SARS-CoV-2: An Update on Genomics, Risk Assessment, Potential Therapeutics and Vaccine Development
Abstract
1. Introduction
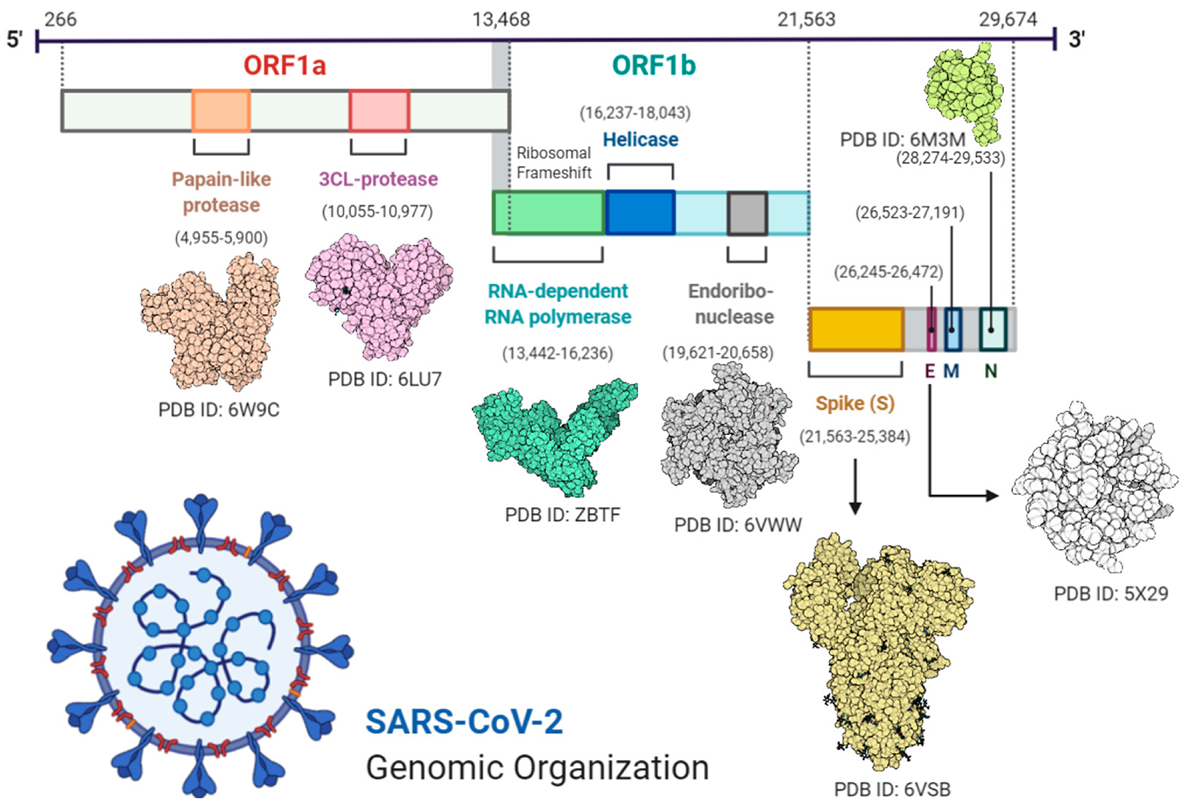
2. Insights into Genomic Organization
2.1. Genome Sequencing
2.2. Phylogenetic Analysis
2.3. Conserved Proteins
2.4. Receptor Binding Domain (RBD)
3. SARS-CoV-2 Recent Mutations
4. Pathophysiology and Epidemiology
5. Transmission Dynamics
6. Clinical Symptoms
6.1. Mild Disease
6.2. Severe Disease
6.3. Critical Disease
7. Duration of SARS-CoV-2 Replication
8. Risk Factors
9. Treatment
9.1. Antiviral Treatments
9.2. Potential Therapeutic Compounds and Drugs
9.3. Nutritional Treatment
10. Platforms for SARS-CoV-2 Vaccine Development
11. Current Status of COVID-19 Vaccine Development
12. Recommended Preventive Measures
- Regular use of face masks [151].
- Wash your hands frequently and sanitize them after close contact with objects and patients. Isolate patients in a separate room and minimize visits to patients [152].
- Avoid personal contact with farm and wild animals [152].
- Avoid close contact with people that have any respiratory illness or symptoms [153].
- Specifically, people with weak immune systems should avoid public gatherings and healthy people should also avoid gatherings to minimize the chances of getting the disease [154].
- People with flu and a cough should avoid close contact with healthy people. While coughing and sneezing they should use disposable tissue/cloth and dispose of them properly. Afterward, wash hands frequently and use sanitizer [155].
- Strict hygiene rules should be followed in hospitals and other health care departments to avoid the spread of disease and to prevent infection [156].
- Some of the vaccines like Pfizer-BioNTech COVID-19 Vaccine and Moderna COVID-19 Vaccine are approved for emergency use; however, they have not been fully evaluated for efficacy against SARS-CoV-2 variants that recently emerged in the UK and South Africa [157].
13. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sohrab, S.S.; Azhar, E.I. Genetic diversity of MERS-CoV spike protein gene in Saudi Arabia. J. Infect. Public Health 2020, 13, 709–717. [Google Scholar] [CrossRef]
- Weiss, S.R.; Leibowitz, J.L. Coronavirus Pathogenesis. Adv. Virus Res. 2011, 81, 85–164. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef]
- Su, S.; Wong, G.; Shi, W.; Liu, J.; Lai, A.C.K.; Zhou, J.; Liu, W.; Bi, Y.; Gao, G.F. Epidemiology, Genetic Recombination, and Pathogenesis of Coronaviruses. Trends Microbiol. 2016, 24, 490–502. [Google Scholar] [CrossRef]
- Yu, F.; Du, L.; Ojcius, D.M.; Pan, C.; Jiang, S. Measures for diagnosing and treating infections by a novel coronavirus responsible for a pneumonia outbreak originating in Wuhan, China Fei. Microbes Infect. 2020, 22, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Cui, W.; Tian, B. The potential intermediate hosts for SARS-CoV-2. Front. Microbiol. 2020, 11, 1814–1820. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Singh, R.; Dar, Z.; Bijarnia, R.K.; Dhingra, N.; Kaur, T. Genetic comparison among various coronavirus strains for the identification of potential vaccine targets of SARS-CoV2. Infect. Genet. Evol. 2020, 89, 104490. [Google Scholar] [CrossRef]
- Zhong, N.; Zheng, B.; Li, Y.; Poon, L.; Lancet, Z.X.-T. Epidemiology and Cause of Severe Acute Respiratory Syndrome (SARS) in Guangdong, People’s Republic of China, in February. Lancet 2003, 362, 1353–1358. [Google Scholar] [CrossRef]
- Ksiazek, T.G.; Erdman, D.; Goldsmith, C.S.; Zaki, S.R.; Peret, T.; Emery, S.; Tong, S.; Urbani, C.; Comer, J.A.; Lim, W.; et al. A novel coronavirus associated with severe acute respiratory syndrome. N. Engl. J. Med. 2003, 348, 1953–1966. [Google Scholar] [CrossRef] [PubMed]
- Drosten, C.; Günther, S.; Preiser, W.; Van der Werf, S.; Brodt, H.R.; Becker, S.; Rabenau, H.; Panning, M.; Kolesnikova, L.; Fouchier, R.A.M.; et al. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 2003, 348, 1967–1976. [Google Scholar] [CrossRef]
- Zaki, A.M.; Van Boheemen, S.; Bestebroer, T.M.; Osterhaus, A.D.M.E.; Fouchier, R.A.M. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 2012, 367, 1814–1820. [Google Scholar] [CrossRef] [PubMed]
- WHO. Summary of Probable SARS Cases with Onset of Illness from 1 November 2002 to 31 July 2003. Available online: https://www.ci.nii.ac.jp (accessed on 1 January 2021).
- Assiri, A.; McGeer, A.; Perl, T.M.; Price, C.S.; Al Rabeeah, A.A.; Cummings, D.A.T.; Alabdullatif, Z.N.; Assad, M.; Almulhim, A.; Makhdoom, H.; et al. Hospital outbreak of middle east respiratory syndrome coronavirus. N. Engl. J. Med. 2013, 369, 407–416. [Google Scholar] [CrossRef]
- Wong, G.; Liu, W.; Liu, Y.; Zhou, B.; Bi, Y.; Microbe, G.G.-C. MERS, SARS, and Ebola: The Role of Super-Spreaders in Infectious Disease. Cell Host Micro. 2015, 18, 398–401. [Google Scholar] [CrossRef] [PubMed]
- Peiris, J.S.M.; Guan, Y.; Yuen, K.Y. Severe acute respiratory syndrome. Nat. Med. 2004, 10, S88–S97. [Google Scholar] [CrossRef] [PubMed]
- Neuman, B.W.; Adair, B.D.; Yoshioka, C.; Quispe, J.D.; Orca, G.; Kuhn, P.; Milligan, R.A.; Yeager, M.; Buchmeier, M.J. Supramolecular Architecture of Severe Acute Respiratory Syndrome Coronavirus Revealed by Electron Cryomicroscopy. J. Virol. 2006, 80, 7918–7928. [Google Scholar] [CrossRef]
- Barcena, M.; Oostergetel, G.T.; Bartelink, W.; Faas, F.G.A.; Verkleij, A.; Rottier, P.J.M.; Koster, A.J.; Bosch, B.J. Cryo-electron tomography of mouse hepatitis virus: Insights into the structure of the coronavirion. Proc. Natl. Acad. Sci. USA 2009, 106, 582–587. [Google Scholar] [CrossRef]
- Tsai, P.H.; Wang, M.L.; Yang, D.M.; Liang, K.H.; Chou, S.J.; Chiou, S.H.; Lin, T.H.; Wang, C.T.; Chang, T.J. Genomic variance of Open Reading Frames (ORFs) and Spike protein in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). J. Chin. Med. Assoc. 2020, 83, 725–732. [Google Scholar] [CrossRef]
- Alamri, M.A.; Qamar, M.T.U.; Mirza, M.U.; Alqahtani, S.M.; Froeyen, M.; Chen, L.-L. Discovery of human coronaviruses pan-papain-like protease inhibitors using computational approaches. J. Pharm. Anal. 2020, 10, 546–559. [Google Scholar] [CrossRef]
- Perlman, S.; Netland, J. Coronaviruses post-SARS: Update on replication and pathogenesis. Nat. Rev. Microbiol. 2009, 7, 439–450. [Google Scholar] [CrossRef]
- Alamri, M.A.; Qamar, M.T.U.; Mirza, M.U.; Bhadane, R.; Alqahtani, S.M.; Muneer, I.; Froeyen, M.; Salo-Ahen, O.M.H. Pharmacoinformatics and molecular dynamics simulation studies reveal potential covalent and FDA-approved inhibitors of SARS-CoV-2 main protease 3CLpro. J. Biomol. Struct. Dyn. 2020. [Google Scholar] [CrossRef]
- Angeletti, S.; Benvenuto, D.; Bianchi, M.; Giovanetti, M.; Pascarella, S.; Ciccozzi, M. COVID-2019: The role of the nsp2 and nsp3 in its pathogenesis. J. Med. Virol. 2020, 92, 584–588. [Google Scholar] [CrossRef] [PubMed]
- Qamar, M.T.U.; Rehman, A.; Tusleem, K.; Ashfaq, U.A.; Qasim, M.; Zhu, X.; Fatima, I.; Shahid, F.; Chen, L.L. Designing of a next generation multiepitope based vaccine (MEV) against SARS-COV-2: Immunoinformatics and in silico approaches. PLoS ONE 2020, 15, e0244176. [Google Scholar] [CrossRef]
- Lu, R.; Zhao, X.; Li, J.; Niu, P.; Yang, B.; Wu, H.; Wang, W.; Song, H.; Huang, B.; Zhu, N.; et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet 2020, 395, 565–574. [Google Scholar] [CrossRef]
- Naqvi, A.A.T.; Fatima, K.; Mohammad, T.; Fatima, U.; Singh, I.K.; Singh, A.; Atif, S.M.; Hariprasad, G.; Hasan, G.M.; Hassan, M.I. Insights into SARS-CoV-2 genome, structure, evolution, pathogenesis and therapies: Structural genomics approach. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2020, 1866, 165878. [Google Scholar] [CrossRef]
- Qamar, M.T.U.; Alqahtani, S.M.; Alamri, M.A.; Chen, L.-L. Structural basis of SARS-CoV-2 3CLpro and anti-COVID-19 drug discovery from medicinal plants. J. Pharm. Anal. 2020, 10, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Muhseen, Z.T.; Hameed, A.R.; Al-Hasani, H.M.H.; Qamar, M.T.U.; Li, G. Promising terpenes as SARS-CoV-2 spike receptor-binding domain (RBD) attachment inhibitors to the human ACE2 receptor: Integrated computational approach. J. Mol. Liq. 2020, 320, 114493. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Kleine-Weber, H.; Pöhlmann, S. A Multibasic Cleavage Site in the Spike Protein of SARS-CoV-2 Is Essential for Infection of Human Lung Cells. Mol. Cell 2020, 78, 779–784. [Google Scholar] [CrossRef]
- Ren, L.L.; Wang, Y.M.; Wu, Z.Q.; Xiang, Z.C.; Guo, L.; Xu, T.; Jiang, Y.Z.; Xiong, Y.; Li, Y.J.; Li, X.W.; et al. Identification of a novel coronavirus causing severe pneumonia in human: A descriptive study. Chin. Med. J. 2020, 133, 1015–1024. [Google Scholar] [CrossRef]
- Gioia, M.; Ciaccio, C.; Calligari, P.; Simone, G.D.; Sbardella, D.; Tundo, G.; Francesco, G.; Di, A.; Di, D. Role of proteolytic enzymes in the COVID-19 infection and promising therapeutic approaches. Biochem. Pharmacol. 2020, 182, 1–22. [Google Scholar] [CrossRef]
- Ceraolo, C.; Giorgi, F.M. Genomic variance of the 2019-nCoV coronavirus. J. Med. Virol. 2020, 92, 522–528. [Google Scholar] [CrossRef]
- Lau, S.K.P.; Li, K.S.M.; Tsang, A.K.L.; Lam, C.S.F.; Ahmed, S.; Chen, H.; Chan, K.-H.; Woo, P.C.Y.; Yuen, K.-Y. Genetic Characterization of Betacoronavirus Lineage C Viruses in Bats Reveals Marked Sequence Divergence in the Spike Protein of Pipistrellus Bat Coronavirus HKU5 in Japanese Pipistrelle: Implications for the Origin of the Novel Middle East Respiratory Sy. J. Virol. 2013, 87, 8638–8650. [Google Scholar] [CrossRef]
- Du, L.; He, Y.; Zhou, Y.; Liu, S.; Zheng, B.J.; Jiang, S. The spike protein of SARS-CoV—A target for vaccine and therapeutic development. Nat. Rev. Microbiol. 2009, 7, 226–236. [Google Scholar] [CrossRef]
- Lan, J.; Ge, J.; Yu, J.; Shan, S.; Zhou, H.; Fan, S.; Zhang, Q.; Shi, X.; Wang, Q.; Zhang, L.; et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature 2020, 581, 215–220. [Google Scholar] [CrossRef]
- Pachetti, M.; Marini, B.; Benedetti, F.; Giudici, F.; Mauro, E.; Storici, P.; Masciovecchio, C.; Angeletti, S.; Ciccozzi, M.; Gallo, R.C.; et al. Emerging SARS-CoV-2 mutation hot spots include a novel RNA-dependent-RNA polymerase variant. J. Transl. Med. 2020, 18, 1–9. [Google Scholar] [CrossRef]
- Sevajol, M.; Subissi, L.; Decroly, E.; Canard, B.; Imbert, I. Insights into RNA synthesis, capping, and proofreading mechanisms of SARS-coronavirus. Virus Res. 2014, 194, 90–99. [Google Scholar] [CrossRef]
- Pathan, R.K.; Biswas, M.; Khandaker, M.U. Time series prediction of COVID-19 by mutation rate analysis using recurrent neural network-based LSTM model. Chaos Solitons Fractals 2020, 138, 110018. [Google Scholar] [CrossRef]
- Fung, T.S.; Liu, D.X. Human Coronavirus: Host-Pathogen Interaction. Annu. Rev. Microbiol. 2019, 73, 529–560. [Google Scholar] [CrossRef]
- Phan, T. Genetic diversity and evolution of SARS-CoV-2. Infect. Genet. Evol. 2020, 81, 104260. [Google Scholar] [CrossRef]
- Rouchka, E.C.; Chariker, J.H.; Chung, D. Variant analysis of 1040 SARS-CoV-2 genomes. PLoS ONE 2020, 15, e0241535. [Google Scholar] [CrossRef]
- Wise, J. Covid-19: New coronavirus variant is identified in UK. BMJ 2020, 371, m4857. [Google Scholar] [CrossRef]
- Qamar, M.T.U.; Shahid, F.; Aslam, S.; Ashfaq, U.A.; Aslam, S.; Fatima, I.; Fareed, M.M.; Zohaib, A.; Chen, L.-L. Reverse vaccinology assisted designing of multiepitope-based subunit vaccine against SARS-CoV-2. Infect. Dis. Poverty 2020, 9, 1–14. [Google Scholar]
- Azer, S.A. COVID-19: Pathophysiology, diagnosis, complications and investigational therapeutics. New Microbes New Infect. 2020, 37, 100738. [Google Scholar] [CrossRef] [PubMed]
- Worldometer Coronavirus Cases. Available online: https://www.worldometers.info/coronavirus/ (accessed on 1 January 2021).
- Yanez, N.D.; Weiss, N.S.; Romand, J.A.; Treggiari, M.M. COVID-19 mortality risk for older men and women. BMC Public Health 2020, 20, 1–7. [Google Scholar] [CrossRef]
- Li, Q.; Guan, X.; Wu, P.; Wang, X.; Zhou, L.; Tong, Y.; Ren, R.; Leung, K.S.M.; Lau, E.H.Y.; Wong, J.Y.; et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus–Infected Pneumonia. N. Engl. J. Med. 2020, 382, 1199–1207. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Ko, J.-H.; Joo, E.-J.; Park, S.-J.; Baek, J.Y.; Kim, W.D.; Jee, J.; Kim, C.J.; Jeong, C.; Kim, Y.-J.; Shon, H.J. Neutralizing antibody production in asymptomatic and mild COVID-19 patients, in comparison with pneumonic COVID-19 patients. J. Clin. Med. 2020, 9, 2268. [Google Scholar] [CrossRef]
- Mokhtari, T.; Hassani, F.; Ghaffari, N.; Ebrahimi, B.; Yarahmadi, A.; Hassanzadeh, G. COVID-19 and multiorgan failure: A narrative review on potential mechanisms. J. Mol. Histol. 2020, 51, 613–628. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Hiyoshi, A.; Montgomery, S. COVID-19 case-fatality rate and demographic and socioeconomic influencers: Worldwide spatial regression analysis based on country-level data. BMJ Open 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72314 Cases from the Chinese Center for Disease Control and Prevention. JAMA J. Am. Med. Assoc. 2020, 323, 1239–1242. [Google Scholar] [CrossRef]
- Gandhi, A.; Sokhi, J.; Lockie, C.; Ward, P.A. Emergency Tracheal Intubation in Patients with COVID-19: Experience from a UK Centre. Anesthesiol. Res. Pract. 2020, 2020, 1–9. [Google Scholar] [CrossRef]
- Meng, L.; Qiu, H.; Wan, L.; Ai, Y.; Xue, Z.; Guo, Q.; Deshpande, R.; Zhang, L.; Meng, J.; Tong, C.; et al. Intubation and Ventilation amid the COVID-19 Outbreak: Wuhan’s Experience. Anesthesiology 2020, 132, 1317–1332. [Google Scholar] [CrossRef] [PubMed]
- Storch, G.A. Diagnostic virology. Clin. Infect. Dis. 2000, 31, 739–751. [Google Scholar] [CrossRef]
- Xu, D.; Zhang, Z.; Jin, L.; Chu, F.; Mao, Y.; Wang, H.; Liu, M.; Wang, M.; Zhang, L.; Gao, G.F.; et al. Persistent shedding of viable SARS-CoV in urine and stool of SARS patients during the convalescent phase. Eur. J. Clin. Microbiol. Infect. Dis. 2005, 24, 165–171. [Google Scholar] [CrossRef]
- Oh, M.; Park, W.B.; Choe, P.G.; Choi, S.-J.; Kim, J.-I.; Chae, J.; Park, S.S.; Kim, E.-C.; Oh, H.S.; Kim, E.J.; et al. Viral Load Kinetics of MERS Coronavirus Infection. N. Engl. J. Med. 2016, 375, 1303–1305. [Google Scholar] [CrossRef]
- Corman, V.M.; Albarrak, A.M.; Omrani, A.S.; Albarrak, M.M.; Farah, M.E.; Almasri, M.; Muth, D.; Sieberg, A.; Meyer, B.; Assiri, A.M.; et al. Viral Shedding and Antibody Response in 37 Patients With Middle East Respiratory Syndrome Coronavirus Infection. Clin. Infect. Dis. 2015, 62, civ951. [Google Scholar] [CrossRef]
- Writing Committee of the WHO Consultation on Clinical Aspects of Pandemic (H1N1) 2009 Influenza. Clinical aspects of pandemic 2009 influenza A (H1N1) virus infection. N. Engl. J. Med. 2010, 362, 1708–1719. [Google Scholar] [CrossRef]
- Di Castelnuovo, A.; Bonaccio, M.; Costanzo, S.; Gialluisi, A.; Antinori, A.; Berselli, N.; Blandi, L.; Bruno, R.; Cauda, R.; Guaraldi, D.; et al. Common cardiovascular risk factors and in-hospital mortality in 3,894 patients with COVID-19: Survival analysis and machine learning-based findings from the multicentre Italian CORIST Study. Nutr. Metabol. Cardiovas. Dis. 2020, 30, 1899–1913. [Google Scholar] [CrossRef]
- Yang, X.; Yu, Y.; Xu, J.; Shu, H.; Xia, J.; Liu, H.; Wu, Y.; Zhang, L.; Yu, Z.; Fang, M.; et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir. Med. 2020, 8, 475–481. [Google Scholar] [CrossRef]
- Sprung, C.L.; Schein, R.M.H.; Balk, R.A. The new sepsis consensus definitions: The good, the bad and the ugly. Intensive Care Med. 2016, 42, 2024–2026. [Google Scholar] [CrossRef] [PubMed]
- Lopes Ferreira, F.; Peres Bota, D.; Bross, A.; Mélot, C.; Vincent, J.L. Serial evaluation of the SOFA score to predict outcome in critically ill patients. J. Am. Med. Assoc. 2001, 286, 1754–1758. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Wang, Y.; Liu, Y.; Liu, X.; Gu, L.; Zhang, X.; Pu, Z.; Yang, G.; Liu, B.; Mie, Q.; et al. Disease Severity and Clinical Outcomes of Community Acquired Pneumonia Caused by Non-Influenza Respiratory Viruses: A Multicenter Prospective Registry Study from CAP-China Network. Eur. Resp. J. 2019, 54, 1802406. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.W.M.; Ng, C.K.; Chan, Y.H.; Mok, Y.W.; Lee, S.; Chu, Y.; Law, W.L.; Lee, M.P. Short term outcome and risk factors for adverse clinical outcomes in adults with severe acute respiratory syndrome (SARS). Thorax 2003, 58, 686–689. [Google Scholar] [CrossRef]
- Arabi, Y.M.; Balkhy, H.H.; Hayden, F.G.; Bouchama, A.; Luke, T.; Baillie, J.K.; Al-Omari, A.; Hajeer, A.H.; Senga, M.; Denison, M.R.; et al. Middle east respiratory syndrome. N. Engl. J. Med. 2017, 376, 584–594. [Google Scholar] [CrossRef] [PubMed]
- Opal, S.M.; Girard, T.D.; Wesley Ely, E. The Immunopathogenesis of Sepsis in Elderly Patients. Clin. Infec. Dis. 2005, 41, S504–S512. [Google Scholar] [CrossRef] [PubMed]
- Marrie, T.J.; Shariatzadeh, M.R. Community-Acquired Pneumonia Requiring Admission to an Intensive Care Unit. Medicine 2007, 86, 103–111. [Google Scholar] [CrossRef]
- Corrales-Medina, V.F.; Musher, D.M.; Shachkina, S.; Chirinos, J.A. Acute pneumonia and the cardiovascular system. Lancet 2013, 381, 496–505. [Google Scholar] [CrossRef]
- Lei, J.; Kusov, Y.; Hilgenfeld, R. Nsp3 of coronaviruses: Structures and functions of a large multi-domain protein. Antivir. Res. 2018, 149, 58–74. [Google Scholar] [CrossRef]
- Milbrandt, E.B.; Reade, M.C.; Lee, M.; Shook, S.L.; Angus, D.C.; Kong, L.; Carter, M.; Yealy, D.M.; Kellum, J.A. Prevalence and significance of coagulation abnormalities in community-acquired pneumonia. Mol. Med. 2009, 15, 438–445. [Google Scholar] [CrossRef]
- Rodelo, J.R.; De La Rosa, G.; Valencia, M.L.; Ospina, S.; Arango, C.M.; Gómez, C.I.; García, A.; Nuñez, E.; Jaimes, F.A. D-dimer is a significant prognostic factor in patients with suspected infection and sepsis. Am. J. Emerg. Med. 2012, 30, 1991–1999. [Google Scholar] [CrossRef]
- Chen, L.; Xiong, J.; Bao, L.; Shi, Y. Convalescent plasma as a potential therapy for COVID-19. Lancet Infect. Dis. 2020, 20, 398–400. [Google Scholar] [CrossRef]
- Khalili, J.S.; Zhu, H.; Mak, N.S.A.; Yan, Y.; Zhu, Y. Novel coronavirus treatment with ribavirin: Groundwork for an evaluation concerning COVID-19. J. Med. Virol. 2020, 92, 740–746. [Google Scholar] [CrossRef]
- Lu, H. Drug treatment options for the 2019-new coronavirus (2019-nCoV). Biosci. Trends 2020, 14, 69–71. [Google Scholar] [CrossRef]
- Prescott, H.C.; Rice, T.W. Corticosteroids in COVID-19 ARDS: Evidence and Hope during the Pandemic. JAMA J. Am. Med. Assoc. 2020, 324, 1292–1295. [Google Scholar] [CrossRef]
- Guan, W.-J.; Ni, Z.-Y.; Hu, Y.; Liang, W.-H.; Ou, C.-Q.; He, J.-X.; Liu, L.; Shan, H.; Lei, C.-L.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Tan, Q.; Duan, L.; Ma, Y.; Wu, F.; Huang, Q.; Mao, K.; Xiao, W.; Xia, H.; Zhang, S.; Zhou, E.; et al. Is oseltamivir suitable for fighting against COVID-19: In silico assessment, in vitro and retrospective study. Bioorg. Chem. 2020, 104, 104257. [Google Scholar] [CrossRef] [PubMed]
- Marciniec, K.; Beberok, A.; Pęcak, P.; Boryczka, S.; Wrześniok, D. Ciprofloxacin and moxifloxacin could interact with SARS-CoV-2 protease: Preliminary in silico analysis. Pharmacol. Rep. 2020, 72, 1553–1561. [Google Scholar] [CrossRef] [PubMed]
- Sultana, J.; Cutroneo, P.M.; Crisafulli, S.; Puglisi, G.; Caramori, G.; Trifirò, G. Azithromycin in COVID-19 Patients: Pharmacological Mechanism, Clinical Evidence and Prescribing Guidelines. Drug Saf. 2020, 43, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Gordon, C.J.; Tchesnokov, E.P.; Feng, J.Y.; Porter, D.P.; Gotte, M. The antiviral compound remdesivir potently inhibits RNA-dependent RNA polymerase from Middle East respiratory syndrome coronavirus. J. Biol. Chem. 2020, 295, 4773–4779. [Google Scholar] [CrossRef]
- Agostini, M.L.; Andres, E.L.; Sims, A.C.; Graham, R.L.; Sheahan, T.P.; Lu, X.; Smith, E.C.; Case, J.B.; Feng, J.Y.; Jordan, R.; et al. Coronavirus susceptibility to the antiviral remdesivir (GS-5734) is mediated by the viral polymerase and the proofreading exoribonuclease. MBio 2018, 9, e00221-18. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Wang, Y.; Wen, D.; Liu, W.; Wang, J.; Fan, G.; Ruan, L.; Song, B.; Cai, Y.; Wei, M.; et al. A Trial of Lopinavir–Ritonavir in Adults Hospitalized with Severe Covid-19. N. Engl. J. Med. 2020, 382, 1787–1799. [Google Scholar] [CrossRef]
- Yan, Y.; Zou, Z.; Sun, Y.; Li, X.; Xu, K.F.; Wei, Y.; Jin, N.; Jiang, C. Anti-malaria drug chloroquine is highly effective in treating avian influenza A H5N1 virus infection in an animal model. Cell Res. 2013, 23, 300–302. [Google Scholar] [CrossRef]
- Wang, M.; Cao, R.; Zhang, L.; Yang, X.; Liu, J.; Xu, M.; Shi, Z.; Hu, Z.; Zhong, W.; Xiao, G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020, 30, 269–271. [Google Scholar] [CrossRef]
- Zhonghua, J.; He, H.; Hu, X.; Za, Z. Expert consensus on chloroquine phosphate for the treatment of novel coronavirus pneumonia. Bibl. Nac. Med. 2020, 43, 185–188. [Google Scholar]
- Gautret, P.; Lagier, J.-C.; Parola, P.; Meddeb, L.; Mailhe, M.; Doudier, B.; Courjon, J.; Giordanengo, V.; Vieira, V.E.; Dupont, H.T. Hydroxychloroquine and azithromycin as a treatment of COVID-19: Results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents 2020, 56, 105949. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Singh, A.; Shaikh, A.; Singh, R.; Misra, A. Chloroquine and hydroxychloroquine in the treatment of COVID-19 with or without diabetes: A systematic search and a narrative review with a special reference to India and other developing countries. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Ye, F.; Zhang, M.; Cui, C.; Huang, B.; Niu, P.; Liu, X.; Zhao, L.; Dong, E.; Song, C. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin. Infect. Dis. 2020, 71, 732–739. [Google Scholar] [CrossRef]
- Liu, J.; Cao, R.; Xu, M.; Wang, X.; Zhang, H.; Hu, H.; Li, Y.; Hu, Z.; Zhong, W.; Wang, M. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020, 6, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Cvetkovic, R.S.; Goa, K.L. Lopinavir/ritonavir: A review of its use in the management of HIV infection. Drugs 2003, 63, 769–802. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.M.; Cheng, C.C.; Hung, F.N.; Wong, M.M.L.; Chan, H.; Chan, S.; Kao, Y.T.; Poon, L.L.M.; Wong, L.P.; Guan, Y.; et al. Role of lopinavir/ritonavir in the treatment of SARS: Initial virological and clinical findings. Thorax 2004, 59, 252–256. [Google Scholar] [CrossRef]
- Arabi, Y.M.; Asiri, A.Y.; Assiri, A.M.; Aziz Jokhdar, H.A.; Alothman, A.; Balkhy, H.H.; AlJohani, S.; Al Harbi, S.; Kojan, S.; Al Jeraisy, M.; et al. Treatment of Middle East respiratory syndrome with a combination of lopinavir/ritonavir and interferon-β1b (MIRACLE trial): Statistical analysis plan for a recursive two-stage group sequential randomized controlled trial. Trials 2020, 21, 8. [Google Scholar] [CrossRef]
- Lim, J.; Jeon, S.; Shin, H.Y.; Kim, M.J.; Seong, Y.M.; Lee, W.J.; Choe, K.W.; Kang, Y.M.; Lee, B.; Park, S.J. Case of the index patient who caused tertiary transmission of coronavirus disease 2019 in Korea: The application of lopinavir/ritonavir for the treatment of COVID-19 pneumonia monitored by quantitative RT-PCR. J. Korean Med. Sci. 2020, 35, e79. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, X.; Lu, Y.; Chen, F.; Zhang, W. Clinical characteristics and therapeutic procedure for four cases with 2019 novel coronavirus pneumonia receiving combined Chinese and Western medicine treatment. Biosci. Trends 2020, 14, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Piao, W.; Shirey, K.A.; Ru, L.W.; Lai, W.; Szmacinski, H.; Snyder, G.A.; Sundberg, E.J.; Lakowicz, J.R.; Vogel, S.N.; Toshchakov, V.Y. A Decoy Peptide that Disrupts TIRAP Recruitment to TLRs Is Protective in a Murine Model of Influenza. Cell Rep. 2015, 11, 1941–1952. [Google Scholar] [CrossRef]
- Perrin-Cocon, L.; Aublin-Gex, A.; Sestito, S.E.; Shirey, K.A.; Patel, M.C.; André, P.; Blanco, J.C.; Vogel, S.N.; Peri, F.; Lotteau, V. TLR4 antagonist FP7 inhibits LPS-induced cytokine production and glycolytic reprogramming in dendritic cells, and protects mice from lethal influenza infection. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef]
- Lansbury, L.; Rodrigo, C.; Leonardi-Bee, J.; Nguyen-Van-Tam, J.; Lim, W.S. Corticosteroids as adjunctive therapy in the treatment of influenza. Cochrane Database Syst. Rev. 2019. [Google Scholar] [CrossRef]
- Baughn, L.B.; Sharma, N.; Elhaik, E.; Sekulic, A.; Bryce, A.H.; Fonseca, R. Targeting TMPRSS2 in SARS-CoV-2 Infection. Mayo Clin. Proc. 2020, 95, 1989–1999. [Google Scholar] [CrossRef]
- Li, G.; De Clercq, E. Therapeutic options for the 2019 novel coronavirus (2019-nCoV). Nat. Rev. Drug Discov. 2020, 19, 149–150. [Google Scholar] [CrossRef]
- Zumla, A.; W Chan, J.F.; Azhar, E.I.; C Hui, D.S.; Yuen, K.-Y. Coronaviruses—Drug discovery and therapeutic options. Nat. Rev. Drug Discov. 2016, 15, 327–347. [Google Scholar] [CrossRef] [PubMed]
- Holshue, M.L.; DeBolt, C.; Lindquist, S.; Lofy, K.H.; Wiesman, J.; Bruce, H.; Spitters, C.; Ericson, K.; Wilkerson, S.; Tural, A.; et al. First case of 2019 novel coronavirus in the United States. N. Engl. J. Med. 2020, 382, 929–936. [Google Scholar] [CrossRef]
- Beigel, J.H.; Nam, H.H.; Adams, P.L.; Krafft, A.; Ince, W.L.; El-Kamary, S.S.; Sims, A.C. Advances in Respiratory Virus Therapeutics–A Meeting Report from the 6th Isirv Antiviral Group Conference. Antivir. Res. 2019, 167, 45–67. [Google Scholar] [CrossRef] [PubMed]
- Richardson, P.; Griffin, I.; Tucker, C.; Smith, D.; Oechsle, O.; Phelan, A.; Stebbing, J. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet 2020, 395, e30–e31. [Google Scholar] [CrossRef]
- Hoang, B.X.; Shaw, D.G.; Fang, W.; Han, B. A Possible application of high dose vitamin C in the prevention and therapy for Coronavirus Infections. J. Glob. Antimicrob. Resist. 2020, 23, 256–262. [Google Scholar] [CrossRef]
- Abobaker, A.; Alzwi, A.; Alraied, A.H.A. Overview of the possible role of vitamin C in management of COVID-19. Pharmacol. Rep. 2020, 72, 1517–1528. [Google Scholar] [CrossRef] [PubMed]
- Schooley, R.T. The human microbiome: Implications for health and disease, including HIV infection. Top. Antivir. Med. 2018, 26, 75–78. [Google Scholar] [PubMed]
- Saul, A.W. Nutritional treatment of coronavirus. Orthomol. Med. News Serv. 2020, 16, 22. [Google Scholar]
- Skrajnowska, D.; Bobrowska-Korczak, B. Role of zinc in immune system and anti-cancer defense mechanisms. Nutrients 2019, 11, 2273. [Google Scholar] [CrossRef]
- Guillin, O.M.; Vindry, C.; Ohlmann, T.; Chavatte, L. Selenium, Selenoproteins and Viral Infection. Nutrients 2019, 11, 2120. [Google Scholar] [CrossRef] [PubMed]
- Harthill, M. Review: Micronutrient selenium deficiency influences evolution of some viral infectious diseases. Biol. Trace Elem. Res. 2011, 143, 1325–1336. [Google Scholar] [CrossRef] [PubMed]
- Stachowska, E.; Folwarski, M.; Jamioł-Milc, D.; Maciejewska, D.; Skonieczna-Żydecka, K. Nutritional support in coronavirus 2019 disease. Medicina (B Aires) 2020, 56, 289. [Google Scholar] [CrossRef]
- Lauring, A.S.; Jones, J.O.; Andino, R. Rationalizing the development of live attenuated virus vaccines. Nat. Biotechnol. 2010, 28, 573–579. [Google Scholar] [CrossRef]
- Pulendran, B.; Ahmed, R. Immunological mechanisms of vaccination. Nat. Immunol. 2011, 12, 509–517. [Google Scholar] [CrossRef]
- Lee, J.; Arun Kumar, S.; Jhan, Y.Y.; Bishop, C.J. Engineering DNA vaccines against infectious diseases. Acta Biomater. 2018, 80, 31–47. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, H.R.; Gilbride, C.; Allen, E.; Belij-Rammerstorfer, S.; Bissett, C.; Ewer, K.; Lambe, T. The early landscape of coronavirus disease 2019 vaccine development in the UK and rest of the world. Immunology 2020, 160, 223–232. [Google Scholar] [CrossRef]
- Haque, A.; Pant, A.B. Efforts at COVID-19 Vaccine Development: Challenges and Successes. Vaccines 2020, 8, 739. [Google Scholar] [CrossRef]
- Luo, F.; Liao, F.L.; Wang, H.; Tang, H.B.; Yang, Z.Q.; Hou, W. Evaluation of Antibody-Dependent Enhancement of SARS-CoV Infection in Rhesus Macaques Immunized with an Inactivated SARS-CoV Vaccine. Virol. Sin. 2018, 33, 201–204. [Google Scholar] [CrossRef]
- Lin, J.T.; Zhang, J.S.; Su, N.; Xu, J.G.; Wang, N.; Chen, J.T.; Chen, X.; Liu, Y.X.; Gao, H.; Jia, Y.P.; et al. Safety and immunogenicity from a Phase I trial of inactivated severe acute respiratory syndrome coronavirus vaccine. Antivir. Ther. 2007, 12, 1107–1113. [Google Scholar]
- Wang, H.; Zhang, Y.; Huang, B.; Deng, W.; Quan, Y.; Wang, W.; Xu, W.; Zhao, Y.; Li, N.; Zhang, J.; et al. Development of an Inactivated Vaccine Candidate, BBIBP-CorV, with Potent Protection against SARS-CoV-2. Cell 2020, 182, 713–721.e9. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.T.; Sbrana, E.; Iwata-Yoshikawa, N.; Newman, P.C.; Garron, T.; Atmar, R.L.; Peters, C.J.; Couch, R.B. Immunization with SARS coronavirus vaccines leads to pulmonary immunopathology on challenge with the SARS virus. PLoS ONE 2012, 7. [Google Scholar] [CrossRef]
- Bolles, M.; Deming, D.; Long, K.; Agnihothram, S.; Whitmore, A.; Ferris, M.; Funkhouser, W.; Gralinski, L.; Totura, A.; Heise, M.; et al. A Double-Inactivated Severe Acute Respiratory Syndrome Coronavirus Vaccine Provides Incomplete Protection in Mice and Induces Increased Eosinophilic Proinflammatory Pulmonary Response upon Challenge. J. Virol. 2011, 85, 12201–12215. [Google Scholar] [CrossRef]
- Karch, C.; Burkhard, P. Vaccine Technologies: From Whole Organisms to Rationally Designed Protein Assemblies. Biochem. Pharmacol. 2016, 120, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Plotkin, S.; Robinson, J.; Cunningham, G.; Vaccine, R.I. The Complexity and Cost of Vaccine Manufacturing–An Overview. Vaccine 2017, 35, 4064–4071. [Google Scholar] [CrossRef] [PubMed]
- Wrapp, D.; Wang, N.; Corbett, K.S.; Goldsmith, J.A.; Hsieh, C.; Abiona, O.; Graham, B.S.; McLellan, J.S. Cryo-EM Structure of the 2019-nCoV Spike in the Prefusion Conformation. Science 2020, 367, 1260–1263. [Google Scholar] [CrossRef]
- Cannon, G.; Weissman, D. RNA based vaccines. DNA Cell Biol. 2002, 21, 953–961. [Google Scholar] [CrossRef]
- Blakney, A.K.; McKay, P.F.; Christensen, D.; Yus, B.I.; Aldon, Y.; Follmann, F.; Shattock, R.J. Effects of Cationic Adjuvant Formulation Particle Type, Fluidity and Immunomodulators on Delivery and Immunogenicity of saRNA. J. Control. Rel. 2019, 304, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Geall, A.; Mandl, C.; Ulemr, J.B. RNA: The New Revolution in Nucleic Acid Vaccines. Sem. Immunol. 2013, 25, 152–159. [Google Scholar] [CrossRef]
- Ulmer, J.; Mason, P.; Geall, A.; Vaccine, C.M. RNA-Based Vaccines. Vaccine 2012, 30, 4414–4418. [Google Scholar] [CrossRef] [PubMed]
- Schnee, M.; Vogel, A.B.; Voss, D.; Petsch, B.; Baumhof, P.; Kramps, T.; Stitz, L. An mRNA vaccine encoding rabies virus glycoprotein induces protection against lethal infection in mice and correlates of protection in adult and newborn pigs. PLoS Negl. Trop. Dis. 2016, 10, e0004746. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.; Louder, M.; Holman, L.; Vaccine, I.G. A SARS DNA Vaccine Induces Neutralizing Antibody and Cellular Immune Responses in Healthy Adults in a Phase I Clinical Trial. Vaccine 2008, 26, 6338–6343. [Google Scholar] [CrossRef]
- Kramps, T.; Elbers, K. Introduction to RNA vaccines. In Methods in Molecular Biology; Humana Press Inc.: New York, USA, 2017; Volume 1499, pp. 1–11. [Google Scholar]
- Mulligan, M.J.; Lyke, K.E.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Raabe, V.; Bailey, R.; Swanson, K.A.; et al. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature 2020, 586, 589–593. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Lee, L.Y.Y.; Izzard, L.; Hurt, A.C. A review of DNA vaccines against influenza. Front. Immunol. 2018, 9, 1568. [Google Scholar] [CrossRef]
- Bull, J.J.; Nuismer, S.L.; Antia, R. Recombinant vector vaccine evolution. PLoS Comput. Biol. 2019, 15, e1006857. [Google Scholar] [CrossRef] [PubMed]
- Henao-Restrepo, A.M.; Camacho, A.; Longini, I.M.; Watson, C.H.; Edmunds, W.J.; Egger, M.; Carroll, M.W.; Dean, N.E.; Diatta, I.; Doumbia, M.; et al. Efficacy and effectiveness of an rVSV-vectored vaccine in preventing Ebola virus disease: Final results from the Guinea ring vaccination, open-label, cluster-randomised trial (Ebola Ça Suffit!). Lancet 2017, 389, 505–518. [Google Scholar] [CrossRef]
- Jartti, T.; Jartti, L.; Ruuskanen, O.; Söderlund-Venermo, M. New Respiratory Viral Infections. Curr. Opin. Pulm. Med. 2012, 18, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhang, Z.; Gao, H.; Li, Y.; Hou, L.; Yao, H.; Wu, S.; Liu, J.; Wang, L.; Zhai, Y.; et al. Open-label phase I clinical trial of Ad5-EBOV in Africans in China. Hum. Vaccin. Immunother. 2017, 13, 2078–2085. [Google Scholar] [CrossRef]
- Dudareva, M.; Andrews, L.; Gilbert, S.; Bejon, P.; Vaccine, K.M. Prevalence of Serum Neutralizing Antibodies against Chimpanzee Adenovirus 63 and Human Adenovirus 5 in Kenyan Children, in the Context of Vaccine Vector. Vaccine 2009, 27, 3501–3504. [Google Scholar] [CrossRef]
- Morris, S.J.; Sebastian, S.; Spencer, A.J.; Gilbert, S.C. Simian adenoviruses as vaccine vectors. Future Virol. 2016, 11, 649–659. [Google Scholar] [CrossRef]
- Van Doremalen, N.; Haddock, E.; Feldmann, F.; Meade-White, K.; Bushmaker, T.; Fischer, R.J.; Okumura, A.; Hanley, P.W.; Saturday, G.; Edwards, N.J.; et al. A Single Dose of ChAdOx1 MERS Provides Protective Immunity in Rhesus Macaques. Sci. Adv. 2020, 6, eaba8399. [Google Scholar] [CrossRef]
- Stanley, D.A.; Honko, A.N.; Asiedu, C.; Trefry, J.C.; Lau-Kilby, A.W.; Johnson, J.C.; Hensley, L.; Ammendola, V.; Abbate, A.; Grazioli, F.; et al. Chimpanzee adenovirus vaccine generates acute and durable protective immunity against ebolavirus challenge. Nat. Med. 2014, 20, 1126–1129. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Gui, M.; Wang, X.; Xiang, Y. Cryo-EM structure of the SARS coronavirus spike glycoprotein in complex with its host cell receptor ACE2. PLoS Pathog. 2018, 14, e1007236. [Google Scholar] [CrossRef]
- Le, T.T.; Andreadakis, Z.; Kumar, A.; Gómez Román, R.; Tollefsen, S.; Saville, M.; Mayhew, S. The COVID-19 vaccine development landscape. Nat. Rev. Drug Discov. 2020, 19, 305–306. [Google Scholar] [CrossRef] [PubMed]
- Dearlove, B.; Lewitus, E.; Bai, H.; Li, Y.; Reeves, D.B.; Joyce, M.G.; Scott, P.T.; Amare, M.F.; Vasan, S.; Michael, N.L.; et al. A SARS-CoV-2 vaccine candidate would likely match all currently circulating variants. Proc. Natl. Acad. Sci. USA 2020, 117, 23652–23662. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.H.; Cai, L.; Cheng, Z.S.; Cheng, H.; Deng, T.; Fan, Y.P.; Fang, C.; Huang, D.; Huang, L.Q.; Huang, Q.; et al. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version). Mil. Med. Res. 2020, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Mueller, A.L.; McNamara, M.S.; Sinclair, D.A. Why does COVID-19 disproportionately affect older people? Aging 2020, 12, 9959. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Tang, J.; Wei, F. Updated understanding of the outbreak of 2019 novel coronavirus (2019-nCoV) in Wuhan, China. J. Med. Virol. 2020, 92, 441–447. [Google Scholar] [CrossRef]
- Lee, N.; Hui, D.; Wu, A.; Chan, P.; Cameron, P.; Joynt, G.M.; Ahuja, A.; Yung, M.Y.; Leung, C.B.; To, K.F.; et al. A major outbreak of severe acute respiratory syndrome in Hong Kong. N. Engl. J. Med. 2003, 348, 1986–1994. [Google Scholar] [CrossRef]
- Assiri, A.; Al-Tawfiq, J.A.; Al-Rabeeah, A.A.; Al-Rabiah, F.A.; Al-Hajjar, S.; Al-Barrak, A.; Flemban, H.; Al-Nassir, W.N.; Balkhy, H.H.; Al-Hakeem, R.F.; et al. Epidemiological, Demographic, and Clinical Characteristics of 47 Cases of Middle East Respiratory Syndrome Coronavirus Disease from Saudi Arabia: A Descriptive Study. Lancet Infec. Dis. 2013, 13, 752–761. [Google Scholar] [CrossRef]
- Eikenberry, S.E.; Mancuso, M.; Iboi, E.; Phan, T.; Eikenberry, K.; Kuang, Y.; Kostelich, E.; Gumel, A.B. To mask or not to mask: Modeling the potential for face mask use by the general public to curtail the COVID-19 pandemic. Infect. Dis. Model. 2020, 5, 293–308. [Google Scholar] [CrossRef] [PubMed]
- Finset, A.; Bosworth, H.; Butow, P.; Gulbrandsen, P.; Hulsman, R.L.; Pieterse, A.H.; Street, R.; Tschoetschel, R.; van Weert, J. Effective health communication—A key factor in fighting the COVID-19 pandemic. Patient Educ. Couns. 2020, 103, 873–876. [Google Scholar] [CrossRef] [PubMed]
- Graham Carlos, W.; Dela Cruz, C.S.; Cao, B.; Pasnick, S.; Jamil, S. Novel Wuhan (2019-NCoV) coronavirus. Am. J. Respir. Crit. Care Med. 2020, 201, P7–P8. [Google Scholar] [CrossRef]
- Chakraborty, I.; Maity, P. COVID-19 outbreak: Migration, effects on society, global environment and prevention. Sci. Total Environ. 2020, 728, 138882. [Google Scholar] [CrossRef]
- Ali, I.; Alharbi, O.M.L. COVID-19: Disease, management, treatment, and social impact. Sci. Total Environ. 2020, 728, 138861. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Maqsood, A.; Abduljabbar, T.; Vohra, F. Tobacco smoking a potential risk factor in transmission of COVID-19 infection. Pakistan J. Med. Sci. 2020, 36, S104–S107. [Google Scholar] [CrossRef]
- Knoll, M.D.; Wonodi, C. Oxford–AstraZeneca COVID-19 vaccine efficacy. Lancet 2021, 397, 72–74. [Google Scholar] [CrossRef]
| Coronaviruses Strains | Sequence Similarity |
|---|---|
| SARSr-CoV; RaTG13 | 96.20% |
| bat-SL-CoVZC45 | 88.00% |
| bat-SL-CoVZXC21 | 88.00% |
| SARS-CoV | 82.45% |
| SARS-HCoV Tor2 | 82.00% |
| SARS-HCoV BJ01 | 82.00% |
| MERS-CoV | 69.58% |
| HCoV-OC43 | 68.93% |
| HCoV-HKU1 | 67.59% |
| HCoV-229E | 65.04% |
| HCoV-NL63 | 65.11% |
| Gene | SARS NC_004718.3 | Bat MG772934.1 | Bat DQ022305.2 |
|---|---|---|---|
| ORF1ab | 86.12% | 95.15% | 85.78% |
| ORF3a | 72.36% | 92.00% | 72.99% |
| ORF6 | 68.85% | 93.44% | 67.21% |
| ORF7a | 85.25% | 88.43% | 88.52% |
| ORF7b | 81.40% | 93.02% | 79.07% |
| ORF8 | 30.16% | 94.21% | 57.02% |
| ORF10 | 72.45% | 73.20% | 74.23% |
| S (Spike) | 75.96% | 80.32% | 76.04% |
| E (Envelope) | 94.74% | 100% | 94.74% |
| M (Membrane) | 90.54% | 98.65% | 90.99% |
| N (Nucleo-capsid) | 90.52% | 94.27% | 89.55% |
| Risk Factors | Total Patients (191) | Survivors (137) | Non-Survivors (54) |
|---|---|---|---|
| Comorbidity | 91 (48%) | 55 (40%) | 36 (67%) |
| Hypertension | 58 (30%) | 32 (23%) | 26 (48%) |
| Diabetes | 36 (19%) | 19 (14%) | 17 (31%) |
| Coronary heart disease | 15 (8%) | 2 (1%) | 13 (24%) |
| Chronic obstructive lung disease | 6 (3%) | 2 (1%) | 4 (7%) |
| Carcinoma | 2 (1%) | 2 (1%) | 0 |
| Chronic kidney disease | 2 (1%) | 0 | 2 (4%) |
| Other | 22 (12%) | 11 (8%) | 11 (20%) |
| Laboratory Markers | Survivors | Non-Survivors |
|---|---|---|
| Lymphocytes count | Initially low but with hospitalization (after 7 days) it improved | Lymphopenia observed (a low number of lymphocytes) |
| Blood d-dimer levels | Normal level | A very high level which increased with worsening of the disease |
| High-sensitivity cardiac troponin I | Normal level | A very high level (after 16 days of disease onset) which increased with worsening of the disease. |
| Serum ferritin | Normal level | A very high level which increased with worsening of the disease |
| Lactate dehydrogenase | Increased with the early onset of illness but normalized/decreased after 13 days. | A very high level which increased with worsening of the disease |
| IL-6 | Normal level | A very high level which increased with worsening of the disease |
| SOFA | Low | High |
| Antiviral Compounds | Drug’s Status | Compound’s Functions to Inhibit Viral Action | References |
|---|---|---|---|
| Favipiravir (T-705), a guanine analogue | Approved for influenza treatment | Effectively inhibits the RNA-dependent RNA polymerase of RNA viruses such as influenza, Ebola, yellow fever, chikungunya, norovirus, and enterovirus. | [98] |
| Favipiravir+baloxavir marboxil, favipiravir+ interferon-α | An approved influenza inhibitor | Targeting the cap-dependent endonuclease. | [99] |
| Ribavirin (guanine derivative) | Approved for treating HCV and respiratory syncytial virus (RSV) that has been evaluated in patients with SARS and MERS | The mechanism is not understood yet. | [100] |
| Remdesivir (GS-5734), phosphoramidate prodrug of an adenine derivative | Approved HIV reverse transcriptase inhibitor | Has broad-spectrum activities against RNA viruses such as MERS and SARS in cell cultures and animal models, and has been tested in a clinical trial for Ebola; it also inhibits SARS-CoV-2 in vitro [99], and patients recovered in the US after administration [101]. | [99] |
| Galidesivir (adenosine analogue) | Approved (originally developed for HCV, also has shown antiviral activities in preclinical studies against many RNA viruses, including SARS and MERS). | The mechanism is not understood yet. | [100] |
| Disulfiram (Protease inhibitor) | An approved drug to treat alcohol dependence. | Inhibit the papain-like protease of MERS and SARS. | |
| Lopinavir and ritonavir | Approved HIV protease inhibitors. | Inhibit the 3-chymotrypsin-like protease of SARS and MERS. | [101] |
| Griffithsin (red algae-derived lectin) | Approved to treat HIV. | Binds to oligosaccharides on the surface of various viral glycoproteins, including HIV glycoprotein 120 and SARS-CoV spike glycoprotein. | [100] |
| Pegylated interferon alfa-2a and -2b | Approved to treat HCV and HBV. | Stimulate innate antiviral responses in patients infected with 2019-nCoV. | [99] |
| Chloroquine | Approved immune modulator. | Triggers the Glycosylate viral cell’s receptors and increases endosomal PH while also acting as autophagy inhibitors. | [84] |
| Nitazoxanide | Approved for diarrhea treatment. | The mechanism is not understood yet. | [94] |
| Monoclonal (immunoglobulin G1 (MHAA4549A, VIS410) and polyclonal antibodies (SAB-301) | Approved for influenza, however, trials are continuing against SARS-CoV-2. | Several antibodies have been shown to bind influenza virus haemagglutinin and inhibit virus replication. | [102] |
| Convalescent sera (prepared from a patient’s blood, acts as a type of passive immunization) | Approved (Target cytomegalovirus, hepatitis B virus, and varicella-zoster virus). | The mechanism has not been described yet. | [94] |
| Nafamostat | Potent against MERS-CoV | Prevents membrane fusion | |
| Pathways inhibitors (Fedratinib, Sunitinib, Baricitinib, and Erlotinib) | Approved for medical use. | Inhibits the AAK (AP2-associated protein kinase 1) pathway, which involves endocytosis. Baricitinib also inhibits cyclin G-associated kinase, which is another regulator of endocytosis. | [103] |
| Type of Platform | Name of Candidate Vaccine | Doses | Manufacturer | Status | Countries Authorized for Emergency Use/Trials |
|---|---|---|---|---|---|
| Non-replicating viral vector | Ad5-nCoV | 1 | CanSino Biological Inc./Beijing Institute of Biotechnology | Approved | China and Mexico |
| Sputnik V | 2 | Gamaleya Research Institute; Health Ministry of the Russian Federation | Approved | Russia, Belarus, Argentina, Hungary, UAE, Algeria, Bolivia, Serbia, Palestinian territories, and Iran | |
| AZD1222 | 2 | AstraZeneca + University of Oxford | Approved | UK, Argentina, El Salvador, India, Mexico, Bangladesh, the Dominican Republic, Pakistan, the Philippines, Nepal, Brazil, and Sri Lanka | |
| Ad26.COV2-S | 2 | Janssen Pharmaceutical | Phase III | USA, Brazil, Chile, Colombia, Mexico, Peru, South Africa, Ukraine, and the Philippines | |
| Inactivated virus | BBIBP-CorV | 2 | Sinopharm + China National Biotec Group Co + Wuhan Institute of Biological Products | Approved | Bahrain, China, Egypt, Iraq, Jordan, Pakistan, Seychelles, and the UAE |
| CoronaVac | 2 | Sinovac Research and Development Co., Ltd. | Approved | China, Indonesia, Brazil, and Turkey | |
| WIBP | 2 | Sinopharm + Wuhan Institute of Biological | Phase III | China | |
| Covaxin | 2 | Bharat Biotech International Limited | Approved | India | |
| RNA | Comirnaty (BNT162b2) | 2 | Pfizer/BioNTech + Fosun Pharma | Approved | The UK, Europe, Argentina, Australia, Bahrain, Canada, Chile, Costa Rica, Ecuador, Hong Kong, Iraq, Israel, Jordan, Kuwait, Mexico, Oman, Panama, the Philippines, Qatar, Saudi Arabia, Singapore, the UAE, and the USA |
| mRNA-1273 | 2 | Moderna + National Institute of Allergy and Infectious Diseases (NIAID) | Approved | Austria, Belgium, Bulgaria, Canada, Croatia, Cyprus, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Iceland, Ireland, Israel, Italy, Latvia, Liechtenstein, Lithuania, Luxembourg, Malta, Mongolia, Netherlands, Norway, Poland, Portugal, Romania, Seychelles, Slovakia, Slovenia, Spain, Sweden, Switzerland, the UK, and the USA | |
| Protein subunit | NVX-CoV2373 | 2 | Novavax | Phase III | The UK, the USA |
| ZF2001 | 3 | Anhui Zhifei Longcom Biopharmaceutical + Institute of Microbiology, Chinese Academy of Sciences | Phase III | China | |
| EpiVac-Corona | 2 | Federal Budgetary Research Institution State Research Center of Virology and Biotechnology Russia | Approved | Russia |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mehmood, I.; Ijaz, M.; Ahmad, S.; Ahmed, T.; Bari, A.; Abro, A.; Allemailem, K.S.; Almatroudi, A.; Tahir ul Qamar, M. SARS-CoV-2: An Update on Genomics, Risk Assessment, Potential Therapeutics and Vaccine Development. Int. J. Environ. Res. Public Health 2021, 18, 1626. https://doi.org/10.3390/ijerph18041626
Mehmood I, Ijaz M, Ahmad S, Ahmed T, Bari A, Abro A, Allemailem KS, Almatroudi A, Tahir ul Qamar M. SARS-CoV-2: An Update on Genomics, Risk Assessment, Potential Therapeutics and Vaccine Development. International Journal of Environmental Research and Public Health. 2021; 18(4):1626. https://doi.org/10.3390/ijerph18041626
Chicago/Turabian StyleMehmood, Iqra, Munazza Ijaz, Sajjad Ahmad, Temoor Ahmed, Amna Bari, Asma Abro, Khaled S. Allemailem, Ahmad Almatroudi, and Muhammad Tahir ul Qamar. 2021. "SARS-CoV-2: An Update on Genomics, Risk Assessment, Potential Therapeutics and Vaccine Development" International Journal of Environmental Research and Public Health 18, no. 4: 1626. https://doi.org/10.3390/ijerph18041626
APA StyleMehmood, I., Ijaz, M., Ahmad, S., Ahmed, T., Bari, A., Abro, A., Allemailem, K. S., Almatroudi, A., & Tahir ul Qamar, M. (2021). SARS-CoV-2: An Update on Genomics, Risk Assessment, Potential Therapeutics and Vaccine Development. International Journal of Environmental Research and Public Health, 18(4), 1626. https://doi.org/10.3390/ijerph18041626









