Exposure to Phthalate and Organophosphate Esters via Indoor Dust and PM10 Is a Cause of Concern for the Exposed Saudi Population
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Solvents
2.2. Sampling and Analysis
2.3. Sample Preparation and Quantitative Analysis
2.4. Human Risk Assessment Calculations
2.5. Statistical Analysis
3. Results and Discussion
3.1. OPEs in Indoor PM10 and Dust
3.2. Phthalates in Indoor PM10 and Dust
3.3. Human Risk Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stapleton, H.M.; Klosterhaus, S.; Eagle, S.; Fuh, J.; Meeker, J.D.; Blum, A.; Webster, T.F. Detection of organophosphate flame retardants in furniture foam and US house dust. Environ. Sci. Technol. 2009, 43, 7490–7495. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Kannan, K. Comparative assessment of human exposure to phthalate esters from house dust in China and the United States. Environ. Sci. Technol. 2011, 45, 3788–3794. [Google Scholar] [CrossRef]
- Dodson, R.E.; Perovich, L.J.; Covaci, A.; van den Eede, N.; Ionas, A.C.; Dirtu, A.C.; Brody, J.G.; Rudel, R.A. After the PBDE phase-out: A broad suite of flame retardants in repeat house dust samples from California. Environ. Sci. Technol. 2012, 46, 13056–13066. [Google Scholar] [CrossRef]
- Garcia, M.; Rodriguez, I.; Cela, R. Microwave-assisted extraction of organophosphate flame retardants and plasticizers from indoor dust samples. J. Chromatogr. A 2007, 1152, 280–286. [Google Scholar] [CrossRef]
- Kamrin, M.A. Phthalate risks, phthalate regulation, and public health: A review. J. Toxicol. Environ. Health B Crit. Rev. 2009, 12, 157–174. [Google Scholar] [CrossRef]
- Abdallah, M.A.E.; Covaci, A. Organophosphate flame retardants in indoor dust from Egypt: Implications for human exposure. Environ. Sci. Technol. 2014, 48, 4782–4789. [Google Scholar] [CrossRef]
- Albar, H.M.S.A.; Ali, N.; Shahzad, K.; Ismail, I.M.I.; Rashid, M.I.; Wang, W.; Ali, L.N.; Eqani, S.A.M.A.S. Phthalate esters in settled dust of different indoor microenvironments; source of non-dietary human exposure. Microchem. J. 2017, 132, 227–232. [Google Scholar] [CrossRef]
- Adibi, J.J.; Perera, F.P.; Jedrychowski, W.; Camann, D.E.; Barr, D.; Jacek, R.; Whyatt, R.M. Prenatal exposures to phthalates among women in New York City and Krakow, Poland. Environ. Health Perspect. 2003, 111, 1719–1722. [Google Scholar] [CrossRef] [Green Version]
- Meeker, J.D.; Stapleton, H.M. House dust concentrations of organophosphate flame retardants in relation to hormone levels and semen quality parameters. Environ. Health Perspect. 2010, 118, 318–323. [Google Scholar] [CrossRef] [Green Version]
- Meeker, J.D.; Cooper, E.M.; Stapleton, H.M.; Hauser, R. Exploratory analysis of urinary metabolites of phosphorus-containing flame retardants in relation to markers of male reproductive health. Endocr. Disruptors 2013, 1, e26306. [Google Scholar] [CrossRef] [Green Version]
- Butte, W.; Heinzow, B. Pollutants in house dust as indicators of indoor contamination. Rev. Environ. Contam. Toxicol. 2002, 175, 1–46. [Google Scholar]
- Bergh, C.; Torgrip, R.; Emenius, G.; Östman, C. Organophosphate and phthalate esters in air and settled dust—A multi-location indoor study. Indoor Air 2011, 21, 67–76. [Google Scholar] [CrossRef]
- Ali, N.; Ali, L.; Mehdi, T.; Dirtu, A.C.; Al-Shammari, F.; Neels, H.; Covaci, A. Levels and profiles of organochlorines and flame retardants in car and house dust from Kuwait and Pakistan: Implication for human exposure via dust ingestion. Environ. Int. 2013, 55, 62–70. [Google Scholar] [CrossRef]
- Takeuchi, S.; Kojima, H.; Saito, I.; Jin, K.; Kobayashi, S.; Tanaka-Kagawa, T.; Jinno, H. Detection of 34 plasticizers and 25 flame retardants in indoor air from houses in Sapporo, Japan. Sci. Total Environ. 2014, 491, 28–33. [Google Scholar] [CrossRef]
- Yang, F.; Ding, J.; Huang, W.; Xie, W.; Liu, W. Particle size-specific distributions and preliminary exposure assessments of organophosphate flame retardants in office air particulate matter. Environ. Sci. Technol. 2014, 48, 63–70. [Google Scholar] [CrossRef]
- Ali, N.; Eqani, S.A.M.A.S.; Ismail, I.M.I.; Malarvannan, G.; Kadi, M.W.; Albar, H.M.S.; Rehan, M.; Covaci, A. Brominated and organophosphate flame retardants in indoor dust of Jeddah, Kingdom of Saudi Arabia: Implications for human exposure. Sci. Total Environ. 2016, 569, 269–277. [Google Scholar] [CrossRef]
- Ali, N.; Ismail, I.M.I.; Kadi, M.W.; Albar, H.M.S.A. Currently used organophosphate flame retardants determined in the settled dust of masjids and hotels of Saudi Arabia, a new insight into human health implications of dust exposure. Environ. Sci. Process. Impacts 2018, 20, 798–805. [Google Scholar] [CrossRef]
- Ali, N. Polycyclic aromatic hydrocarbons (PAHs) in indoor air and dust samples of different Saudi microenvironments; health and carcinogenic risk assessment for the general population. Sci. Total Environ. 2019, 696, 133995. [Google Scholar] [CrossRef]
- Kadi, M.W.; Ali, N.; Albar, H.M.S.A. Phthalates and polycyclic aromatic hydrocarbons (PAHs) in the indoor settled carpet dust of mosques, health risk assessment for public. Sci. Total Environ. 2018, 627, 134–140. [Google Scholar] [CrossRef]
- Bergh, C.; Luongo, G.; Wise, S.; Östman, C. Organophosphate and phthalate esters in standard reference material 2585 organic contaminants in house dust. Anal. Bioanal. Chem. 2012, 402, 51–59. [Google Scholar] [CrossRef]
- Ali, N.; Shahzad, K.; Rashid, M.I.; Shen, H.; Ismail, I.M.I.; Eqani, S.A.M.A.S. Currently used organophosphate and brominated flame retardants in the environment of China and other developing countries (2000–2016). Environ. Sci. Pollut. Res. 2017, 24, 18721–18741. [Google Scholar] [CrossRef]
- Doyi, I.N.; Isley, C.F.; Soltani, N.S.; Taylor, M.P. Human exposure and risk associated with trace element concentrations in indoor dust from Australian homes. Environ. Int. 2019, 133, 105125. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. Risk Assessment Guidance for Superfund: Volume III—Part A: Process for Conducting Probabilistic Risk Assessment; EPA 540-R-02-002; US Environmental Protection Agency: Washington, DC, USA, 2001. Available online: https://www.epa.gov/sites/production/files/2015-09/documents/rags3adt_complete.pdf (accessed on 24 December 2020).
- Australian Exposure Factor Guidance Document, Guidelines for Assessing Human Health Risks from Environmental Hazards. Department of Health. 2011. Available online: https://www.health.gov.au/internet/main/publishing.nsf/content/A12B57E41EC9F326CA257BF0001F9E7D/$File/Aust-Exposure-Factor-Guide.pdf (accessed on 24 December 2020).
- Albar, H.M.S.A.; Ali, N.; Eqani, S.A.M.A.S.N.A.; Alhakamy, N.A.; Nazar, E.; Rashid, M.I.; Shahzad, K.; Ismail, I.M.I. Trace metals in different socioeconomic indoor residential settings, implications for human health via dust exposure. Ecotoxicol. Environ. Saf. 2020, 189, 109927. [Google Scholar] [CrossRef]
- ATSDR. Toxicological Profile for di-n-butyl Phthalate. U.S. Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry. 2001. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp135.pdf (accessed on 24 December 2020).
- Mäkinen, M.S.; Mäkinen, M.R.; Koistinen, J.T.; Pasanen, A.L.; Pasanen, P.O.; Kalliokoski, P.J.; Korpi, A.M. Respiratory and dermal exposure to organophosphorus flame retardants and tetrabromobisphenol A at five work environments. Environ. Sci. Technol. 2009, 43, 941–947. [Google Scholar] [CrossRef]
- Cequier, E.; Ionas, A.C.; Covaci, A.; Marcé, R.M.; Becher, G.; Thomsen, C. Occurrence of a broad range of legacy and emerging flame retardants in indoor environments in Norway. Environ. Sci. Technol. 2014, 48, 6827–6835. [Google Scholar] [CrossRef]
- Fromme, H.; Lahrz, T.; Kraft, M.; Fembacher, L.; Mach, C.; Dietrich, S.; Burkardt, R.; Völkel, W.; Göen, T. Organophosphate flame retardants and plasticizers in the air and dust in German daycare centers and human biomonitoring in visiting children (LUPE 3). Environ. Int. 2014, 71, 158–163. [Google Scholar] [CrossRef]
- Kim, U.J.; Wang, Y.; Li, W.; Kannan, K. Occurrence of and human exposure to organophosphate flame retardants/plasticizers in indoor air and dust from various microenvironments in the United States. Environ. Int. 2019, 125, 342–349. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. Flame Retardants Used in Flexible Polyurethane Foam: An Alternatives Assessment Update. 2015. Available online: https://www.epa.gov/sites/production/files/2015-08/documents/ffr_final.pdf (accessed on 24 December 2020).
- U.S. Environmental Protection Agency. TSCA Work Plan Chemical Problem Formulation and Initial Assessment—Chlorinated Phosphate Ester Cluster Flame Retardants. 2015. Available online: www.epa.gov/assessing-and-managing-chemicals-under-tsca/tsca-work-plan-chemical-problemformulation- (accessed on 24 December 2020).
- van der Veen, I.; de Boer, J. Phosphorus flame retardants: Properties, production, environmental occurrence, toxicity, and analysis. Chemosphere 2012, 88, 1119–1153. [Google Scholar] [CrossRef]
- Hauser, R.; Calafat, A.M. Phthalates and human health. Occup. Environ. Med. 2005, 62, 806–818. [Google Scholar] [CrossRef] [Green Version]
- Rowdhwal, S.S.S.; Chen, J. Toxic effects of di-2-ethylhexyl phthalate: An overview. BioMed Res. Int. 2018, 1735068. [Google Scholar] [CrossRef] [Green Version]
- ATSDR. Toxicological Profile for Di (2-ethylhexyl) Phthalate (DEHP). 2019. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp9.pdf (accessed on 24 December 2020).
- WHO. EHC 209: Flame Retardants: Tris-(Chloro-Propyl) Phosphate and Tris-(2-Chloroethyl) Phosphate; WHO: Geneva, Switzerland, 1998. Available online: www.epa.gov/sites/production/files/2015-08/documents/ffr_final.pdf (accessed on 24 December 2020).
- Andresen, J.A.; Grundmann, A.; Bester, K. Organophosphorus flame retardants and plasticizers in surface waters. Sci. Total Environ. 2004, 332, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Butt, C.M.; Hoffman, K.; Chen, A.; Lorenzo, A.; Congleton, J.; Stapleton, H.M. Regional comparison of organophosphate flame retardant (PFR) urinary metabolites and tetrabromobenzoic acid (TBBA) in mother-toddler pairs from California and New Jersey. Environ. Int. 2016, 94, 627–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pepper, I.L.; Gerba, C.P.; Brusseau, M.L. Environmental and Pollution Science. Pollution Science Series; Academic Press: Oxford, UK, 2012; pp. 212–232. [Google Scholar]
- Health Canada. Federal Contaminated Site Risk Assessment in Canada Part I: Guidance on Human Health Preliminary Quantitative Risk Assessment (PQRA); Cat. H46-2/04-367E; Health Canada: Ottawa, ON, Canada, 2004; ISBN 0-662-38244-7.
- Lee, I.; Alakeel, R.; Kim, S.; Al-Sheikh, Y.A.; Al-Mandeel, H.; Alyousef, A.A.; Kho, Y.; Choi, K. Urinary phthalate metabolites among children in Saudi Arabia: Occurrences, risks, and their association with oxidative stress markers. Sci. Total Environ. 2019, 654, 1350–1357. [Google Scholar] [CrossRef] [PubMed]
- The European Chemicals Agency. Available online: https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/10536 (accessed on 24 December 2020).

| Parameters | Children | Adults | Reference |
|---|---|---|---|
| Ingestion rate (Ring) (mg d−1) | 200 | 100 | [18] |
| Inhalation rate (Rinh) (m3 d−1) | 7.6 | 20 | [18] |
| Exposure frequency (EF) (d year−1) | 350 | [23] | |
| Duration of exposure (ED) (years) | 2 | 30 | [24] |
| Exposed skin area (SA) (cm3) | 1600 | 6700 | [24] |
| Dust to skin adherence factor (SL) (mg cm−2) | 0.5 | [24] | |
| Dermal absorption factor (ABSd) | 0.03 | 0.001 | [23] |
| Particle emission factor (PEF) (m3 kg−1) | 1.36 × 109 | [23] | |
| Body weight (BW) (kg) | 15 | 70 | [25] |
| Lifetime (LT) (years) | 70 | [26] | |
| Conversion factor (CF) | 1 × 10−6 | [23] | |
| Dust dermal contact factor -age-adjusted (DFSadj) (mg × year kg−1 d−1) | 362.4 | [23] | |
| Dust ingestion rate age-adjusted (IR) (mg × year kg−1 d−1) | 113 | [23] | |
| Exposure time (ET) (h d−1) | 17.8 | 20 | [24] |
| Average non-carcinogenic exposure time (ATnca) | ED × 365 | [23] | |
| Average carcinogenic exposure time (ATca) | LT × 365 | [23] | |
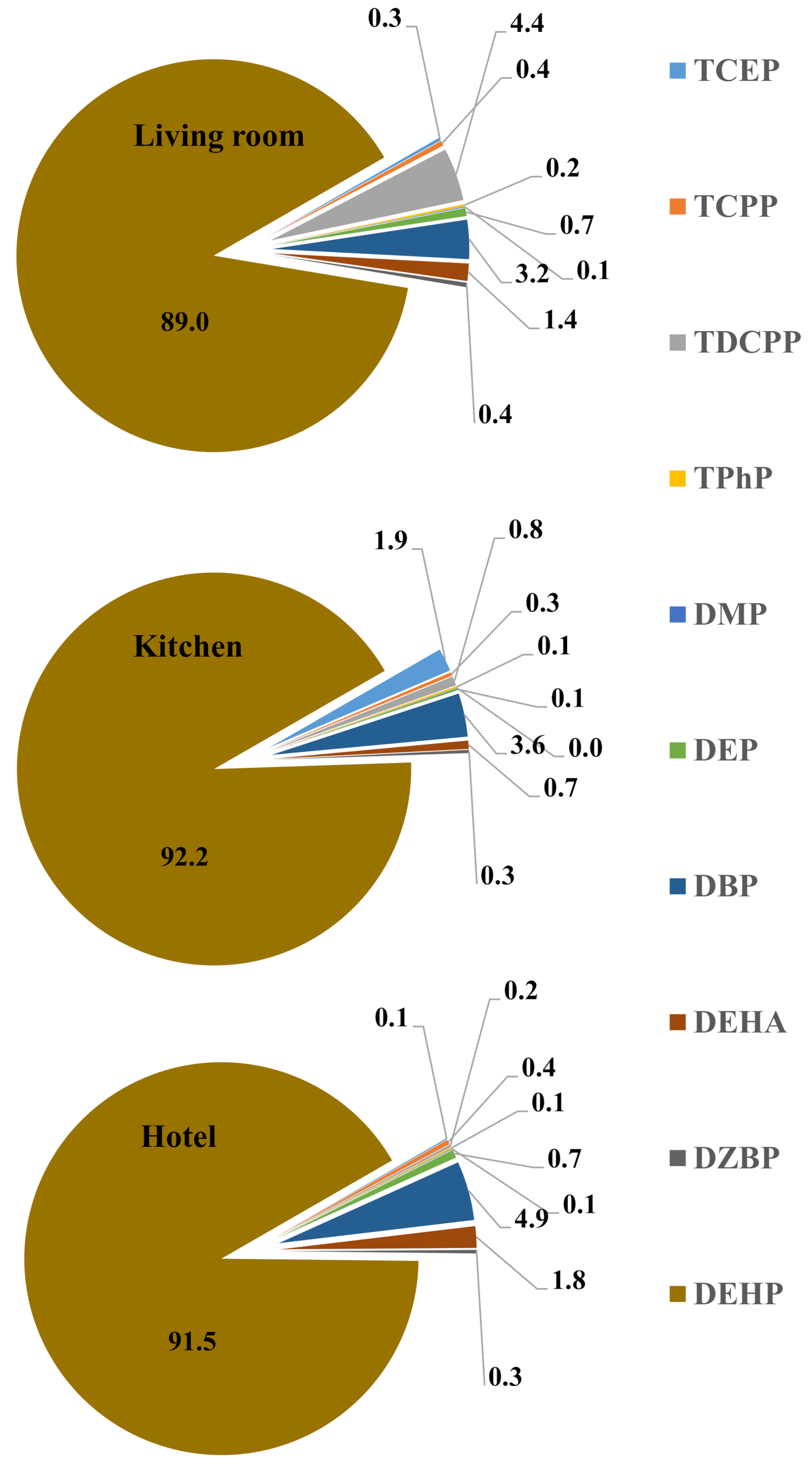
| Analytes | Kitchen (n = 15) | Hotel (n = 10) | Living room (n = 20) | Office (n = 10) | ||||
|---|---|---|---|---|---|---|---|---|
| Mean ± StDev | Median (Min–Max) | Mean ± StDev | Median (Min–Max) | Mean ± StDev | Median (Min–Max) | Mean | Median (Min–Max) | |
| TCEP | 17 ± 25 | 6 (<LOQ–2) | 6 ± 6 | 4 (LOQ–17) | 11 ± 14 | 7 (<LOQ–54) | 2 ± 4 | <LOQ (<LOQ–10) |
| TCPP | 63 ± 62 | 43 (7–180) | 55 ± 58 | 42 (7–185) | 51 ± 45 | 38 (3–155) | 13 ± 8 | 12 (3–25) |
| TDCPP | 2 ± 4 | 0.1 (<LOQ–17) | <LOQ | <LOQ (<LOQ–2) | 1 ± 1 | <LOQ (<LOQ–5) | <LOQ | <LOQ |
| TPhP | 17 ± 12 | 15 (<LOQ–38) | 8 ± 8 | 7 (<LOQ–25) | 16 ± 30 | 9 (<LOQ–138) | 5 ± 4 | 3 (2–12) |
| ∑OPEs | 97 ± 70 | 70 (9–225) | 70 ± 62 | 62 (10–210) | 78 ± 65 | 68 (8–295) | 20 ± 8 | 20 (14–30) |
| DMP | <LOQ | <LOQ | 1 ± 1 | <LOQ (<LOQ–2) | <LOQ | <LOQ | <LOQ | <LOQ |
| DEP | 14 ± 13 | 9 (3–48) | 65 ± 52 | 62 (6–150) | 17 ± 14 | 11 (3 ± 55) | 30 ± 8 | 28 (25–45) |
| DBP | 257 ± 280 | 191 (11–1130) | 275 ± 200 | 255 (1–645) | 320 ± 270 | 250 (17–1010) | 180 ± 60 | 175 (100–260) |
| DEHA | 25 ± 30 | 14 (4–90) | 9 ± 7 | 6 (2–22) | 15 ± 14 | 10 (2 ± 57) | 5 ± 2 | 4 (3–7) |
| DZBP | 44 ± 160 | 1 (<LOQ–600) | 80 ± 220 | 1 (1–625) | 30 ± 125 | 1 (<LOQ–585) | 1 ± 1 | 2 (1–2) |
| DEHP | 695 ± 340 | 610 (100–1150) | 345 ± 300 | 230 (145–1060) | 520 ± 260 | 520 (160–950) | 111 ± 15 | 110 (90–125) |
| ∑Phthalates | 1030 ± 395 | 1000 (275–1700) | 710 ± 315 | 650 (325–1180) | 895 ± 360 | 830 (395–1900) | 330 ± 70 | 315 (245–420) |
| Analytes | AC Filter Dust (n = 20) | Hotel Floor Dust (n = 10) | Household Floor Dust (n = 20) | |||
|---|---|---|---|---|---|---|
| Mean ± StDev | Median (Min–Max) | Mean ± StDev | Median (Min–Max) | Mean ± StDev | Median (Min–Max) | |
| TCEP | 16,100 ± 16,600 | 14,480 (<LOQ–58,200) | 800 ± 1190 | <LOQ (<LOQ–3370) | 2500 ± 6500 | 280 (<LOQ–25,500) |
| TCPP | 2500 ± 1800 | 1750 (670–5850) | 2900 ± 2400 | 2650 (150–7800) | 4100 ± 5400 | 1300 (25–17,300) |
| TDCPP | 6500 ± 5850 | 5950 (650–19,700) | 1600 ± 1800 | 660 (400–5650) | 42,700 ± 157,800 | 1530 (<LOQ–613,000) |
| TPhP | 1190 ± 550 | 920 (790–2500) | 900 ± 250 | 835 (670–1540) | 1730 ± 2490 | 800 (610–9000) |
| ∑OPEs | 263,00 ± 18,100 | 26,250 (3100–69,200) | 6230 ± 4350 | 4632 (1860–16,000) | 53,700 ± 162,200 | 4800 (650–615300) |
| DMP | 360 ± 70 | 340 (280–500) | 400 ± 85 | 410 (315–550) | 710 ± 1100 | 290 (200–3950) |
| DEP | 1250 ± 770 | 1140 (390–2950) | 5090 ± 3030 | 4150 (1380–9000) | 6455 ± 14,520 | 1020 (<LOQ–55,800) |
| DBP | 30,400 ± 15,600 | 26,700 (13,900–56,400) | 36,450 ± 18,650 | 41,450 (4350–57,650) | 104,500 ± 242,000 | 18,850 (<LOQ–952,300) |
| DEHA | 5850 ± 6300 | 3550 (1850–22,550) | 13,350 ± 11,900 | 7810 (400–34,900) | 14,000 ± 45,800 | 1620 (<LOQ–179,400) |
| DZBP | 2250 ± 3750 | 930 (210–12,650) | 2200 ± 2450 | 750 (230–5550) | 3650 ± 10,550 | 460 (90–41,400) |
| DEHP | 784,100 ± 459,500 | 671750 (391,900–1,844,600) | 684,400 ± 396,900 | 745,500 (61,050–1,335,500) | 871,700 ± 1,022,900 | 573,100 (350–3,550,300) |
| ∑Phthalates | 824,100 ± 467,500 | 720,550 (423,100–1,904,500) | 741,900 ± 427,500 | 776,630 (70,400–1,441,700) | 784,100 ± 986,800 | 475,900 (650–3,901,500) |
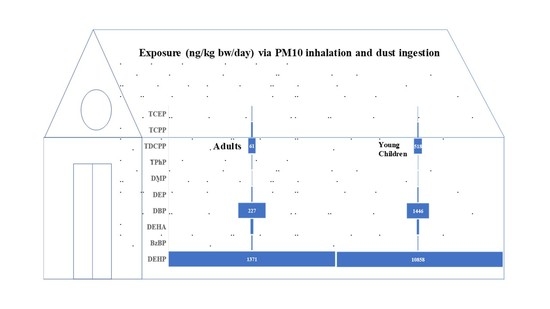
| Analytes | RfD | PM10 | Floor Dust | Inhalation + Ingestion | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Adult Hotel | Adult Households | Young Children Households | Adult Hotel | Adult Households | Young Children Households | Adult Hotel | Adult Households | Young Children Households | ||
| TCEP | 22,000 | 2 | 3 | 6 | 3 | 4 | 30 | 5 | 6 | 35 |
| TCPP | 80,000 | 13 | 12 | 30 | 5 | 6 | 49 | 18 | 18 | 80 |
| TDCPP | 15,000 | 0 | 0 | 1 | 40 | 60 | 515 | 42 | 60 | 520 |
| TPhP | 70,000 | 3 | 4 | 9 | 2 | 2 | 20 | 5 | 6 | 30 |
| DMP | 100,000 | 0 | 0 | 1 | 1 | 1 | 9 | 1 | 1 | 9 |
| DEP | 800,000 | 8 | 4 | 10 | 9 | 9 | 80 | 16 | 15 | 90 |
| DBP | 100,000 | 75 | 75 | 180 | 115 | 150 | 1265 | 190 | 225 | 1450 |
| DEHA | 600,000 | 3 | 4 | 8 | 20 | 20 | 170 | 23 | 25 | 180 |
| DZBP | 200,000 | 11 | 7 | 15 | 4 | 5 | 45 | 16 | 10 | 60 |
| DEHP | 20,000 | 110 | 125 | 290 | 1150 | 1245 | 10,550 | 1260 | 1370 | 10,850 |
| Adults | Children | |||||||
|---|---|---|---|---|---|---|---|---|
| Non-Carcinogenic | CDInca-Ingestion | CDInca-Dermal | CDInca-Inhalation | CDInca-Ingestion | CDInca-Dermal | CDInca-Inhalation | ||
| TCEP | 9.1 × 10−6 | 6.1 × 10−7 | 1.4 × 10−10 | 1.7 × 10−4 | 2.0 × 10−5 | 2.2 × 10−10 | ||
| TCPP | 1.1 × 10−5 | 7.6 × 10−7 | 5.3 × 10−10 | 2.1 × 10−4 | 2.5 × 10−5 | 8.4 × 10−10 | ||
| TDCPP | 1.7 × 10−4 | 1.2 × 10−5 | 1.6 × 10−11 | 3.2 × 10−3 | 3.8 × 10−4 | 2.5 × 10−11 | ||
| TPhP | 5.1 × 10−6 | 3.4 × 10−7 | 1.1 × 10−10 | 9.6 × 10−5 | 1.2 × 10−5 | 1.7 × 10−10 | ||
| DMP | 2.2 × 10−6 | 1.5 × 10−7 | 0.0 × 100 | 4.1 × 10−5 | 4.9 × 10−6 | 0.0 × 100 | ||
| DEP | 2.3 × 10−5 | 1.5 × 10−6 | 1.7 × 10−10 | 4.3 × 10−4 | 5.1 × 10−5 | 2.7 × 10−10 | ||
| DBP | 4.8 × 10−5 | 3.2 × 10−6 | 3.7 × 10−9 | 9.0 × 10−4 | 1.1 × 10−4 | 5.8 × 10−9 | ||
| DEHA | 5.3 × 10−5 | 3.6 × 10−6 | 1.6 × 10−10 | 9.9 × 10−4 | 1.2 × 10−4 | 2.5 × 10−10 | ||
| DZBP | 1.4 × 10−5 | 9.4 × 10−7 | 1.6 × 10−11 | 2.6 × 10−4 | 3.1 × 10−5 | 2.5 × 10−11 | ||
| DEHP | 2.1 × 10−3 | 1.4 × 10−4 | 3.6 × 10−9 | 4.0 × 10−2 | 4.8 × 10−3 | 5.7 × 10−9 | ||
| Hazardous Index | HQ-ingestion | HQ-dermal | HQ-inhalation | HQ-ingestion | HQ-dermal | HQ-inhalation | HI (Adult) | HI (Children) |
| TCEP | 4.1 × 10−4 | 2.8 × 10−5 | 6.4 × 10−9 | 7.7 × 10−3 | 9.3 × 10−4 | 1.0 × 10−8 | 4.4 × 10−4 | 8.7 × 10−3 |
| TCPP | 1.4 × 10−4 | 9.5 × 10−6 | 6.6 × 10−9 | 2.7 × 10−3 | 3.2 × 10−4 | 1.0 × 10−8 | 1.5 × 10−4 | 3.0 × 10−3 |
| TDCPP | 1.1 × 10−2 | 7.7 × 10−4 | 1.1 × 10−9 | 2.1 × 10−1 | 2.6 × 10−2 | 1.7 × 10−9 | 1.2 × 10−2 | 2.4 × 10−1 |
| TPhP | 7.3 × 10−5 | 4.9 × 10−6 | 1.6 × 10−9 | 1.4 × 10−3 | 1.6 × 10−4 | 2.5 × 10−9 | 7.8 × 10−5 | 1.5 × 10−3 |
| DMP | 2.2 × 10−5 | 1.5 × 10−6 | 0.0 × 100 | 4.1 × 10−4 | 4.9 × 10−5 | 0.0 × 100 | 2.3 × 10−5 | 4.6 × 10−4 |
| DEP | 2.9 × 10−5 | 1.9 × 10−6 | 2.1 × 10−10 | 5.3 × 10−4 | 6.4 × 10−5 | 3.3 × 10−10 | 3.0 × 10−5 | 6.0 × 10−4 |
| DBP | 4.8 × 10−4 | 3.2 × 10−5 | 3.7 × 10−8 | 9.0 × 10−3 | 1.1 × 10−3 | 5.8 × 10−8 | 5.1 × 10−4 | 1.0 × 10−2 |
| DEHA | 8.9 × 10−5 | 5.9 × 10−6 | 2.7 × 10−10 | 1.7 × 10−3 | 2.0 × 10−4 | 4.2 × 10−10 | 9.5 × 10−5 | 1.9 × 10−3 |
| DZBP | 7.0 × 10−5 | 4.7 × 10−6 | 8.1 × 10−11 | 1.3 × 10−3 | 1.6 × 10−4 | 1.3 × 10−10 | 7.5 × 10−5 | 1.5 × 10−3 |
| DEHP | 1.1 × 10−1 | 7.2 × 10−3 | 1.8 × 10−7 | 2.0 × 100 | 2.4 × 10−1 | 2.9 × 10−7 | 1.1 × 10−1 | 2.2 × 100 |
| Carcinogenic | CDIca-Ingestion | CDIca-Dermal | CDIca-Inhalation | CDIca-Ingestion | CDIca-Dermal | CDIca-Inhalation | ILRC (Adult) | ILRC (Children) |
| TCEP | 2.1 × 10−5 | 6.6 × 10−8 | 8.8 × 10−9 | 2.1 × 10−5 | 2.0 × 10−6 | 5.2 × 10−10 | 4.1 × 10−7 | 4.8 × 10−8 |
| DEHA | 1.2 × 10−4 | 3.9 × 10−7 | 1.0 × 10−8 | 1.2 × 10−4 | 1.2 × 10−5 | 6.0 × 10−10 | 1.4 × 10−7 | 1.4 × 10−8 |
| DEHP | 4.8 × 10−3 | 1.5 × 10−5 | 2.3 × 10−7 | 4.8 × 10−3 | 4.6 × 10−4 | 1.4 × 10−8 | 1.5 × 10−5 | 1.5 × 10−6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, N.; Alhakamy, N.A.; Ismail, I.M.I.; Nazar, E.; Summan, A.S.; Shah Eqani, S.A.M.A.; Malarvannan, G. Exposure to Phthalate and Organophosphate Esters via Indoor Dust and PM10 Is a Cause of Concern for the Exposed Saudi Population. Int. J. Environ. Res. Public Health 2021, 18, 2125. https://doi.org/10.3390/ijerph18042125
Ali N, Alhakamy NA, Ismail IMI, Nazar E, Summan AS, Shah Eqani SAMA, Malarvannan G. Exposure to Phthalate and Organophosphate Esters via Indoor Dust and PM10 Is a Cause of Concern for the Exposed Saudi Population. International Journal of Environmental Research and Public Health. 2021; 18(4):2125. https://doi.org/10.3390/ijerph18042125
Chicago/Turabian StyleAli, Nadeem, Nabil A. Alhakamy, Iqbal M. I. Ismail, Ehtisham Nazar, Ahmed Saleh Summan, Syed Ali Musstjab Akbar Shah Eqani, and Govindan Malarvannan. 2021. "Exposure to Phthalate and Organophosphate Esters via Indoor Dust and PM10 Is a Cause of Concern for the Exposed Saudi Population" International Journal of Environmental Research and Public Health 18, no. 4: 2125. https://doi.org/10.3390/ijerph18042125