schachtrupp, A. Systemic toxicity of di-2-ethylhexyl terephthalate (DEHT) in rodents following four weeks of intravenous exposure. Toxicol. Lett. 2011, 205, 8–14. [Google Scholar] [CrossRef]
- Deyo, J.A. Carcinogenicity and chronic toxicity of di-2-ethylhexyl terephthalate (DEHT) following a 2-year dietary exposure in Fischer 344 rats. Food Chem. Toxicol. 2008, 46, 990–1005. [Google Scholar] [CrossRef] [PubMed]
- Campioli, E.; Lau, M.; Papadopoulos, V. Effect of subacute and prenatal DINCH plasticizer exposure on rat dams and male offspring hepatic function: The role of PPAR-α. Environ. Res. 2019, 179, 108773. [Google Scholar] [CrossRef] [PubMed]
- Den Braver-Sewradj, S.P.; Piersma, A.; Hessel, E.V.S. An update on the hazard of and exposure to diethyl hexyl phthalate (DEHP) alternatives used in medical devices. Crit. Rev. Toxicol. 2020, 50, 650–672. [Google Scholar] [CrossRef] [PubMed]
- Christia, C.; Poma, G.; Harrad, S.; de Wit, C.A.; Sjostrom, Y.; Leonards, P.; Lamoree, M.; Covaci, A. Occurrence of legacy and alternative plasticizers in indoor dust from various EU countries and implications for human exposure via dust ingestion and dermal absorption. Environ. Res. 2019, 171, 204–212. [Google Scholar] [CrossRef]
- Rodríguez-Carmona, Y.; Ashrap, P.; Calafat, A.M.; Ye, X.; Rosario, Z.; Bedrosian, L.D.; Huerta-Montanez, G.; Vélez-Vega, C.M.; Alshawabkeh, A.; Cordero, J.F.; et al. Determinants and characterization of exposure to phthalates, DEHTP and DINCH among pregnant women in the PROTECT birth cohort in Puerto Rico. J. Expo. Sci. Environ. Epidemiol. 2020, 30, 56–69. [Google Scholar] [CrossRef]
- Babich, M.A.; Bevington, C.; Dreyfus, M.A. Plasticizer migration from children’s toys, child care articles, art materials, and school supplies. Regul. Toxicol. Pharmacol. 2020, 111, 104574. [Google Scholar] [CrossRef]
- Ionas, A.C.; Ulevicus, J.; Gómez, A.B.; Brandsma, S.H.; Leonards, P.E.G.; van de Bor, M.; Covaci, A. Children’s exposure to polybrominated diphenyl ethers (PBDEs) through mouthing toys. Environ. Int. 2016, 87, 101–107. [Google Scholar] [CrossRef]
- Yuan, B.; Tay, J.H.; Papadopoulou, E.; Haug, L.S.; Padilla-Sánchez, J.A.; de Wit, C.A. Complex Mixtures of Chlorinated Paraffins Found in Hand Wipes of a Norwegian Cohort. Environ. Sci. Technol. Lett. 2020, 7, 198–205. [Google Scholar] [CrossRef] [Green Version]
- USEPA. Exposure Factors Handbook; EPA/600/R-09/052F; USEPA: Washington, DC, USA, 2011.
- Abdallah, M.A.-E.; Pawar, G.; Harrad, S. Evaluation of 3D-human skin equivalents for assessment of human dermal absorption of some brominated flame retardants. Environ. Int. 2015, 84, 64–70. [Google Scholar] [CrossRef]
- Abdallah, M.A.; Pawar, G.; Harrad, S. Human dermal absorption of chlorinated organophosphate flame retardants; implications for human exposure. Toxicol. Appl. Pharmacol. 2016, 291, 28–37. [Google Scholar] [CrossRef]
- Frederiksen, M.; Vorkamp, K.; Jensen, N.M.; Sørensen, J.A.; Knudsen, L.E.; Sørensen, L.S.; Webster, T.F.; Nielsen, J.B. Dermal uptake and percutaneous penetration of ten flame retardants in a human skin ex vivo model. Chemosphere 2016, 162, 308–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdallah, M.A.E.; Harrad, S. Dermal contact with furniture fabrics is a significant pathway of human exposure to brominated flame retardants. Environ. Int. 2018, 118, 26–33. [Google Scholar] [CrossRef] [PubMed]

| Code | Product | Polymer | Country of Manufacture |
|---|---|---|---|
| YM-1 | Yoga mat | PVC | China |
| YM-2 | Yoga mat | PVC | n.a. |
| BB-1 | Beach ball | PVC | China |
| BB-2 | Beach ball | PVC | China |
| PM-1 | Inflatable pool mat | PVC | Hong Kong |
| PM-2 | Inflatable pool mat | PVC | China |
| PM-3 | Inflatable pool mat | PVC | China |
| PM-4 | Inflatable pool mat | PVC | United Kingdom |
| CH | Can holder | PVC | China |
| JR-1 | Jump rope | PVC | Taiwan |
| JR-2 | Jump rope | PVC | China |
| JR-3 | Jump rope | PVC | China |
| JR-4 | Jump rope | PVC | China |
| EC-1 | Electrical cable | PVC | China |
| EC-2 | Electrical cable | PVC | China |
| FF-1 | Flip flop | PVC | China |
| FF-2 | Flip flop | PVC | China |
| FF-3 | Flip flop | PVC | China |
| FF-4 | Flip flop | PVC | China |
| FF-5 | Flip flop | Rubber | Bangladesh |
| RD-1 | Rubber duck | Rubber | China |
| RD-2 | Rubber duck | Rubber | China |
| RD-3 | Rubber duck | Rubber | n.a. |
| CC-1 | Corner cover | Rubber | Denmark |
| CC-2 | Corner cover | Rubber | The Netherlands |
| CL | Clothesline | n.a. | China |
| ASM-1 | Anti-slip mat | n.a. | The Netherlands |
| ASM-2 | Anti-slip mat | n.a. | China |
| Sample | ΣC10 | ΣC11 | ΣC12 | ΣC13 | ΣSCCP | Cl% | ΣC14 | ΣC15 | ΣC16 | ΣC17 | ΣMCCP | Cl% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| YM-1 | 5.6 | 1.0 | 32 | <LOQ | 39 | 64.6 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | - |
| YM-2 | 5.0 | <LOQ | 39 | <LOQ | 44 | 65.3 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | - |
| BB-1 | 2.5 | <LOQ | 1.0 | <LOQ | 3.5 | 61.7 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | - |
| BB-2 | 1.9 | <LOQ | <LOQ | <LOQ | 1.9 | 61.0 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | - |
| PM-1 | 2.2 | <LOQ | <LOQ | <LOQ | 2.2 | 60.9 | 7.0 | 5.1 | 2.2 | <LOQ | 14 | 51.1 |
| PM-2 | 1.3 | <LOQ | <LOQ | <LOQ | 1.3 | 60.9 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | - |
| PM-3 | 7.8 | 2.2 | <LOQ | <LOQ | 10 | 57.6 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | - |
| PM-4 | 2.0 | <LOQ | <LOQ | <LOQ | 2.0 | 60.9 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | - |
| CH | 1.2 | <LOQ | <LOQ | <LOQ | 1.2 | 63.1 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | - |
| JR-1 | 1.6 | <LOQ | <LOQ | <LOQ | 1.6 | 57.3 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | - |
| JR-2 | 49 | 22 | 8.1 | 2.6 | 82 | 53.4 | 9.0 | 3.0 | 2.1 | <LOQ | 14 | 50.6 |
| JR-3 | 2.5 | <LOQ | <LOQ | <LOQ | 2.5 | 60.6 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | - |
| JR-4 | 2.5 | <LOQ | <LOQ | <LOQ | 2.5 | 61.3 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | - |
| EC-1 | 1.5 | <LOQ | <LOQ | <LOQ | 1.5 | 60.9 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | - |
| EC-2 | 1.4 | <LOQ | <LOQ | <LOQ | 1.4 | 60.9 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | - |
| FF-1 | 2.5 | <LOQ | <LOQ | <LOQ | 2.5 | 60.9 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | - |
| FF-2 | 7.3 | 1.8 | <LOQ | <LOQ | 9.1 | 57.5 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | - |
| FF-3 | 1.7 | <LOQ | <LOQ | <LOQ | 1.7 | 60.7 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | - |
| FF-4 | 1.6 | <LOQ | <LOQ | <LOQ | 1.6 | 60.9 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | - |
| FF-5 | 4.0 | <LOQ | <LOQ | <LOQ | 4.0 | 59.3 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | - |
| RD-1 | 5.6 | <LOQ | 1.8 | <LOQ | 7.4 | 60.0 | 130 | 83 | 79 | 53 | 350 | 51.0 |
| RD-2 | 4.9 | <LOQ | <LOQ | <LOQ | 4.9 | 60.8 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | - |
| RD-3 | 6.7 | <LOQ | 1.3 | <LOQ | 8.0 | 59.7 | 5.8 | <LOQ | <LOQ | <LOQ | 5.8 | 54.4 |
| CC-1 | 2.0 | <LOQ | <LOQ | <LOQ | 2.0 | 55.8 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | - |
| CC-2 | <10 | <LOQ | <LOQ | <LOQ | <LOQ | - | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | - |
| CL | 36,000 | 34,000 | 31,000 | 29,000 | 130,000 | 57.0 | 1000 | 990 | 890 | 640 | 3500 | 56.7 |
| ASM-1 | 3.2 | 1.6 | 52 | <LOQ | 57 | 65.3 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | - |
| ASM-2 | 7.5 | 1.4 | 31 | <LOQ | 40 | 64.4 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | - |
| %DF | 93 | 21 | 32 | 7.0 | 96 | - | 18 | 14 | 14 | 7.0 | 18 | - |
| Median | 2.5 | <LOQ | <LOQ | <LOQ | 2.5 | - | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | - |
| Min | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | - | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | - |
| Max | 36,000 | 34,000 | 31,000 | 29,000 | 130,000 | - | 1000 | 990 | 890 | 640 | 3500 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
McGrath, T.J.; Poma, G.; Matsukami, H.; Malarvannan, G.; Kajiwara, N.; Covaci, A. Short- and Medium-Chain Chlorinated Paraffins in Polyvinylchloride and Rubber Consumer Products and Toys Purchased on the Belgian Market. Int. J. Environ. Res. Public Health 2021, 18, 1069. https://doi.org/10.3390/ijerph18031069
McGrath TJ, Poma G, Matsukami H, Malarvannan G, Kajiwara N, Covaci A. Short- and Medium-Chain Chlorinated Paraffins in Polyvinylchloride and Rubber Consumer Products and Toys Purchased on the Belgian Market. International Journal of Environmental Research and Public Health. 2021; 18(3):1069. https://doi.org/10.3390/ijerph18031069
Chicago/Turabian StyleMcGrath, Thomas J., Giulia Poma, Hidenori Matsukami, Govindan Malarvannan, Natsuko Kajiwara, and Adrian Covaci. 2021. "Short- and Medium-Chain Chlorinated Paraffins in Polyvinylchloride and Rubber Consumer Products and Toys Purchased on the Belgian Market" International Journal of Environmental Research and Public Health 18, no. 3: 1069. https://doi.org/10.3390/ijerph18031069
APA StyleMcGrath, T. J., Poma, G., Matsukami, H., Malarvannan, G., Kajiwara, N., & Covaci, A. (2021). Short- and Medium-Chain Chlorinated Paraffins in Polyvinylchloride and Rubber Consumer Products and Toys Purchased on the Belgian Market. International Journal of Environmental Research and Public Health, 18(3), 1069. https://doi.org/10.3390/ijerph18031069








