Heavy-Metal Phytoremediation from Livestock Wastewater and Exploitation of Exhausted Biomass
Abstract
1. Sustainability in Animal Production
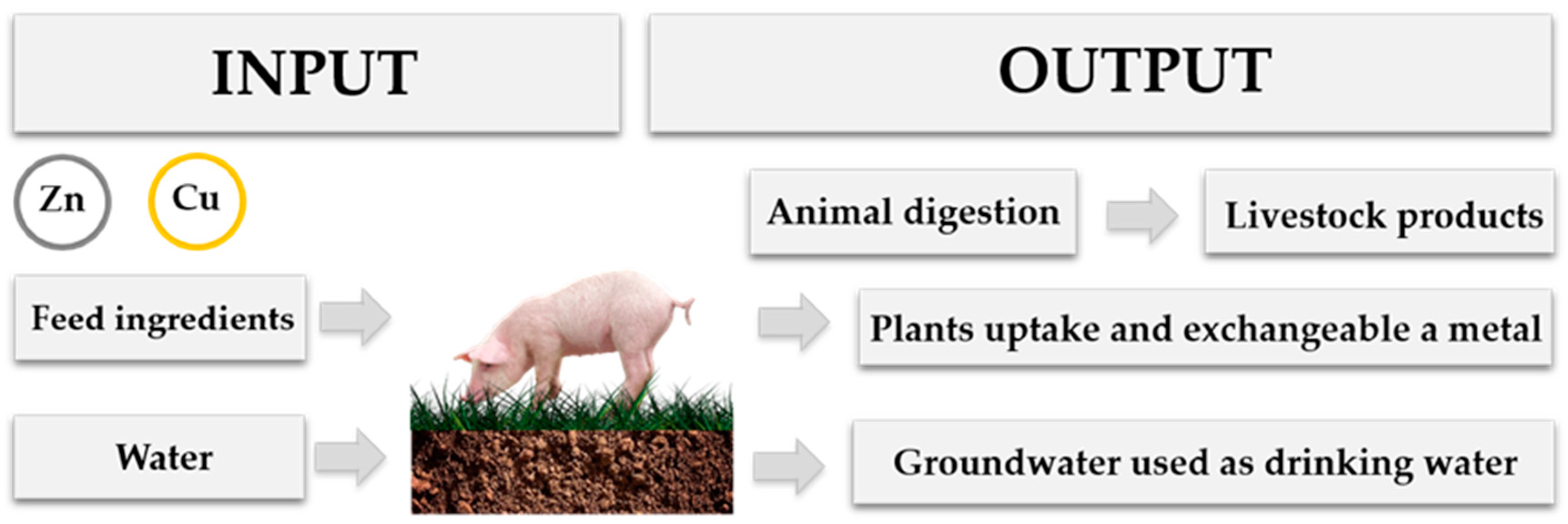
2. The Importance of Heavy-Metals Use in Intensive Animal Production
3. The Importance of Zinc and Copper as Alternative to Antibiotics in Animal Feeding
4. Heavy Metals and Their Impact on the Environment
5. How Can Plants Remove Metals from Livestock Wastewater?
5.1. How Plants Function in the Phytoremediation of Heavy Metals
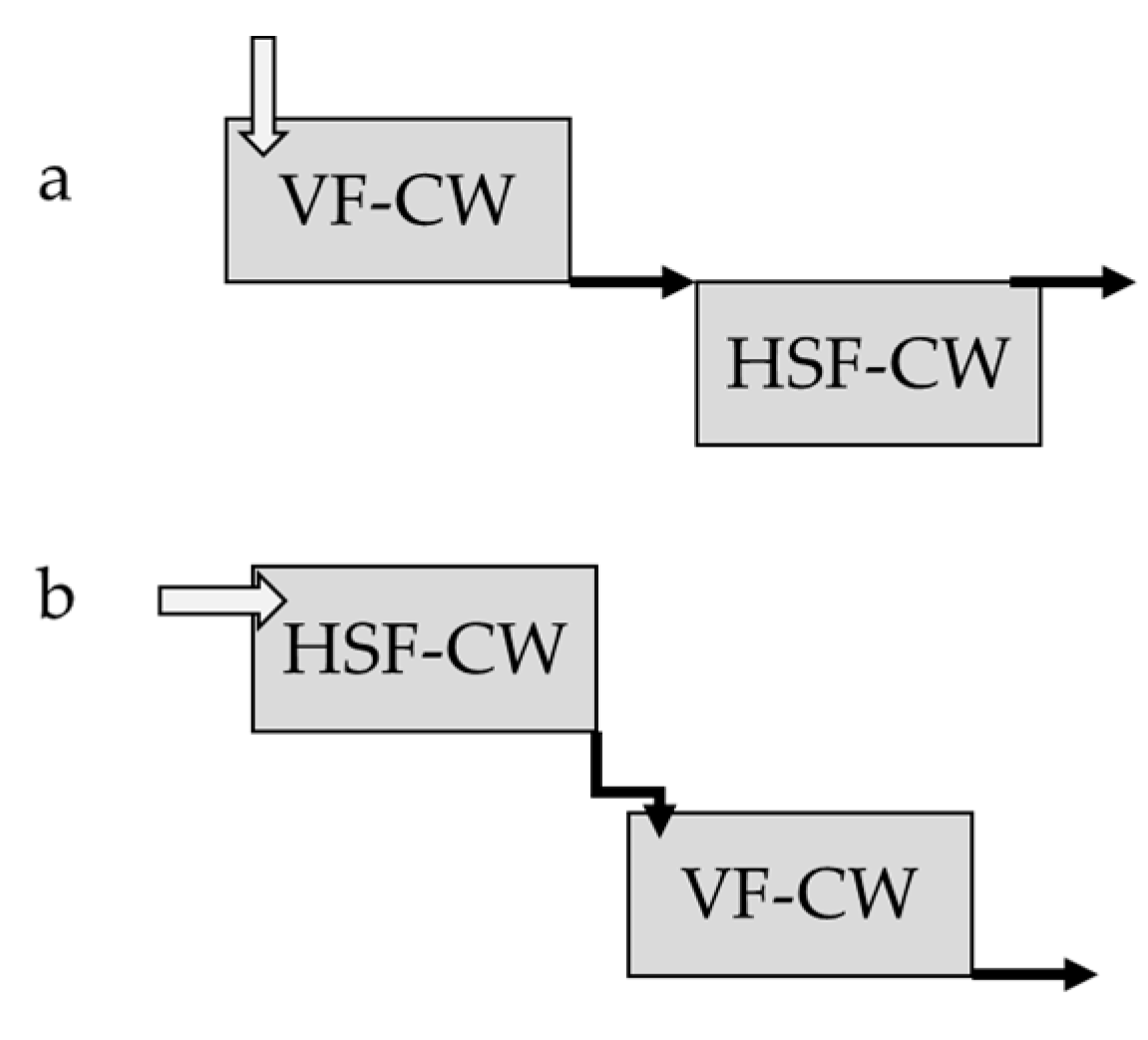
5.2. CWs in the Phytoremediation of Heavy Metals from Livestock Wastewater
6. Plant Reuse after Phytoremediation
6.1. Incineration
6.2. Biotechnological Process
7. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Kesavan, P.C.; Swaminathan, M.S. Strategies and models for agricultural sustainability in developing Asian countries. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 877–891. [Google Scholar] [CrossRef]
- Pretty, J. Agricultural sustainability: Concepts, principles and evidence. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 447–465. [Google Scholar] [CrossRef]
- Velten, S.; Leventon, J.; Jager, N.; Newig, J. What Is Sustainable Agriculture? A Systematic Review. Sustainability 2015, 7, 7833–7865. [Google Scholar] [CrossRef]
- Hysa, E.; Kruja, A.; Rehman, N.U.; Laurenti, R. Circular Economy Innovation and Environmental Sustainability Impact on Economic Growth: An Integrated Model for Sustainable Development. Sustainability 2020, 12, 4831. [Google Scholar] [CrossRef]
- Raubenheimer, D.; Simpson, S.J.; Mayntz, D. Nutrition, ecology and nutritional ecology: Toward an integrated framework. Funct. Ecol. 2009, 23, 4–16. [Google Scholar] [CrossRef]
- Bhargava, A.; Carmona, F.F.; Bhargava, M.; Srivastava, S. Approaches for enhanced phytoextraction of heavy metals. J. Environ. Manag. 2012, 105, 103–120. [Google Scholar] [CrossRef]
- Govind, P.; Madhuri, S. Heavy metals causing toxicity in animals and fishers. J. Anim. Sci. 2014, 2, 17–23. [Google Scholar]
- Dai, S.Y.; Jones, B.; Lee, K.-M.; Li, W.; Post, L.; Herrman, T.J. Heavy metal contamination of animal feed in Texas. JRS 2016, 1, 21–32. [Google Scholar]
- Giromini, C.; Rebucci, R.; Fusi, E.; Rossi, L.; Saccone, F.; Baldi, A. Cytotoxicity, apoptosis, DNA damage and methylation in mammary and kidney epithelial cell lines exposed to ochratoxin A. Cell Biol. Toxicol. 2016, 32, 249–258. [Google Scholar] [CrossRef]
- Santos, D.; Luzio, R.V.A.; Félix, L. Chapter five—Zebrafish early life stages for toxicological screening: Insights from molecular and biochemical markers. Adv. Mol. Toxicol. 2018, 12, 151–179. [Google Scholar]
- Rossi, L.; Dell’Orto, V.; Vagni, S.; Sala, V.; Reggi, S.; Baldi, A. Protective effect of oral administration of transgenic tobacco seeds against verocytotoxic Escherichia coli strain in piglets. Vet. Res. Commun. 2014, 38, 39–49. [Google Scholar] [CrossRef]
- Hejna, M.; Gottardo, D.; Baldi, A.; Dell’Orto, V.; Cheli, F.; Zaninelli, M.; Rossi, L. Review: Nutritional ecology of heavy metals. Animal 2018, 12, 2156–2170. [Google Scholar] [CrossRef]
- Fink-Gremmels, J. Animal Feed Contamination. Effects on Livestock and Food Safety; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Cambridge, UK, 2012. [Google Scholar]
- Suttle, N.F. Mineral Nutrition of Livestock, 4th ed.; CABI: Oxfordshire, UK, 2010. [Google Scholar]
- López-Alonso, M.; García-Vaquero, M.; Benedito, J.L.; Castillo, C.; Miranda, M. Trace mineral status and toxic metal accumulation in extensive and intensive pigs in NW Spain. Livest. Sci. 2012, 146, 47–53. [Google Scholar] [CrossRef]
- European Commission. European Parliament and of the Council. Directive 2002/32/EC of 7 May 2002 on Undesirable Substances in Animal Feed. Off. J. Eur. Commun. 2002, L140, 10–21. [Google Scholar]
- Hejna, M.; Moscatelli, A.; Onelli, E.; Baldi, A.; Pilu, S.; Rossi, L. Evaluation of concentration of heavy metals in animal rearing system. Ital. J. Anim. Sci. 2019, 18, 1372–1384. [Google Scholar] [CrossRef]
- Kim, K.; He, Y.; Xiong, X.; Ehrlich, A.; Li, X.; Raybould, H.; Atwill, E.R.; Maga, E.A.; Jørgensen, J.; Liu, Y. Dietary supplementation of Bacillus subtilis influenced intestinal health of weaned pigs experimentally infected with a pathogenic E. coli. J. Anim. Sci. Biotechnol. 2019, 10, 52. [Google Scholar] [CrossRef]
- WHO. Guidelines on Use of Medically Important Antimicrobials in Food-Producing Animals; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- European Parliament and the Council. Regulation (EC) No. 1831/2003 of 22 September 2003 on Additives for Use in Animal Nutrition. Off. J. Eur. Union 2003, 268, 29–43. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32003R1831 (accessed on 6 September 2020).
- Commission Regulation (EC) No. 1334/2003 of 25 July 2003 Amending the Conditions for Authorisation of a Number of Additives in Feedingstuffs Belonging to the Group of Trace Elements. Off. J. Eur. Union 2003, 187, 11–15. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32003R1334 (accessed on 10 September 2020).
- Sales, J. Effects of Pharmacological Concentrations of Dietary Zinc Oxide on Growth of Post-weaning Pigs: A Meta-analysis. Biol. Trace Elem. Res. 2013, 152, 343–349. [Google Scholar] [CrossRef]
- Walk, C.L.; Wilcock, P.R.; Magowan, E. Evaluation of the effects of pharmacological zinc oxide and phosphorus source on weaned piglet growth performance, plasma minerals and mineral digestibility. Animal 2015, 9, 1145–1152. [Google Scholar] [CrossRef]
- Polen, T.; Voia, O.S. Copper effect of feed supplementation on growth performance in fattening pigs. Anim. Sci. J. 2015, 48, 28–30. [Google Scholar]
- Suleiman, N.; Ibitoye, E.B.; Jimoh, A.A.; Sani, Z.A. Assessment of heavy metals in chicken feeds available in Sokoto, Nigeria. Sokoto J. Vet. Sci. 2015, 13, 17–21. [Google Scholar]
- Adewole, D.; Kim, I.H.; Nyachoti, C.M. Gut Health of Pigs: Challenge Models and Response Criteria with a Critical Analysis of the Effectiveness of Selected Feed Additives—A Review. Asian Australas. J. Anim. Sci. 2015, 29, 909–924. [Google Scholar] [CrossRef]
- Nicholson, F.A.; Smith, S.R.; Alloway, B.J.; Carlton-Smith, C.; Chambers, B.J. An inventory of heavy metals inputs to agricultural soils in England and Wales. Sci. Total Environ. 2003, 311, 205–219. [Google Scholar] [CrossRef]
- Luo, L.; Ma, Y.; Zhang, S.; Wei, D.; Zhu, Y.-G. An inventory of trace element inputs to agricultural soils in China. J. Environ. Manag. 2009, 90, 2524–2530. [Google Scholar] [CrossRef]
- Zhang, F.; Li, Y.; Yang, M.; Li, W. Content of Heavy Metals in Animal Feeds and Manures from Farms of Different Scales in Northeast China. Int. J. Environ. Res. Public Health 2012, 9, 2658–2668. [Google Scholar] [CrossRef]
- Jakubus, M.; Dach, J.; Starmans, D. Biovailability of copper and zinc in pig and cattle slurries. Fresenius Environ. Bull. 2013, 22, 995–1002. [Google Scholar]
- Chardon, X.; Rigolot, C.; Leterme, P.; Paillat, J.; Delaby, L.; Garcia, F.; Peyraud, J.; Poupa, J.; Morvan, T.; Faverdin, P.; et al. MELODIE: A whole-farm model to study the dynamics of nutrients in dairy and pig farms with crops. Animal 2012, 6, 1711–1721. [Google Scholar] [CrossRef]
- Ingelmo, F.; Molina, M.J.; Soriano, M.D.; Gallardo, A.; Lapeña, L. Influence of organic matter transformations on the bioavailability of heavy metals in a sludge based compost. J. Environ. Manag. 2012, 95, S104–S109. [Google Scholar] [CrossRef]
- Wang, H.; Dong, Y.; Yang, Y.S.; Toor, G.S.; Zhang, X. Changes in heavy metal contents in animal feeds and manures in an intensive animal production region of China. J. Environ. Sci. 2013, 25, 2435–2442. [Google Scholar] [CrossRef]
- Belon, E.; Boisson, M.; Deportes, I.; Eglin, T.; Feix, I.; Bispo, A.; Galsomies, L.; Leblond, S.; Guellier, C. An inventory of trace elements inputs to French agricultural soils. Sci. Total Environ. 2012, 439, 87–95. [Google Scholar] [CrossRef]
- Moral, R.; Moreno-Caselles, J.; Perez-Murcia, M.; Pérez-Espinosa, A.; Rufete, B.; Paredes, C. Characterisation of the organic matter pool in manures. Bioresour. Technol. 2005, 96, 153–158. [Google Scholar] [CrossRef]
- Gul, S.; Naz, A.; Fareed, I.; Khan, A.; Irshad, M. Speciation of heavy metals during co-composting of livestock manure. Pol. J. Chem. Technol. 2015, 17, 19–23. [Google Scholar] [CrossRef]
- Lyubenova, L.; Schreoder, P. Plants for wastewater treatment—Effects of heavy metals on the detoxification system of Typha latifolia. Bioresour. Technol. 2011, 996–1004. [Google Scholar] [CrossRef]
- Yazdankhah, S.; Rudi, K.; Bernhoft, A. Zinc and copper in animal feed-development of resistance and co-resistance to antimicrobial agents in bacteria of animal origin. Microb. Ecol. Health Dis. 2014, 25. [Google Scholar] [CrossRef]
- Seiler, C.; Berendonk, T.U. Heavy metal driven co-selection of antibiotic resistance in soil and water bodies impacted by agriculture and aquaculture. Front. Microbiol. 2012, 3, 399. [Google Scholar] [CrossRef]
- Aarestrup, F.M.; Cavaco, L.; Hasman, H. Decreased susceptibility to zinc chloride is associated with methicillin resistant Staphylococcus aureus CC398 in Danish swine. Vet. Microbiol. 2010, 142, 455–457. [Google Scholar] [CrossRef]
- Cavaco, L.M.; Hasman, H.; Stegger, M.; Andersen, P.S.; Skov, R.; Fluit, A.C.; Ito, T.; Aarestrup, F.M. Cloning and Occurrence of czrC, a Gene Conferring Cadmium and Zinc Resistance in Methicillin-Resistant Staphylococcus aureus CC398 Isolates. Antimicrob. Agents Chemother. 2010, 54, 3605–3608. [Google Scholar] [CrossRef]
- Järup, L. Hazards of heavy metal contamination. Br. Med. Bull. 2003, 68, 167–182. [Google Scholar] [CrossRef]
- European Medicine Agency (EMA). Questions and Answers on Veterinary Medicinal Products Containing Zinc Oxide to Be Administered Orally to Food-Producing Species; EMA No. 394961; EMA: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Commission Implementing Regulation (EU) 2018/1039 of 23 July 2018 concerning the authorisation of Copper(II) diacetate monohydrate, Copper(II) carbonate dihydroxy monohydrate, Copper(II) chloride dihydrate, Copper(II) oxide, Copper(II) sulphate pentahydrate, Copper(II) chelate of amino acids hydrate, Copper(II) chelate of protein hydrolysates, Copper(II) chelate of glycine hydrate (solid) and Copper(II) chelate of glycine hydrate (liquid) as feed additives for all animal species and amending Regulations (EC) No 1334/2003, (EC) No 479/2006 and (EU) No. 349/2010 and Implementing Regulations (EU) No. 269/2012, (EU) No. 1230/2014 and (EU) 2016/2261. Off. J. Eur. Union 2018, 186, 3–24. Available online: https://eur-lex.europa.eu/eli/reg_impl/2018/1039/oj (accessed on 5 September 2020).
- Lee, C.-Y.; Lee, C.-C.; Lee, F.-Y.; Tseng, S.-K.; Liao, C.-J. Performance of subsurface flow constructed wetland taking pretreated swine effluent under heavy loads. Bioresour. Technol. 2004, 92, 173–179. [Google Scholar] [CrossRef]
- Meers, E.; Rousseau, D.P.L.; Blomme, N.; Lesage, E.; Du Laing, G.; Tack, F.M.G.; Verloo, M.G. Tertiary treatment of the liquid fraction of pig manure with Phragmites australis. Water Air Soil Pollut. 2005, 160, 15–26. [Google Scholar] [CrossRef]
- Knight, R.L.; Payne, V.W.; Borer, R.E.; Clarke, R.A.; Pries, J.H. Constructed wetlands for livestock wastewater management. Ecol. Eng. 2000, 15, 41–55. [Google Scholar] [CrossRef]
- Almeida, C.M.R.; Santos, F.; Ferreira, A.C.F.; Gomes, C.R.; Basto, M.C.P.; Mucha, A.P. Constructed wetlands for the removal of metals from livestock wastewater—Can the presence of veterinary antibiotics affect removals? Ecotoxicol. Environ. Saf. 2016, 137, 143–148. [Google Scholar] [CrossRef]
- Hejna, M.; Moscatelli, A.; Stroppa, N.; Onelli, E.; Pilu, S.; Baldi, A.; Rossi, L. Bioaccumulation of heavy metals from wastewater through a Typha latifolia and Thelypteris palustris phytoremediation system. Chemosphere 2020, 241, 125018. [Google Scholar] [CrossRef]
- Stroppa, N.; Onelli, E.; Hejna, M.; Rossi, L.; Gagliardi, A.; Bini, L.; Baldi, A.; Moscatelli, A. Typha latifolia and Thelypteris palustris behavior in a pilot system for the refinement of livestock wastewaters: A case of study. Chemosphere 2020, 240, 124915. [Google Scholar] [CrossRef]
- Ali, S.; Abbas, Z.; Rizwan, M.; Zaheer, I.E.; Yavaş, I.; Ünay, A.; Abdel-Daim, M.M.; Bin-Jumah, M.; Hasanuzzaman, M.; Kalderis, D. Application of Floating Aquatic Plants in Phytoremediation of Heavy Metals Polluted Water: A Review. Sustainability 2020, 12, 1927. [Google Scholar] [CrossRef]
- Yan, A.; Wang, Y.; Tan, S.N.; Yusof, M.L.M.; Ghosh, S.; Chen, Z. Phytoremediation: A Promising Approach for Revegetation of Heavy Metal-Polluted Land. Front. Plant Sci. 2020, 11, 359. [Google Scholar] [CrossRef]
- Kadlec, R.H.; Knight, R.L.; Vymazal, J.; Brix, H.; Cooper, P.; Haberl, R. Constructed Wetlands for Pollution Control: Processes, Performance, Design and Operation; Scientific and Technical Report; IWA Publishing: London, UK, 2000; Volume 8, pp. 1–15. [Google Scholar]
- Stefanakis, A.; Akratos, C.; Tsihrintzis, V. Constructed Wetlands Classification. In Vertical Flow Constructed Wetlands; Elsevier: Amsterdam, The Netherlands, 2014; pp. 17–25. [Google Scholar]
- Fitch, M.W. Constructed Wetlands. Compr. Water Qual. Purif. 2014, 3, 268–295. [Google Scholar] [CrossRef]
- Jutsz, A.M.; Gnida, A. Mechanisms of stress avoidance and tolerance by plants used in phytoremediation of heavy metals. Arch. Environ. Prot. 2015, 41, 104–114. [Google Scholar] [CrossRef]
- Dalvi, A.A.; Bhalerao, S.A. Response of plants towards heavy metal toxicity: An overview of avoidance, tolerance and uptake mechanism. Ann. Plant Sci. 2013, 2, 362–368. [Google Scholar]
- Miransari, M. Hyperaccumulators, arbuscular mycorrhizal fungi and stress of heavy metals. Biotechnol. Adv. 2011, 29, 645–653. [Google Scholar] [CrossRef]
- Memon, A.R.; Schröder, P. Implications of metal accumulation mechanisms to phytoremediation. Environ. Sci. Pollut. Res. 2009, 16, 162–175. [Google Scholar] [CrossRef]
- Krzeslowska, M. The wall cell in plant cell response to trace metals: Polysaccharide remodeling and its role in defense strategy. Acta Physiol. Plant. 2011, 33, 35–51. [Google Scholar] [CrossRef]
- Colzi, I.; Arnetoli, M.; Gallo, A.; Doumett, S.; Del Bubba, M.; Pignattelli, S.; Gabbrielli, R.; Gonnelli, C. Copper tolerance strategies involving the root cell wall pectins in Silene paradoxa L. Environ. Exp. Bot. 2012, 78, 91–98. [Google Scholar] [CrossRef]
- Le Gall, H.; Philippe, F.; Domon, J.-M.; Gillet, F.; Pelloux, J.; Rayon, C. Cell Wall Metabolism in Response to Abiotic Stress. Plants 2015, 4, 112–166. [Google Scholar] [CrossRef]
- Oves, M.; Saghir Khan, M.; Huda Qari, A.; Nadeen Felemban, M.; Almeelbi, T. Heavy metals: Biological importance and detoxification strategies. J. Biorem. Biodegrad. 2016, 7, 334. [Google Scholar] [CrossRef]
- Meier, S.; Alvear, M.; Borie, F.; Aguilera, P.; Ginocchio, R.; Cornejo, P. Influence of copper on root exudate patterns in some metallophytes and agricultural plants. Ecotoxicol. Environ. Saf. 2012, 75, 8–15. [Google Scholar] [CrossRef]
- Bradley, R.; Burt, A.J.; Read, D.J. The biology of mycorrhiza in the ericaceae. VIII. The role of mycorrhizal infection in heavy metal resistance. New Phytol. 1982, 91, 197–209. [Google Scholar] [CrossRef]
- Tiwari, S.; Lata, C. Heavy Metal Stress, Signaling, and Tolerance Due to Plant-Associated Microbes: An Overview. Front. Plant Sci. 2018, 9, 452. [Google Scholar] [CrossRef]
- Göhre, V.; Paszkowski, U. Contribution of the arbuscular mycorrhizal symbiosis to heavy metal phytoremediation. Planta 2006, 223, 1115–1122. [Google Scholar] [CrossRef]
- Cicatelli, A.; Torrigiani, P.; Todeschini, V.; Biondi, S.; Castiglione, S.; Lingua, G. Arbuscular mycorrhizal fungi as a tool to ameliorate the phytoremediation potential of poplar: Biochemical and molecular aspects. iForest 2014, 7, 333–341. [Google Scholar] [CrossRef]
- Fasani, E.; Manara, A.; Martini, F.; Furini, A.; DalCorso, G. The potential of genetic engineering of plants for the remediation of soils contaminated with heavy metals. Plant, Cell Environ. 2018, 41, 1201–1232. [Google Scholar] [CrossRef]
- Doganlar, Z.B. Metal accumulation and physiological responses induced by copper and cadmium in Lemna gibba, L. minor and Spirodela polyrhiza. Chem. Speciat. Bioavailab. 2013, 25, 79–88. [Google Scholar] [CrossRef]
- Hasan, K.; Cheng, Y.; Kanwar, M.K.; Chu, X.-Y.; Ahammed, G.J.; Qi, Z.-Y. Responses of Plant Proteins to Heavy Metal Stress—A Review. Front. Plant Sci. 2017, 8, 1492. [Google Scholar] [CrossRef]
- Petraglia, A.; De Benedictis, M.; Degola, F.; Pastore, G.; Calcagno, M.; Ruotolo, R.; Mengoni, A.; Di Toppi, L.S. The capability to synthesize phytochelatins and the presence of constitutive and functional phytochelatin synthases are ancestral (plesiomorphic) characters for basal land plants. J. Exp. Bot. 2014, 65, 1153–1163. [Google Scholar] [CrossRef]
- Yadav, S.K. Heavy metals toxicity in plants: An overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. S. Afr. J. Bot. 2010, 76, 167–179. [Google Scholar] [CrossRef]
- Singh, S.; Parihar, P.; Singh, R.; Singh, V.P.; Prasad, S.M. Heavy Metal Tolerance in Plants: Role of Transcriptomics, Proteomics, Metabolomics, and Ionomics. Front. Plant Sci. 2016, 6, 1143. [Google Scholar] [CrossRef]
- Kumar, S.; Trivedi, P.K. Heavy Metal Stress Signaling in Plants. In Plant Metal Interaction-Emerging Remediation Techniques; Ahmad, P., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 585–603. [Google Scholar]
- Dubey, S.; Shri, M.; Misra, P.; Lakhwani, D.; Bag, S.K.; Asif, M.H.; Trivedi, P.K.; Tripathi, R.D.; Chakrabarty, D. Heavy metals induce oxidative stress and genome-wide modulation in transcriptome of rice root. Funct. Integr. Genom. 2014, 14, 401–417. [Google Scholar] [CrossRef]
- Collin, V.C.; Eymery, F.; Genty, B.; Rey, P.; Havaux, M. Vitamin E is essential for the tolerance of Arabidopsis thaliana to metal-induced oxidative stress. Plant Cell Environ. 2008, 31, 244–257. [Google Scholar] [CrossRef]
- Lingua, G.; Bona, E.; Todeschini, V.; Cattaneo, C.; Marsano, F.; Berta, G.; Cavaletto, M. Effects of Heavy Metals and Arbuscular Mycorrhiza on the Leaf Proteome of a Selected Poplar Clone: A Time Course Analysis. PLoS ONE 2012, 7, e38662. [Google Scholar] [CrossRef]
- Rao, K.P.; Vani, G.; Kumar, K.; Wankhede, D.P.; Misra, M.; Gupta, M.; Sinha, A.K. Arsenic stress activates MAP kinase in rice roots and leaves. Arch. Biochem. Biophys. 2011, 506, 73–82. [Google Scholar] [CrossRef]
- Viehweger, K. How plants cope with heavy metals. Bot. Stud. 2014, 55, 1–12. [Google Scholar] [CrossRef]
- Seth, C.S. A Review on Mechanisms of Plant Tolerance and Role of Transgenic Plants in Environmental Clean-up. Bot. Rev. 2012, 78, 32–62. [Google Scholar] [CrossRef]
- Harrington, R.; McInnes, R. Integrated Constructed Wetlands (ICW) for livestock wastewater management. Bioresour. Technol. 2009, 100, 5498–5505. [Google Scholar] [CrossRef]
- Sukumaran, D. Phytoremediation of Heavy Metals from Industrial Effluent Using Constructed Wetland Technology. Appl. Ecol. Environ. Sci. 2013, 1, 92–97. [Google Scholar] [CrossRef]
- Mora-Ravelo, S.G.; Alarcón, A.; Rocandio-Rodríguez, M.; Vanoye-Eligio, V. Bioremediation of wastewater for reutilization in agricultural systems: A review. Appl. Ecol. Environ. Res. 2016, 15, 33–50. [Google Scholar] [CrossRef]
- Rai, P.K. Heavy metals/metalloids remediation from wastewater using free floating macrophytes of a natural wetland. Environ. Technol. Innov. 2019, 15, 100393. [Google Scholar] [CrossRef]
- Morari, F.; Ferro, N.D.; Cocco, E. Municipal Wastewater Treatment with Phragmites australis L. and Typha latifolia L. for Irrigation Reuse. Boron and Heavy Metals. Water Air Soil Pollut. 2015, 226, 1–4. [Google Scholar] [CrossRef]
- Cortes-Esquivel, J.A.; Giácoman-Vallejos, G.; Barceló-Quintal, I.D.; Méndez-Novelo, R.; Ponce-Caballero, M.C. Heavy Metals Removal from Swine Wastewater Using Constructed Wetlands with Horizontal Sub-Surface Flow. J. Environ. Prot. 2012, 3, 871–877. [Google Scholar] [CrossRef]
- Weis, J.S.; Weis, P. Metal uptake, transport and release by wetland plants: Implications for phytoremediation and restoration. Environ. Int. 2004, 30, 685–700. [Google Scholar] [CrossRef]
- Yadav, A.K.; Abbassi, R.; Kumar, N.; Satya, S.; Sreekrishnan, T.; Mishra, B. The removal of heavy metals in wetland microcosms: Effects of bed depth, plant species, and metal mobility. Chem. Eng. J. 2012, 211, 501–507. [Google Scholar] [CrossRef]
- Salt, E.; Blaylock, M.; Kumar, N.P.B.A.; Dushenkov, V.; Ensley, D.; Chet, I.; Raskin, I. Phytoremediation: A novel strategy for the removal of toxic metals from the environment using plants. Biotechnology 1995, 13, 468–474. [Google Scholar]
- Brisson, J.; Cogliastro, A.; Robert, M. Controlling Speckled Alder (Alnus incana ssp. rugosa) Invasion in a Wetland Reserve of Southern Québec. Nat. Areas J. 2006, 26, 78–83. [Google Scholar] [CrossRef]
- Lissy, P.N.M.; Madhu, G. Removal of heavy metals from wastewater using water hyacinth. ACEEE Int. J. Transp. Urb. Dev. 2011, 1, 48–52. [Google Scholar]
- Ajayi, T.O.; Ogunbayio, A.O. Achieving Environmental Sustainability in Wastewater Treatment by Phytoremediation with Water Hyacinth (Eichhornia Crassipes). J. Sustain. Dev. 2012, 5, 80–90. [Google Scholar] [CrossRef]
- Barya, M.P.; Gupta, D.; Shukla, R.; Thakur, T.K.; Mishra, V.K. Phytoremediation of heavy metals from mixed domestic sewage through vertical-flow constructed wetland planted with Canna Indica and Acorus Calamus. Curr. World Environ. 2020, 15. Available online: https://www.cwejournal.org/vol15no3/phytoremediation-of-heavy-metals-from-mixed-domestic-sewage-through-vertical--flow-constructed-wetland-planted-with-canna-indica-and-acorus-calamus/ (accessed on 19 October 2020).
- Soda, S.; Hamada, T.; Yamaoka, Y.; Ike, M.; Nakazato, H.; Saeki, Y.; Kasamatsu, T.; Sakurai, Y. Constructed wetlands for advanced treatment of wastewater with a complex matrix from a metal-processing plant: Bioconcentration and translocation factors of various metals in Acorus gramineus and Cyperus alternifolius. Ecol. Eng. 2012, 39, 63–70. [Google Scholar] [CrossRef]
- Fediuc, E.; Erdei, L. Physiological and biochemical aspects of cadmium toxicity and protective mechanisms induced in Phragmites australis and Typha latifolia. J. Plant Physiol. 2002, 159, 265–271. [Google Scholar] [CrossRef]
- Higuchi, K.; Kanai, M.; Tsuchiya, M.; Ishii, H.; Shibuya, N.; Fujita, N.; Nakamura, Y.; Suzui, N.; Fujimaki, S.; Miwa, E. Common reed accumulates starch in its stem by metabolic adaptation under Cd stress conditions. Front. Plant Sci. 2015, 6, 138. [Google Scholar] [CrossRef]
- Yang, J.; Ye, Z. Metal accumulation and tolerance in wetland plants. Front. Biol. China 2009, 4, 282–288. [Google Scholar] [CrossRef]
- Yang, Y.-Y.; Jung, J.-Y.; Song, W.-Y.; Suh, H.-S.; Lee, Y. Identification of Rice Varieties with High Tolerance or Sensitivity to Lead and Characterization of the Mechanism of Tolerance. Plant Physiol. 2000, 124, 1019–1026. [Google Scholar] [CrossRef]
- Gibson, S.I. Control of plant development and gene expression by sugar signaling. Curr. Opin. Plant Biol. 2005, 8, 93–102. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, C. Zinc distribution and zinc-binding forms in Phragmites australis under zinc pollution. J. Plant Physiol. 2008, 165, 697–704. [Google Scholar] [CrossRef]
- Dronnet, V.M.; Renard, C.M.G.C.; Axelos, M.A.V.; Thibault, J.F. Heavy metals binding by pectins: Selectivity, quantification and characterization. Carbohydr. Polym. 1996, 30, 253–263. [Google Scholar] [CrossRef]
- Eticha, D.; Stass, A.; Horst, W.J. Cell-wall pectin and its degree of methylation in the maize root-apex: Significance for genotypic differences in aluminium resistance. Plant Cell Environ. 2005, 28, 1410–1420. [Google Scholar] [CrossRef]
- Sandoval, L.; Zamora-Castro, S.A.; Vidal-Álvarez, M.; Marín-Muñiz, J.L. Role of Wetland Plants and Use of Ornamental Flowering Plants in Constructed Wetlands for Wastewater Treatment: A Review. Appl. Sci. 2019, 9, 685. [Google Scholar] [CrossRef]
- Vigil, M.; Pérez, M.F.M.; Huerta, G.M.M.; Cabal, J.V. Álvarez Is phytoremediation without biomass valorization sustainable? Comparative LCA of landfilling vs. anaerobic co-digestion. Sci. Total Environ. 2015, 505, 844–850. [Google Scholar] [CrossRef]
- Anderson, C.W.N.; Brooks, R.; Chiarucci, A.; Lacoste, C.; Leblanc, M.; Robinson, B.H.; Simcock, R.; Stewart, R.B. Phytomining for nickel, thallium and gold. J. Geochem. Explor. 1999, 67, 407–415. [Google Scholar] [CrossRef]
- Novo, L.A.B.; Castro, P.M.L.; Alvarenga, P.; Da Silva, E.F. Phytomining of Rare and Valuable Metals. Phytoremediation 2017, 13, 469–486. [Google Scholar] [CrossRef]
- Weyens, N.; van der Lelie, D.; Taghavi, S.; Newman, L.; Vangronsveld, J. Exploiting plant–microbe partnerships to improve biomass production and remediation. Trends Biotechnol. 2009, 27, 591–598. [Google Scholar] [CrossRef]
- Gomes, H.I. Phytoremediation for bioenergy: Challenges and opportunities. Environ. Technol. Rev. 2012, 1, 59–66. [Google Scholar] [CrossRef]
- Bauddh, K.; Singh, B.; Korstad, J. Phytoremediation Potential of Bioenergy Plants; Springer Nature: Singapore, 2017. [Google Scholar]
- Bridgwater, A.V.; Meier, D.; Radlein, D. An overview of fast pyrolysis of biomass. Org. Geochem. 1999, 30, 1479–1493. [Google Scholar] [CrossRef]
- Salvo, M.; Rizzo, S.; Caldirola, M.; Novajra, G.; Canonico, F.; Bianchi, M.; Ferraris, M. Biomass ash as supplementary cementitious material (SCM). Adv. Appl. Ceram. 2015, 114 (Suppl. 1), S3–S10. [Google Scholar] [CrossRef]
- Rajamma, R.; Labrincha, J.A.; Ferreira, V.M. Alkali activation of biomass fly ash–metakaolin blends. Fuel 2014, 98, 265–271. [Google Scholar] [CrossRef]
- Luukkonen, T.; Heponiemi, A.; Runtti, H.; Pesonen, J.; Yliniemi, J.; Lassi, U. Application of alkali-activated materials for water and wastewater treatment: A review. Rev. Environ. Sci. Biotechnol. 2019, 18, 271–297. [Google Scholar] [CrossRef]
- European Commission. Study on the EU’s List of Critical Raw Materials (2020); Final Report; Publications Office of the European Union: Brusseles, Belgium, 2020; ISBN 978-92-76-21049-8. [Google Scholar] [CrossRef]
- Edmondson, J.; Holland, A. Materials for Electric Vehicles: Electric Motors, Battery Cells & Packs, HV Cabling 2020–2030. Available online: https://www.idtechex.com/en/research-report/materials-for-electric-vehicles-2020-2030/770 (accessed on 11 October 2020).
- Ebin, B.; Isik, M. Pyrometallurgical Processes for the Recovery of Metals from WEEE. In WEEE Recycling Research, Development, and Policies; Chagnes, A., Cote, G., Ekberg, C., Nilsson, M., Retegan, T., Eds.; Elservier: Amsterdam, The Netherlands, 2016. [Google Scholar] [CrossRef]
- Iannicelli-Zubiani, E.M.; Stampino, P.G.; Cristiani, C.; Dotelli, G. Enhanced lanthanum adsorption by amine modified activated carbon. Chem. Eng. J. 2018, 341, 75–82. [Google Scholar] [CrossRef]
- Iannicelli-Zubiani, E.M.; Cristiani, C.; Dotelli, G.; Stampino, P.G. Recovery of valuable metals from electronic scraps by clays and organo-clays: Study on bi-ionic model solutions. Waste Manag. 2017, 60, 582–590. [Google Scholar] [CrossRef]
- Mata-Alvarez, J.; Macé, S.; Llabrés, P. Anaerobic digestion of organic solid wastes. An overview of research achievements and perspectives. Bioresour. Technol. 2000, 74, 3–16. [Google Scholar] [CrossRef]
- Tafdrup, S. Centralized biogas plants combine agricultural and environmental benefits with energy production. Water Sci. Technol. 1994, 30, 133–141. [Google Scholar] [CrossRef]
- Verma, V.; Singh, Y.; Rai, J. Biogas production from plant biomass used for phytoremediation of industrial wastes. Bioresour. Technol. 2007, 98, 1664–1669. [Google Scholar] [CrossRef]
- Cao, Z.; Wang, S.; Wang, T.; Chang, Z.; Shen, Z.; Chen, Y. Using Contaminated Plants Involved in Phytoremediation for Anaerobic Digestion. Int. J. Phytoremediation 2015, 17, 201–207. [Google Scholar] [CrossRef]
- Sotenko, M.; Coles, S.; Barker, G.; Song, L.; Jiang, Y.; Longhurst, P.J.; Romanova, T.; Shuvaeva, O.; Kirwan, K. Phytoremediation-biorefinery tandem for effective clean-up of metal contaminated soil and biomass valorisation. Int. J. Phytoremediation 2017, 19, 965–975. [Google Scholar] [CrossRef]
- Dumont, B.; Fortun-Lamothe, L.; Jouven, M.; Thomas, M.A.; Tichit, M. Prospects from agroecology and industrial ecology for animal production in the 21st century. Animal 2012, 7, 1028–1043. [Google Scholar] [CrossRef]
- Lyubenova, L.; Kuhn, A.J.; Höltkemeier, A.; Schröder, P. Root exudation pattern of Typha latifolia L. plants after copper exposure. Plant Soil 2013, 370, 187–195. [Google Scholar] [CrossRef]
- Calheiros, C.S.C.; Pereira, S.I.A.; Franco, A.R.; Castro, P.M.L. Diverse Arbuscular Mycorrhizal Fungi (AMF) Communities Colonize Plants Inhabiting a Constructed Wetland for Wastewater Treatment. Water 2019, 11, 1535. [Google Scholar] [CrossRef]
| Essential Elements (authorized in animal nutrition according to EC N°1831/2003) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Co (cobalt) | Cr (chromium) | Cu (copper) | Fe (iron) | Mn (manganese) | Mo (molybdenum) | Ni (nickel) | Se (selenium) | Zn (zinc) |
| Nonessential elements (undesirable elements according to 2002/32/EC) | ||||||||
| As (arsenic) | Cd (cadmium) | Hg (mercury) | Pb (lead) | |||||
| Area | Heavy Metal | Source of Heavy Metals | ||||
|---|---|---|---|---|---|---|
| Swine Slurry | Cattle Slurry | Poultry Slurry | ||||
| England | Zn | mg/kg d.w. | 650.0 | 170.0 | 217.0 | |
| Cu | 470.0 | 45.0 | 32.0 | |||
| Netherlands | Zn | mg·kg−1 | 186.2 | 73.7 | - | |
| Cu | 644.7 | 296.3 | - | |||
| China | Zn | mg/kg d.w. | 843.3 | 151.9 | 308.9 | |
| Cu | 472.6 | 46.5 | 102.0 | |||
| China | Zn | mg/kg d.w. | a S | 119.1 | 674.7 | 268.2 |
| b M | 126.3 | 476.0 | 241.7 | |||
| c L | 136.1 | 691.6 | 384.2 | |||
| Cu | S | 30.8 | 958.8 | 51.6 | ||
| M | 31.0 | 420.4 | 57.2 | |||
| L | 31.4 | 612.2 | 87.1 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hejna, M.; Onelli, E.; Moscatelli, A.; Bellotto, M.; Cristiani, C.; Stroppa, N.; Rossi, L. Heavy-Metal Phytoremediation from Livestock Wastewater and Exploitation of Exhausted Biomass. Int. J. Environ. Res. Public Health 2021, 18, 2239. https://doi.org/10.3390/ijerph18052239
Hejna M, Onelli E, Moscatelli A, Bellotto M, Cristiani C, Stroppa N, Rossi L. Heavy-Metal Phytoremediation from Livestock Wastewater and Exploitation of Exhausted Biomass. International Journal of Environmental Research and Public Health. 2021; 18(5):2239. https://doi.org/10.3390/ijerph18052239
Chicago/Turabian StyleHejna, Monika, Elisabetta Onelli, Alessandra Moscatelli, Maurizio Bellotto, Cinzia Cristiani, Nadia Stroppa, and Luciana Rossi. 2021. "Heavy-Metal Phytoremediation from Livestock Wastewater and Exploitation of Exhausted Biomass" International Journal of Environmental Research and Public Health 18, no. 5: 2239. https://doi.org/10.3390/ijerph18052239
APA StyleHejna, M., Onelli, E., Moscatelli, A., Bellotto, M., Cristiani, C., Stroppa, N., & Rossi, L. (2021). Heavy-Metal Phytoremediation from Livestock Wastewater and Exploitation of Exhausted Biomass. International Journal of Environmental Research and Public Health, 18(5), 2239. https://doi.org/10.3390/ijerph18052239










