Soil Lead (Pb) in New Orleans: A Spatiotemporal and Racial Analysis
Abstract
:1. Introduction
2. Methods
2.1. Soil Lead Data
2.2. Children’s Blood Pb Data
2.3. Interpolated Soil Pb and Blood Pb Data
2.4. Spatial–Temporal Analysis
2.5. Statistical Analyses
3. Results
Environmental Signaling Over Time and Distance
4. Discussion
4.1. Spatial–Temporal Distribution of Soil Pb and Blood Pb: Environmental Injustice for Black Populations
4.2. Potential Implications for COVID-19
4.3. Actions for Primary Prevention for Soil Pb
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stanaway, J.D.; Afshin, A.; Gakidou, E.; Lim, S.S.; Abate, D.; Abate, K.H.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; et al. Global, Regional, and National Comparative Risk Assessment of 84 Behavioural, Environmental and Occupational, and Metabolic Risks or Clusters of Risks for 195 Countries and Territories, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1923–1994. [Google Scholar] [CrossRef] [Green Version]
- Lanphear, B.P.; Rauch, S.; Auinger, P.; Allen, R.W.; Hornung, R.W. Low-Level Lead Exposure and Mortality in US Adults: A Population-Based Cohort Study. Lancet Public Health 2018, 3, e177–e184. [Google Scholar] [CrossRef]
- O’Connor, D.; Hou, D.; Ok, Y.S.; Lanphear, B.P. The Effects of Iniquitous Lead Exposure on Health. Nat. Sustain. 2020, 3, 77–79. [Google Scholar] [CrossRef]
- Sampson, R.J.; Winter, A.S. The Racial Ecology of Lead Poisoning: Toxic Inequality in Chicago Neighborhoods, 1995–2013. Du Bois Rev. Soc. Sci. Res. Race 2016, 13, 261–283. [Google Scholar] [CrossRef] [Green Version]
- Lanphear, B.P.; Weitzman, M.; Eberly, S. Racial Differences in Urban Children’s Environmental Exposures to Lead. Am. J. Public Health 1996, 86, 1460–1463. [Google Scholar] [CrossRef]
- Leech, T.G.J.; Adams, E.A.; Weathers, T.D.; Staten, L.K.; Filippelli, G.M. Inequitable Chronic Lead Exposure: A Dual Legacy of Social and Environmental Injustice. Fam. Community Health 2016, 39, 151. [Google Scholar] [CrossRef]
- Whitehead, L.S.; Buchanan, S.D. Childhood Lead Poisoning: A Perpetual Environmental Justice Issue? J. Public Health Manag. Pract. 2019, 25, S115. [Google Scholar] [CrossRef]
- Abadin, H.; Ashizawa, A.; Llados, F.; Stevens, Y.W. Toxicological Profile for Lead; U.S. Department of Health and Human Services, Public Health Service: Atlanta, GA, USA, 2020; p. 583.
- Parsons, P.J.; McIntosh, K.G. Human Exposure to Lead and New Evidence of Adverse Health Effects: Implications for Analytical Measurements. Powder Diffr. 2010, 25, 175–181. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention CDC—Lead—Blood Lead Levels in Children. Available online: https://www.cdc.gov/nceh/lead/prevention/blood-lead-levels.htm (accessed on 14 September 2019).
- Mielke, H.W.; Anderson, J.C.; Berry, K.J.; Mielke, P.W.; Chaney, R.L.; Leech, M. Lead Concentrations in Inner-City Soils As a Factor in the Child Lead Problem. Am. J. Public Health 1983, 73, 1366. [Google Scholar] [CrossRef] [Green Version]
- Mielke, H.W. Lead Dust Contaminated U.S.A. Communities: Comparison of Louisiana and Minnesota. Appl. Geochem. 1993, 8, 257–261. [Google Scholar] [CrossRef]
- Lark, R.M.; Scheib, C. Land Use and Lead Content in the Soils of London. Geoderma 2013, 209–210, 65–74. [Google Scholar] [CrossRef] [Green Version]
- Masri, S.; LeBrón, A.; Logue, M.; Valencia, E.; Ruiz, A.; Reyes, A.; Lawrence, J.M.; Wu, J. Social and Spatial Distribution of Soil Lead Concentrations in the City of Santa Ana, California: Implications for Health Inequities. Sci. Total Environ. 2020, 743, 140764. [Google Scholar] [CrossRef] [PubMed]
- Mielke, H.W.; Gonzales, C.R.; Powell, E.T.; Laidlaw, M.A.S.; Berry, K.J.; Mielke, P.W.; Egendorf, S.P. The Concurrent Decline of Soil Lead and Children’s Blood Lead in New Orleans. PNAS 2019, 116, 22058–22064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- NHGIS, N.H.G.I.S. Census Tract Boundary Files. Year 2000 and 2010 TIGER/Line Features for Jefferson, Orleans, and St. Bernard Parishes in Louisiana, USA; US Census Bureau: Sutherland, MD, USA, 2020. [Google Scholar]
- Mielke, H.W.; Gonzales, C.; Powell, E.; Mielke, P.W. Changes of Multiple Metal Accumulation (MMA) in New Orleans Soil: Preliminary Evaluation of Differences between Survey I (1992) and Survey II (2000). Int. J. Environ. Res. Public Health 2005, 2, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Jenness, J. Center of Mass (Center_of_mass.Avx) Extension for ArcView 3.x.; Jenness Enterprises: Flagstaff, AZ, USA, 2006. [Google Scholar]
- Golden Software LLC. Surfer 17® 17.1.288 (64-bit) Dec 11 2019. Golden Software LLC: 809 14th Street, Golden, CO, USA. Available online: www.goldensoftware.com (accessed on 30 June 2020).
- ESRI. ArcGIS Desktop 10; Environmental Systems Research Institute: Redlands, CA, USA, 2011. [Google Scholar]
- Reich, A.A. Transportation Efficiency. Strateg. Plan. Energy Environ. 2012, 32, 32–43. [Google Scholar] [CrossRef]
- Manson, S.; Schroeder, J.; Van Riper, D.; Ruggles, S. Integrated Public Use Microdata Series (IPUMS) National Historical Geographic Information System: Version 14.0 [Database]; IPUMS: Minneapolis, MN, USA, 2019. [Google Scholar]
- Cade, B.S.; Richard, J. User Manual for Blossom Statistical Software; U.S. Geological Survey: Reston, VA, USA, 2001; p. 128. [Google Scholar]
- Berry, K.J.; Johnston, J.E.; Mielke, P.W. A Chronicle of Permutation Statistical Methods: 1920–2000, and Beyond; Springer International Publishing: Cham, Switzerland, 2014; ISBN 978-3-319-02743-2. [Google Scholar]
- Mielke, P.W., Jr.; Berry, K.J. Permutation Methods: A Distance Function Approach. In Springer Series in Statistics, 2nd ed.; Springer: New York, NY, USA, 2007; ISBN 978-0-387-69811-3. [Google Scholar]
- Berry, K.J.; Mielke, P.W. A Generalization of Cohen’s Kappa Agreement Measure to Interval Measurement and Multiple Raters. Educ. Psychol. Meas. 1988, 48, 921–933. [Google Scholar] [CrossRef]
- IBM Corp. IBM SPSS Statistics for Macintosh; IBM Corporation: Armonk, NY, USA, 2015. [Google Scholar]
- CDC. Centers for Disease Control and Prevention (CDC) Response to Advisory Committee on Childhood Lead Poisoning Prevention Recommendations in “Low Level Lead Exposure Harms Children: A Renewed Call of Primary Prevention”. Department of Health and Human Services, CDC; 2012. Available online: http://www.cdc.gov/nceh/lead/acclpp/cdc_response_lead_exposure_recs.pdf (accessed on 30 June 2020).
- Bullard, R.D. Confronting Environmental Racism: Voices from the Grassroots; South End Press: Boston, MA, USA, 1993; ISBN 978-0-89608-446-9. [Google Scholar]
- Campanella, R.; Mielke, H.W. Human Geography of New Orleans’ High-Lead Geochemical Setting. Environ. Geochem. Health 2008, 30, 531–540. [Google Scholar] [CrossRef]
- McClintock, N. A Critical Physical Geography of Urban Soil Contamination. Geoforum 2015, 65, 69–85. [Google Scholar] [CrossRef] [Green Version]
- Mielke, H.W.; Laidlaw, M.A.S.; Gonzales, C.R. Estimation of Leaded (Pb) Gasoline’s Continuing Material and Health Impacts on 90 US Urbanized Areas. Environ. Int. 2011, 37, 248–257. [Google Scholar] [CrossRef]
- Pellow, D. Garbage Wars: The Struggle for Environmental Justice in Chicago; MIT Press: Cambridge, MA, USA, 2002; ISBN 978-0-262-66187-4. [Google Scholar]
- Massey, D.S. Segregation and Stratification: A Biosocial Perspective. Du Bois Rev. 2004, 1, 7–25. [Google Scholar] [CrossRef]
- Morello-Frosch, R.; Lopez, R. The Riskscape and the Color Line: Examining the Role of Segregation in Environmental Health Disparities. Environ. Res. 2006, 102, 181–196. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.L.; Homa, D.M.; Meyer, P.A.; Brody, D.J.; Caldwell, K.L.; Pirkle, J.L.; Brown, M.J. Trends in Blood Lead Levels and Blood Lead Testing Among US Children Aged 1 to 5 Years, 1988–2004. Pediatrics 2009, 123, e376–e385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stretesky, P.B. The Distribution of Air Lead Levels across U.S. Counties: Implications for the Production of Racial Inequality. Sociol. Spectr. 2003, 23, 91–118. [Google Scholar] [CrossRef]
- Laidlaw, M.A.S.; Filippelli, G.M.; Sadler, R.C.; Gonzales, C.R.; Ball, A.S.; Mielke, H.W. Children’s Blood Lead Seasonality in Flint, Michigan (USA), and Soil-Sourced Lead Hazard Risks. Int. J. Environ. Res. Public Health 2016, 13, 358. [Google Scholar] [CrossRef] [Green Version]
- Pulido, L. Flint, Environmental Racism, and Racial Capitalism. Capital. Nat. Soc. 2016, 27, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Markowitz, G.; Rosner, D. Lead Wars: The Politics of Science and the Fate of America’s Children; University of California Press: Berkeley, CA, USA, 2013. [Google Scholar]
- Benfer, E.A. Contaminated Childhood: How The United States Failed to Prevent the Chronic Lead Poisoning of Low-Income Children and Communities of Color. Harv. Environ. Law Rev. 2017, 41, 70. [Google Scholar]
- Bellinger, D.C. Childhood Lead Exposure and Adult Outcomes. JAMA 2017, 317, 1219–1220. [Google Scholar] [CrossRef]
- Guan, W.; Liang, W.; Zhao, Y.; Liang, H.; Chen, Z.; Li, Y.; Liu, X.; Chen, R.; Tang, C.; Wang, T.; et al. Comorbidity and Its Impact on 1590 Patients with COVID-19 in China: A Nationwide Analysis. Eur. Respir. J. 2020, 55. [Google Scholar] [CrossRef] [Green Version]
- Ebhardt, T.; Remondini, C.; Bertacche, M. 99% of Those Who Died From Virus Had Other Illness, Italy Says. 18 March 2020. Available online: https://thebridgelifeinthemix.info/wp-content/uploads/2020/04/SB-7.-www_bloomberg_com_news_articles_2020_03_18_99_of_those_who_d.pdf (accessed on 30 June 2020).
- Fox, S.E.; Akmatbekov, A.; Harbert, J.L.; Li, G.; Quincy Brown, J.; Vander Heide, R.S. Pulmonary and Cardiac Pathology in African American Patients with COVID-19: An Autopsy Series from New Orleans. Lancet Respir. Med. 2020, 8, 681–686. [Google Scholar] [CrossRef]
- Apostoli, P.; Maranelli, G.; Dei Cas, L.; Micciolo, R. Blood Lead and Blood Pressure: A Cross Sectional Study in a General Population Group. Cardiologia 1990, 35, 597–603. [Google Scholar]
- De Almeida Lopes, A.C.B.; Silbergeld, E.K.; Navas-Acien, A.; Zamoiski, R.; da Cunha Martins, A., Jr.; Camargo, A.E.I.; Urbano, M.R.; Mesas, A.E.; Paoliello, M.M.B. Association between Blood Lead and Blood Pressure: A Population-Based Study in Brazilian Adults. Environ. Health 2017, 16, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menke, A.; Muntner, P.; Batuman, V.; Silbergeld, E.K.; Guallar, E. Blood Lead Below 0.48 Μmol/L (10 Μg/DL) and Mortality Among US Adults. Circulation 2006, 114, 1388–1394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schober, S.E.; Mirel Lisa, B.; Graubard Barry, I.; Brody Debra, J.; Flegal Katherine, M. Blood Lead Levels and Death from All Causes, Cardiovascular Disease, and Cancer: Results from the NHANES III Mortality Study. Environ. Health Perspect. 2006, 114, 1538–1541. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.K.; Chang, Y.S.; Ahn, C.W. Effects of Blood Lead Levels on Airflow Limitations in Korean Adults: Findings from the 5th KNHNES 2011. Environ. Res. 2015, 136, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Pugh Smith, P.; Nriagu, J.O. Lead Poisoning and Asthma among Low-Income and African American Children in Saginaw, Michigan. Environ. Res. 2011, 111, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Muntner, P.; He, J.; Vupputuri, S.; Coresh, J.; Batuman, V. Blood Lead and Chronic Kidney Disease in the General United States Population: Results from NHANES III. Kidney Int. 2003, 63, 1044–1050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pollack, A.Z.; Mumford, S.L.; Mendola, P.; Perkins, N.J.; Rotman, Y.; Wactawski-Wende, J.; Schisterman, E.F. Kidney Biomarkers Associated with Blood Lead, Mercury, and Cadmium in Premenopausal Women: A Prospective Cohort Study. J. Toxicol. Environ. Health Part A 2015, 78, 119–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boskabady, M.; Marefati, N.; Farkhondeh, T.; Shakeri, F.; Farshbaf, A.; Boskabady, M.H. The Effect of Environmental Lead Exposure on Human Health and the Contribution of Inflammatory Mechanisms, a Review. Environ. Int. 2018, 120, 404–420. [Google Scholar] [CrossRef]
- Mishra, K.P. Lead Exposure and Its Impact on Immune System: A Review. Toxicol. In Vitro 2009, 23, 969–972. [Google Scholar] [CrossRef]
- Roper, W.L.; Houk, V.N.; Falk, H.; Binder, S. Preventing Lead Poisoning in Young Children: A Statement by the Centers for Disease Control, October 1991; Centers for Disease Control: Atlanta, GA, USA, 1991. [Google Scholar]
- Aghdam, K.A.; Zand, A.; Sanjari, M.S. Bilateral Optic Disc Edema in a Patient with Lead Poisoning. J. Ophthalmic Vis. Res. 2019, 14, 513–517. [Google Scholar] [CrossRef]
- Laidlaw, M.A.S.; Filippelli, G.M.; Brown, S.; Paz-Ferreiro, J.; Reichman, S.M.; Netherway, P.; Truskewycz, A.; Ball, A.S.; Mielke, H.W. Case Studies and Evidence-Based Approaches to Addressing Urban Soil Lead Contamination. Appl. Geochem. 2017, 83, 14–30. [Google Scholar] [CrossRef] [Green Version]
- Ottesen, R.T.; Alexander, J.; Langedal, M.; Haugland, T.; Høygaard, E. Soil Pollution in Day-Care Centers and Playgrounds in Norway: National Action Plan for Mapping and Remediation. Environ. Geochem. Health 2008, 30, 623–637. [Google Scholar] [CrossRef] [PubMed]
- Egendorf, S.P.; Cheng, Z.; Deeb, M.; Flores, V.; Paltseva, A.; Walsh, D.; Groffman, P.; Mielke, H.W. Constructed Soils for Mitigating Lead (Pb) Exposure and Promoting Urban Community Gardening: The New York City Clean Soil Bank Pilot Study. Landsc. Urban Plan. 2018, 175, 184–194. [Google Scholar] [CrossRef]
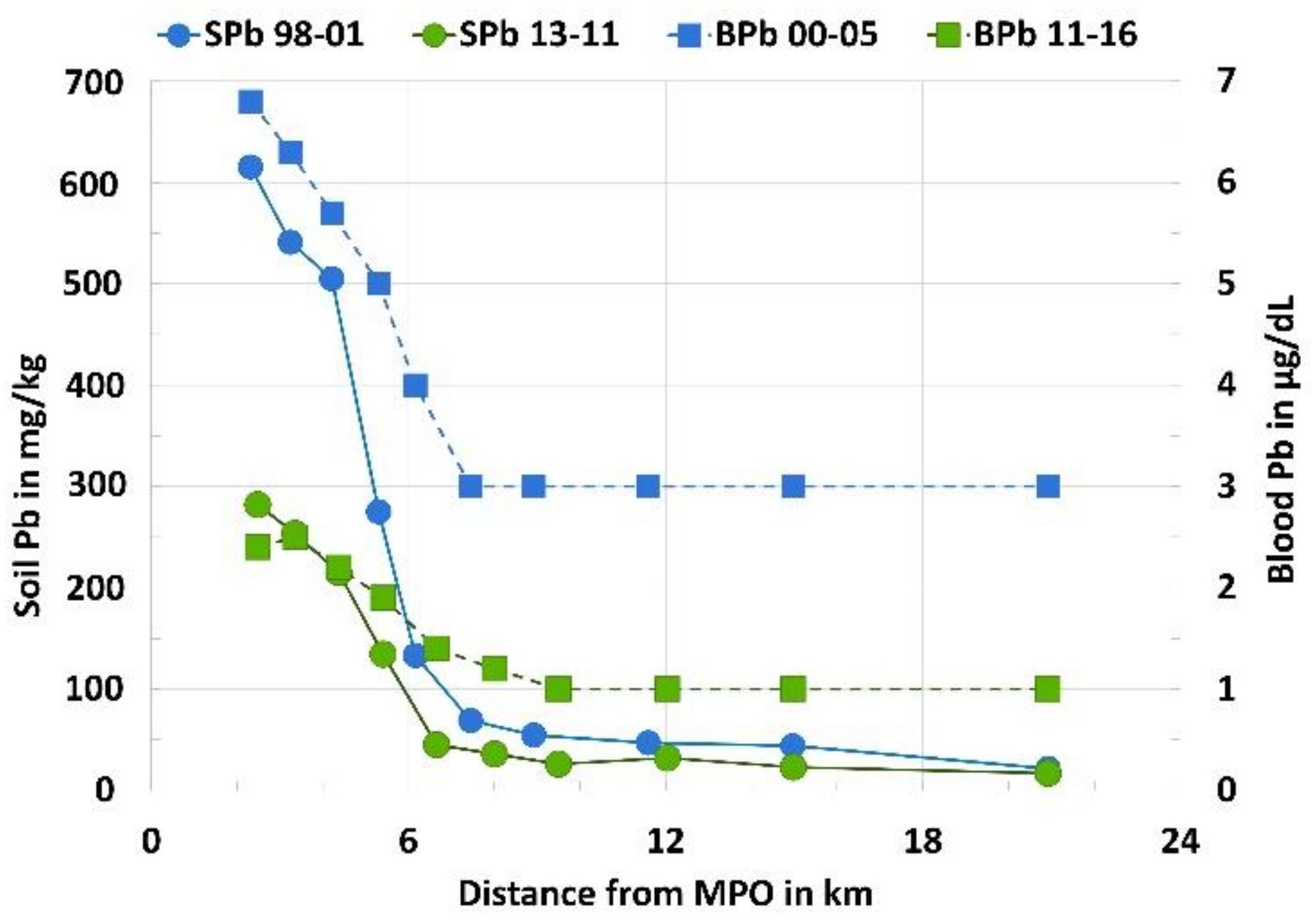
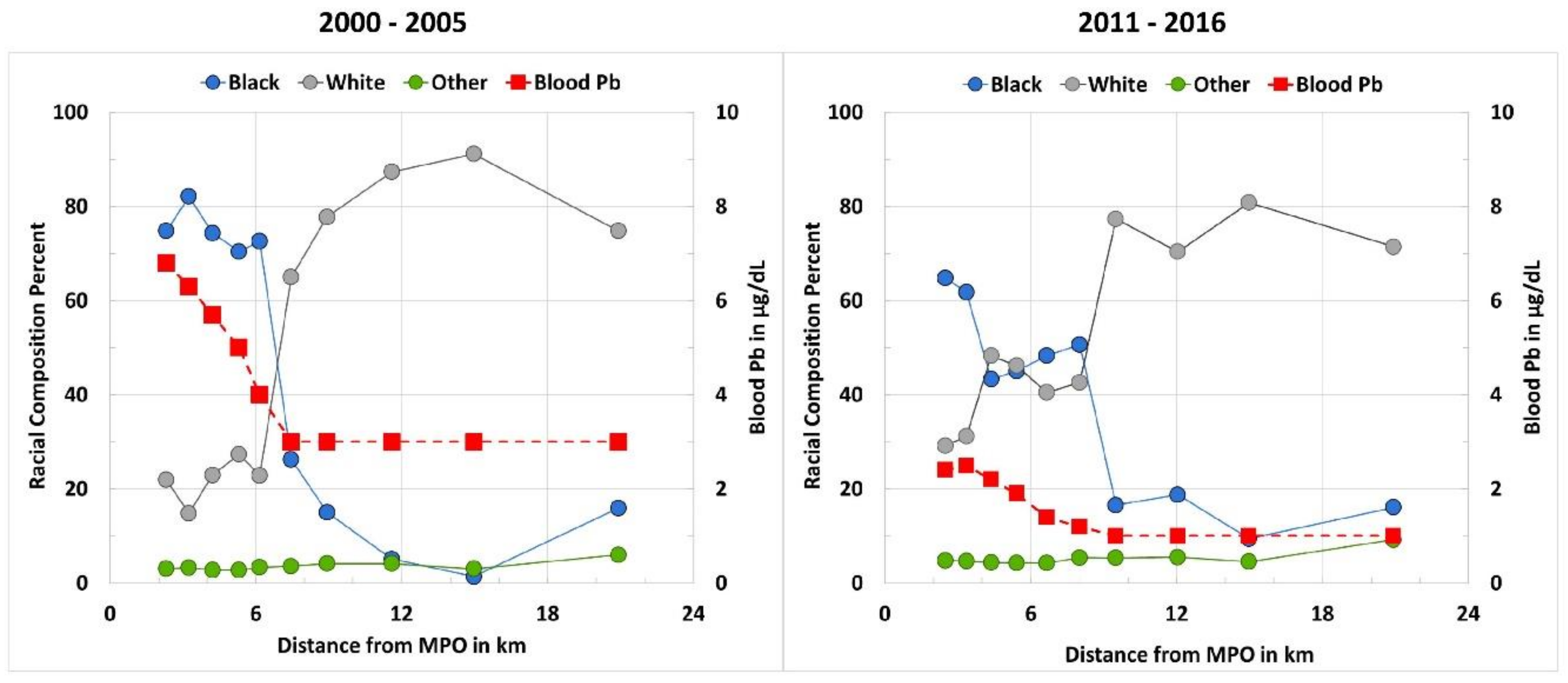
| Dist. MPO km | Area km2 | Pop.km2 | % White | % Black | % Other | SPb mg/kg | BPb µg/dL | Household Income US$ | |
|---|---|---|---|---|---|---|---|---|---|
| 2000 | |||||||||
| NEAR | |||||||||
| N of Census Tracts | 147 | 147 | 147 | 147 | 147 | 147 | 147 | 147 | 147 |
| Minimum | 0.0 | 0.1 | 294 | 0.0 | 0.9 | 0.3 | 35 | 3.0 | 4621 |
| 50% | 3.8 | 0.6 | 3892 | 23.0 | 72.7 | 3.1 | 410 | 5.7 | 21,981 |
| Maximum | 6.2 | 6.4 | 15,527 | 97.4 | 99.5 | 24.8 | 1774 | 10.6 | 109,721 |
| FAR | |||||||||
| N of Census Tracts | 147 | 147 | 147 | 147 | 147 | 147 | 147 | 147 | 146 |
| Minimum | 6.2 | 0.5 | 22 | 0.0 | 0.0 | 0.0 | 6 | 2.1 | 16,250 |
| 50% | 10.2 | 1.6 | 2388 | 78.1 | 12.7 | 4.2 | 44 | 3.0 | 37,919 |
| Maximum | 20.9 | 18.8 | 6476 | 98.7 | 100.0 | 21.5 | 237 | 5.0 | 146,158 |
| p-Value | 2.5 × 10−53 | 1.7 × 10−26 | 1.4 × 10−19 | 5.2 × 10−14 | 1.8 × 10−14 | 4.3 × 10−5 | 4.8 × 10−40 | 2.1 × 10−42 | 2.4 × 10−22 |
| Mielke’s R | 0.425 | 0.143 | 0.127 | 0.130 | 0.138 | 0.028 | 0.349 | 0.411 | 0.149 |
| 2015 | |||||||||
| NEAR | |||||||||
| N of Census Tracts | 143 | 143 | 143 | 143 | 143 | 143 | 143 | 143 | 141 |
| Minimum | 0.4 | 0.16 | 307 | 0.0 | 0.4 | 0.0 | 16 | 1.0 | 8738 |
| 50% | 3.9 | 0.64 | 2813 | 40.8 | 51.5 | 4.4 | 187 | 2.1 | 30,917 |
| Maximum | 6.7 | 5.05 | 7702 | 97.0 | 100.0 | 21.1 | 910 | 4.9 | 155,714 |
| FAR | |||||||||
| N of Census Tracts | 143 | 143 | 143 | 143 | 143 | 143 | 143 | 143 | 143 |
| Minimum | 6.7 | 0.46 | 98 | 0.0 | 0.0 | 0.0 | 6 | 0.5 | 18,114 |
| 50% | 10.6 | 1.44 | 2170 | 71.5 | 17.0 | 5.5 | 25 | 1.0 | 44,357 |
| Maximum | 20.9 | 18.65 | 6900 | 99.4 | 100.0 | 24.9 | 127 | 3.3 | 161,250 |
| p-Value | 4.2 × 10−53 | 8.7 × 10−22 | 1.2 × 10−9 | 5.8 × 10−5 | 2.0 × 10−5 | 0.016 | 1.4 × 10−34 | 2.6 × 10−35 | 5.1 × 10−6 |
| Mielke’s R | 0.437 | 0.115 | 0.062 | 0.036 | 0.042 | 0.010 | 0.301 | 0.335 | 0.035 |
| SECTOR MEDIANS | ||||||||||
| 2000–2005 | N | Main PO | Main PO | Pop. | SPb | BPb | Household | |||
| Sector | CTs | MIN DIST km | MAX DIST km | Density | % White | % Black | % Other | Soil Pb (interp.) | Blood Pb (interp.) | Income US$ |
| 1 | 30 | 0.00 | 2.32 | 4506 | 21.90 | 74.80 | 3.08 | 615 | 6.8 | 11,950 |
| 2 | 29 | 2.36 | 3.25 | 5122 | 14.77 | 82.11 | 3.27 | 541 | 6.3 | 21,619 |
| 3 | 29 | 3.27 | 4.22 | 4129 | 22.99 | 74.37 | 2.81 | 505 | 5.7 | 21,456 |
| 4 | 30 | 4.30 | 5.31 | 3786 | 27.31 | 70.46 | 2.73 | 275 | 5.0 | 23,608 |
| 5 | 29 | 5.31 | 6.16 | 2589 | 22.85 | 72.60 | 3.37 | 133 | 4.0 | 27,526 |
| 6 | 29 | 6.19 | 7.45 | 2344 | 64.97 | 26.29 | 3.62 | 69 | 3.0 | 33,839 |
| 7 | 30 | 7.57 | 8.93 | 2377 | 77.75 | 15.04 | 4.13 | 55 | 3.0 | 40,014 |
| 8 | 29 | 8.97 | 11.60 | 2388 | 87.36 | 5.11 | 4.16 | 47 | 3.0 | 34,250 |
| 9 | 29 | 11.81 | 14.97 | 2577 | 91.18 | 1.38 | 3.03 | 44 | 3.0 | 40,086 |
| 10 | 30 | 14.97 | 20.92 | 1990 | 74.79 | 15.92 | 6.03 | 22 | 3.0 | 39,699 |
| TOTAL | 294 | |||||||||
| SECTOR MEDIANS | ||||||||||
| 2011–2015 | N | Main PO | Main PO | Pop. | SPb | BPb | Household | |||
| Sector | CTs | MIN DIST km | MAX DIST km | Density | % White | % Black | % Other | Soil Pb (interp.) | Blood Pb (interp.) | Income US$ |
| 1 | 29 | 0.38 | 2.49 | 3610 | 29.17 | 64.74 | 4.78 | 282 | 2.4 | 25,389 |
| 2 | 28 | 2.50 | 3.36 | 3632 | 31.16 | 61.83 | 4.67 | 254 | 2.5 | 28,927 |
| 3 | 29 | 3.38 | 4.38 | 2707 | 48.33 | 43.29 | 4.37 | 215 | 2.2 | 34,583 |
| 4 | 28 | 4.38 | 5.41 | 2582 | 46.18 | 45.07 | 4.30 | 135 | 1.9 | 34,822 |
| 5 | 29 | 5.43 | 6.66 | 1981 | 40.48 | 48.30 | 4.27 | 45 | 1.4 | 41,172 |
| 6 | 28 | 6.73 | 8.01 | 2076 | 42.63 | 50.68 | 5.39 | 36 | 1.2 | 38,312 |
| 7 | 29 | 8.05 | 9.50 | 1980 | 77.36 | 16.56 | 5.35 | 26 | 1.0 | 44,984 |
| 8 | 28 | 9.56 | 12.04 | 2216 | 70.40 | 18.77 | 5.49 | 32 | 1.0 | 39,224 |
| 9 | 29 | 12.24 | 14.98 | 2233 | 80.83 | 9.44 | 4.54 | 23 | 1.0 | 52,422 |
| 10 | 29 | 15.03 | 20.92 | 2040 | 71.46 | 16.07 | 9.22 | 17 | 1.0 | 47,679 |
| TOTAL | 286 | |||||||||
| Pre-Existing Condition for COVID-19 Comorbidity | Association with Pb Exposure | References |
|---|---|---|
| High blood pressure (Hypertension) | Most studied cardiovascular outcome of Pb exposure, effects may occur at BPb ≤ 5 µg/dL. | Apostoli et al., 1990 [46]; Bertin de Almeida Lopes et al., 2017 [47] |
| Coronary heart disease (CHD) | A positive dose–response relationship at BPb ≤ 10 µg/dL is an all-cause mortality and mortality cause of coronary heart disease. | Menke et al., 2006 [48]; Schober et al., 2006 [49] |
| Chronic obstructive pulmonary disease (COPD) | Over a BPb range of ≤10 µg/dL and BPb > 50 µg/dL, workers had decreased pulmonary function, obstructive pulmonary disease, increased asthma, and shortness of breath compared to controls. | Chung et al., 2015 [50]; Pugh Smith and Nriagu, 2011 [51] |
| Chronic kidney disease (CKD) | Exposure of BPb (≤ 10 ≥ 50 µg/dL) alters kidney function and chronic kidney disease (CKD). Renal nephrotoxicity severity is associated with increasing BPb. | Muntner et al., 2003 [52]; Pollack, 2015 [53] |
| Inflammation, immune system disorders and lymphatic system dysfunction | Elevated BPb (≥10 µg/dL) is an important factor in autoimmune diseases, chronic inflammation, and edema. | Boskabady et al., 2018 [54]; Mishra et al., 2009 [55]; CDC, 1991 [56]; Aghdam et al., 2019 [57] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Egendorf, S.P.; Mielke, H.W.; Castorena-Gonzalez, J.A.; Powell, E.T.; Gonzales, C.R. Soil Lead (Pb) in New Orleans: A Spatiotemporal and Racial Analysis. Int. J. Environ. Res. Public Health 2021, 18, 1314. https://doi.org/10.3390/ijerph18031314
Egendorf SP, Mielke HW, Castorena-Gonzalez JA, Powell ET, Gonzales CR. Soil Lead (Pb) in New Orleans: A Spatiotemporal and Racial Analysis. International Journal of Environmental Research and Public Health. 2021; 18(3):1314. https://doi.org/10.3390/ijerph18031314
Chicago/Turabian StyleEgendorf, Sara Perl, Howard W. Mielke, Jorge A. Castorena-Gonzalez, Eric T. Powell, and Christopher R. Gonzales. 2021. "Soil Lead (Pb) in New Orleans: A Spatiotemporal and Racial Analysis" International Journal of Environmental Research and Public Health 18, no. 3: 1314. https://doi.org/10.3390/ijerph18031314









