Phytoremediation and Microorganisms-Assisted Phytoremediation of Mercury-Contaminated Soils: Challenges and Perspectives
Abstract
:1. Introduction
2. Hg Uptake and Detoxification in Plants
3. Phytoremediation of Hg-Contaminated Soils
3.1. Phytoextraction
3.2. Phytostabilization
3.3. Phytovolatilization
4. Microorganisms-Assisted Hg Phytoremediation
4.1. Bacteria-Assisted Hg Phytoremediation
4.1.1. Rhizobial Associations
4.1.2. Non-Rhizobial Associations
4.1.3. Bacterial Siderophores, IAA, NH3
4.2. Fungi
5. Are Transgenic Plants Ready for Hg Phytoremediation?
6. Challenges and Perspectives for Hg Phytoremediation
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- ATSDR Agency for Toxic Substances and Disease Registry. ATSDR’s Substance Priority List. Available online: www.atsdr.cdc.gov/spl/index.html (accessed on 28 February 2021).
- Mason, R.P.; Reinfelder, J.R.; Morel, F.M.M. Bioaccumulation of Mercury and Methylmercury. In Mercury as a Global Pollutant, Proceedings of the Third International Conference Held in Whistler, British Columbia, 10–14 July 1994; Porcella, D.B., Huckabee, J.W., Wheatley, B., Eds.; Springer: Dordrecht, The Netherlands, 1995; pp. 915–921. [Google Scholar] [CrossRef]
- Silver, S.; Hobman, J.L. Mercury Microbiology: Resistance Systems, Environmental Aspects, Methylation,and Human Health. In Molecular Microbiology of Heavy Metals; Nies, D.H., Silver, S., Eds.; Springer: Berlin/Heidelberg, Germnay, 2007; pp. 357–370. [Google Scholar] [CrossRef]
- Skyllberg, U. Chemical Speciation of Mercury in Soil and Sediment. In Environmental Chemistry and Toxicology of Mercury; Liu, G., Cai, Y., O’Driscoll, N., Eds.; John Wiley and Sons, Inc.: Hoboken, NJ, USA, 2011; pp. 219–258. [Google Scholar] [CrossRef]
- Kumari, S.; Jamwal, R.; Mishra, N.; Singh, D.K. Recent developments in environmental mercury bioremediation and its toxicity: A review. Environ. Nanotechnol. Monit. Manag. 2020, 13. [Google Scholar] [CrossRef]
- Kocman, D.; Wilson, S.J.; Amos, H.M.; Telmer, K.H.; Steenhuisen, F.; Sunderland, E.M.; Mason, R.P.; Outridge, P.; Horvat, M. Toward an Assessment of the Global Inventory of Present-Day Mercury Releases to Freshwater Environments. Int. J. Environ. Res. Public Health 2017, 14, 138. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Strategic Planning for Implementation of the Health-Related Articles of the Minamata Convention on Mercury; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Cao, L.; Liu, J.; Dou, S.; Huang, W. Biomagnification of methylmercury in a marine food web in Laizhou Bay (North China) and associated potential risks to public health. Mar. Pollut. Bull. 2020, 150, 110762. [Google Scholar] [CrossRef]
- Beckers, F.; Rinklebe, J. Cycling of mercury in the environment: Sources, fate, and human health implications: A review. Crit. Rev. Environ. Sci. Technol. 2017, 47, 693–794. [Google Scholar] [CrossRef]
- AMAP/UN Environment. Technical Background Report to the Global Mercury Assessment 2018; Arctic Monitoring and Assessment Programme, Oslo, Norway; UN Environment Programme, Chemicals and Health Branch: Geneva, Switzerland, 2019. [Google Scholar]
- Tang, W.-L.; Liu, Y.-R.; Guan, W.-Y.; Zhong, H.; Qu, X.-M.; Zhang, T. Understanding mercury methylation in the changing environment: Recent advances in assessing microbial methylators and mercury bioavailability. Sci. Total Environ. 2020, 714, 136827. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, Q.; Cheng, M.; He, Y.; Chen, L.; Zhang, H.; Cao, H.; Shen, H.; Zhang, W.; Tao, S.; et al. Rice life cycle-based global mercury biotransport and human methylmercury exposure. Nat. Commun. 2019, 10, 5164. [Google Scholar] [CrossRef]
- Yu, X.; Khan, S.; Khan, A.; Tang, Y.; Nunes, L.M.; Yan, J.; Ye, X.; Li, G. Methyl mercury concentrations in seafood collected from Zhoushan Islands, Zhejiang, China, and their potential health risk for the fishing community: Capsule: Methyl mercury in seafood causes potential health risk. Environ. Int. 2020, 137, 105420. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Habibullah-Al-Mamun, M.; Han, J.; Wang, L.; Zhu, Y.; Xu, X.; Li, N.; Qiu, G. Total mercury and methylmercury in rice: Exposure and health implications in Bangladesh. Environ. Pollut. 2020, 265, 114991. [Google Scholar] [CrossRef] [PubMed]
- Abeysinghe, K.S.; Qiu, G.; Goodale, E.; Anderson, C.W.N.; Bishop, K.; Evers, D.C.; Goodale, M.W.; Hintelmann, H.; Liu, S.; Mammides, C.; et al. Mercury flow through an Asian rice-based food web. Environ. Pollut. 2017, 229, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Broussard, L.A.; Hammett-Stabler, C.A.; Winecker, R.E.; Ropero-Miller, J.D. The Toxicology of Mercury. Lab. Med. 2002, 33, 614–625. [Google Scholar] [CrossRef]
- Fisher, J.F.; World Health Organization; International Programme on Chemical Safety. Elemental Mercury and Inorganic Mercury Compounds: Human Health Aspects; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Genchi, G.; Sinicropi, M.S.; Carocci, A.; Lauria, G.; Catalano, A. Mercury Exposure and Heart Diseases. Int. J. Environ. Res. Public Health 2017, 14, 74. [Google Scholar] [CrossRef] [Green Version]
- Cappelletti, S.; Piacentino, D.; Fineschi, V.; Frati, P.; D’Errico, S.; Aromatario, M. Mercuric chloride poisoning: Symptoms, analysis, therapies, and autoptic findings. A review of the literature. Crit. Rev. Toxicol. 2019, 49, 329–341. [Google Scholar] [CrossRef]
- ATSDR. Minimal Risk Levels (MRLs) for Hazardous Substances. Available online: https://wwwn.cdc.gov/TSP/MRLS/mrlsListing.aspx (accessed on 18 February 2021).
- Zahir, F.; Rizwi, S.J.; Haq, S.K.; Khan, R.H. Low dose mercury toxicity and human health. Env. Toxicol Pharm. 2005, 20, 351–360. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, C.; Liu, H.; Li, P.; Hu, X.; Wang, H.; Chan, H.M.; Feng, X. Impact of low-level mercury exposure on intelligence quotient in children via rice consumption. Ecotoxicol. Environ. Saf. 2020, 202, 110870. [Google Scholar] [CrossRef]
- ATSDR Agency for Toxic Substances and Disease Registry. Toxicological Profile for Mercury; Department of Health and Human Services, Public Health Service: Atlanta, GA, USA, 1999.
- Rice, K.M.; Walker, E.M.; Wu, M.; Gillette, C.; Blough, E.R. Environmental Mercury and Its Toxic Effects. J. Prev. Med. Public Health 2014, 47, 74–83. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, Y.; Wang, F.; Luo, Z.; Guo, S.; Strahle, U. Toxicity of mercury: Molecular evidence. Chemosphere 2020, 245, 125586. [Google Scholar] [CrossRef]
- Pirrone, N.; Cinnirella, S.; Feng, X.; Finkelman, R.B.; Friedli, H.R.; Leaner, J.; Mason, R.; Mukherjee, A.B.; Stracher, G.B.; Streets, D.G.; et al. Global mercury emissions to the atmosphere from anthropogenic and natural sources. Atmos. Chem. Phys. 2010, 10, 5951–5964. [Google Scholar] [CrossRef] [Green Version]
- Bishop, K.; Shanley, J.B.; Riscassi, A.; de Wit, H.A.; Eklof, K.; Meng, B.; Mitchell, C.; Osterwalder, S.; Schuster, P.F.; Webster, J.; et al. Recent advances in understanding and measurement of mercury in the environment: Terrestrial Hg cycling. Sci. Total Environ. 2020, 721, 137647. [Google Scholar] [CrossRef]
- Amos, H.M.; Jacob, D.J.; Streets, D.G.; Sunderland, E.M. Legacy impacts of all-time anthropogenic emissions on the global mercury cycle. Glob. Biogeochem. Cycles 2013, 27, 410–421. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Jaeglé, L.; Thompson, L.; Streets, D.G. Six centuries of changing oceanic mercury. Glob. Biogeochem. Cycles 2014, 28, 1251–1261. [Google Scholar] [CrossRef]
- Kocman, D.; Horvat, M.; Pirrone, N.; Cinnirella, S. Contribution of contaminated sites to the global mercury budget. Enviorn. Res. 2013, 125, 160–170. [Google Scholar] [CrossRef]
- Reis, A.T.; Rodrigues, S.M.; Araujo, C.; Coelho, J.P.; Pereira, E.; Duarte, A.C. Mercury contamination in the vicinity of a chlor-alkali plant and potential risks to local population. Sci. Total Environ. 2009, 407, 2689–2700. [Google Scholar] [CrossRef]
- Fernández-Martínez, R.; Esbrí, J.M.; Higueras, P.; Rucandio, I. Comparison of mercury distribution and mobility in soils affected by anthropogenic pollution around chloralkali plants and ancient mining sites. Sci. Total Environ. 2019, 671, 1066–1076. [Google Scholar] [CrossRef]
- Mahbub, K.R.; Bahar, M.M.; Labbate, M.; Krishnan, K.; Andrews, S.; Naidu, R.; Megharaj, M. Bioremediation of mercury: Not properly exploited in contaminated soils! Appl. Microbiol. Biotechnol. 2017, 101, 963–976. [Google Scholar] [CrossRef] [Green Version]
- Raj, D.; Maiti, S.K. Sources, toxicity, and remediation of mercury: An essence review. Environ. Monit. Assess. 2019, 191, 566. [Google Scholar] [CrossRef]
- Suchara, I.; Sucharová, J. Mercury distribution around the Spolana chlor-alkali plant (central Bohemia, Czech Republic) after a catastrophic flood, as revealed by bioindicators. Environ. Pollut. 2008, 151, 352–361. [Google Scholar] [CrossRef]
- Grangeon, S.; Guédron, S.; Asta, J.; Sarret, G.; Charlet, L. Lichen and soil as indicators of an atmospheric mercury contamination in the vicinity of a chlor-alkali plant (Grenoble, France). Ecol. Indic. 2012, 13, 178–183. [Google Scholar] [CrossRef]
- Frentiu, T.; Pintican, B.P.; Butaciu, S.; Mihaltan, A.I.; Ponta, M.; Frentiu, M. Determination, speciation and distribution of mercury in soil in the surroundings of a former chlor-alkali plant: Assessment of sequential extraction procedure and analytical technique. Chem. Cent. J. 2013, 7, 178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navrátil, T.; Šimeček, M.; Shanley, J.B.; Rohovec, J.; Hojdová, M.; Houška, J. The history of mercury pollution near the Spolana chlor-alkali plant (Neratovice, Czech Republic) as recorded by Scots pine tree rings and other bioindicators. Sci. Total Environ. 2017, 586, 1182–1192. [Google Scholar] [CrossRef] [PubMed]
- Esbrí, J.M.; Cacovean, H.; Higueras, P. Usage Proposal of a common urban decorative tree (Salix alba L.) to monitor the dispersion of gaseous mercury: A case study from Turda (Romania). Chemosphere 2018, 193, 74–81. [Google Scholar] [CrossRef]
- Zhu, W.; Li, Z.; Li, P.; Yu, B.; Lin, C.-J.; Sommar, J.; Feng, X. Re-emission of legacy mercury from soil adjacent to closed point sources of Hg emission. Environ. Pollut. 2018, 242, 718–727. [Google Scholar] [CrossRef]
- Quiñones, M.A.; Ruiz-Díez, B.; Fajardo, S.; López-Berdonces, M.A.; Higueras, P.L.; Fernández-Pascual, M. Lupinus albus plants acquire mercury tolerance when inoculated with an Hg-resistant Bradyrhizobium strain. Plant Physiol. Biochem. 2013, 73, 168–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathew, D.C.; Ho, Y.-N.; Gicana, R.G.; Mathew, G.M.; Chien, M.-C.; Huang, C.-C. A Rhizosphere-associated symbiont, Photobacterium spp. strain MELD1, and its targeted synergistic activity for phytoprotection against mercury. PLoS ONE 2015, 10, e0121178. [Google Scholar] [CrossRef] [PubMed]
- Plociniczak, T.; Sinkkonen, A.; Romantschuk, M.; Sulowicz, S.; Piotrowska-Seget, Z. Rhizospheric Bacterial Strain Brevibacterium casei MH8a Colonizes Plant Tissues and Enhances Cd, Zn, Cu Phytoextraction by White Mustard. Front. Plant Sci. 2016, 7, 101. [Google Scholar] [CrossRef] [PubMed]
- Kodre, A.; Arčon, I.; Debeljak, M.; Potisek, M.; Likar, M.; Vogel-Mikuš, K. Arbuscular mycorrhizal fungi alter Hg root uptake and ligand environment as studied by X-ray absorption fine structure. Environ. Exp. Bot. 2017, 133, 12–23. [Google Scholar] [CrossRef]
- Rahimi Tamandegani, P.; Zafari, D. Evaluation of different Fusarium species–wheat interactions effect on Cd biosorption by wheat seedlings. Int. J. Environ. Sci. Technol. 2019, 16, 1873–1884. [Google Scholar] [CrossRef]
- Mariano, C.; Mello, I.S.; Barros, B.M.; da Silva, G.F.; Terezo, A.J.; Soares, M.A. Mercury alters the rhizobacterial community in Brazilian wetlands and it can be bioremediated by the plant-bacteria association. Environ. Sci. Pollut. Res. 2020, 27, 13550–13564. [Google Scholar] [CrossRef] [PubMed]
- Mello, I.S.; Targanski, S.; Pietro-Souza, W.; Frutuoso Stachack, F.F.; Terezo, A.J.; Soares, M.A. Endophytic bacteria stimulate mercury phytoremediation by modulating its bioaccumulation and volatilization. Ecotoxicol. Environ. Saf. 2020, 202, 110818. [Google Scholar] [CrossRef]
- Reeves, R.D.; Baker, A.J.M.; Jaffré, T.; Erskine, P.D.; Echevarria, G.; van der Ent, A. A global database for plants that hyperaccumulate metal and metalloid trace elements. New Phytol. 2018, 218, 407–411. [Google Scholar] [CrossRef] [Green Version]
- Millán, R.; Lominchar, M.A.; Rodríguez-Alonso, J.; Schmid, T.; Sierra, M.J. Riparian vegetation role in mercury uptake (Valdeazogues River, Almadén, Spain). J. Geochem. Explor. 2014, 140, 104–110. [Google Scholar] [CrossRef]
- Ekyastuti Wiwik, A.D.; Emi, R. Prospect of indigenous plant species for revegetation in the tailings area of ex community gold mine. Biodiversitas J. Biol. Divers. 2016, 17, 764–768. [Google Scholar] [CrossRef]
- Marrugo-Negrete, J.; Marrugo-Madrid, S.; Pinedo-Hernández, J.; Durango-Hernández, J.; Díez, S. Screening of native plant species for phytoremediation potential at a Hg-contaminated mining site. Sci. Total Environ. 2016, 542, 809–816. [Google Scholar] [CrossRef]
- Sasmaz, M.; Akgül, B.; Yıldırım, D.; Sasmaz, A. Mercury uptake and phytotoxicity in terrestrial plants grown naturally in the Gumuskoy (Kutahya) mining area, Turkey. Int. J. Phytoremediat. 2016, 18, 69–76. [Google Scholar] [CrossRef]
- Xun, Y.; Feng, L.; Li, Y.; Dong, H. Mercury accumulation plant Cyrtomium macrophyllum and its potential for phytoremediation of mercury polluted sites. Chemosphere 2017, 189, 161–170. [Google Scholar] [CrossRef]
- Umlaufová, M.; Száková, J.; Najmanová, J.; Sysalová, J.; Tlustoš, P. The soil-plant transfer of risk elements within the area of an abandoned gold mine in Libčice, Czech Republic. J. Environ. Sci. Health Part A 2018, 53, 1267–1276. [Google Scholar] [CrossRef]
- Mbanga, O.; Ncube, S.; Tutu, H.; Chimuka, L.; Cukrowska, E. Mercury accumulation and biotransportation in wetland biota affected by gold mining. Environ. Monit. Assess. 2019, 191, 186. [Google Scholar] [CrossRef] [PubMed]
- Mello, I.S.; Pietro-Souza, W.; Barros, B.M.; da Silva, G.F.; Campos, M.L.; Soares, M.A. Endophytic bacteria mitigate mercury toxicity to host plants. Symbiosis 2019, 79, 251–262. [Google Scholar] [CrossRef]
- Petelka, J.; Abraham, J.; Bockreis, A.; Deikumah, J.P.; Zerbe, S. Soil heavy metal(loid) pollution and phytoremediation potential of native plants on a former gold mine in Ghana. Water Air Soil Pollut. 2019, 230, 267. [Google Scholar] [CrossRef] [Green Version]
- Frossard, A.; Hartmann, M.; Frey, B. Tolerance of the forest soil microbiome to increasing mercury concentrations. Soil Biol. Biochem. 2017, 105, 162–176. [Google Scholar] [CrossRef]
- Frossard, A.; Donhauser, J.; Mestrot, A.; Gygax, S.; Bååth, E.; Frey, B. Long- and short-term effects of mercury pollution on the soil microbiome. Soil Biol. Biochem. 2018, 120, 191–199. [Google Scholar] [CrossRef]
- Wang, L.; Wang, L.-A.; Zhan, X.; Huang, Y.; Wang, J.; Wang, X. Response mechanism of microbial community to the environmental stress caused by the different mercury concentration in soils. Ecotoxicol. Environ. Saf. 2020, 188, 109906. [Google Scholar] [CrossRef] [PubMed]
- Hoque, E.; Fritscher, J. A new mercury-accumulating Mucor hiemalis strain EH8 from cold sulfidic spring water biofilms. MicrobiologyOpen 2016, 5, 763–781. [Google Scholar] [CrossRef] [Green Version]
- Figueiredo, N.; Serralheiro, M.L.; Canário, J.; Duarte, A.; Hintelmann, H.; Carvalho, C. Evidence of mercury methylation and demethylation by the estuarine microbial communities obtained in stable Hg isotope studies. Int. J. Environ. Res. Public Health 2018, 15, 2141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinto, A.P.; Simes, I.; Mota, A.M. Cadmium Impact on Root Exudates of Sorghum and Maize Plants: A Speciation Study. J. Plant Nutr. 2008, 31, 1746–1755. [Google Scholar] [CrossRef]
- Montiel-Rozas, M.M.; Madejón, E.; Madejón, P. Effect of heavy metals and organic matter on root exudates (low molecular weight organic acids) of herbaceous species: An assessment in sand and soil conditions under different levels of contamination. Environ. Pollut. 2016, 216, 273–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esteban, E.; Moreno, E.; Peñalosa, J.; Cabrero, J.I.; Millán, R.; Zornoza, P. Short and long-term uptake of Hg in white lupin plants: Kinetics and stress indicators. Environ. Exp. Bot. 2008, 62, 316–322. [Google Scholar] [CrossRef]
- Castro, R.; Pereira, S.; Lima, A.; Corticeiro, S.; Válega, M.; Pereira, E.; Duarte, A.; Figueira, E. Accumulation, distribution and cellular partitioning of mercury in several halophytes of a contaminated salt marsh. Chemosphere 2009, 76, 1348–1355. [Google Scholar] [CrossRef]
- Carrasco-Gil, S.; Álvarez-Fernández, A.; Sobrino-Plata, J.; Millán, R.; Carpena-Ruiz, R.O.; Leduc, D.L.; Andrews, J.C.; Abadía, J.; Hernández, L.E. Complexation of Hg with phytochelatins is important for plant Hg tolerance. Plant Cell Environ. 2011, 34, 778–791. [Google Scholar] [CrossRef]
- Chen, J.; Yang, Z.M. Mercury toxicity, molecular response and tolerance in higher plants. BioMetals 2012, 25, 847–857. [Google Scholar] [CrossRef]
- Riddle, S.G.; Tran, H.H.; Dewitt, J.G.; Andrews, J.C. Field, laboratory, and X-ray absorption spectroscopic studies of mercury accumulation by water hyacinths. Environ. Sci. Technol. 2002, 36, 1965–1970. [Google Scholar] [CrossRef]
- Rajan, M.; Darrow, J.; Hua, M.; Barnett, B.; Mendoza, M.; Greenfield, B.K.; Andrews, J.C. Hg L3 XANES Study of mercury methylation in shredded Eichhornia crassipes. Environ. Sci. Technol. 2008, 42, 5568–5573. [Google Scholar] [CrossRef] [PubMed]
- Patty, C.; Barnett, B.; Mooney, B.; Kahn, A.; Levy, S.; Liu, Y.; Pianetta, P.; Andrews, J.C. Using X-ray microscopy and Hg L3 XANES to study Hg binding in the rhizosphere of Spartina cordgrass. Environ. Sci. Technol. 2009, 43, 7397–7402. [Google Scholar] [CrossRef]
- Carrasco-Gil, S.; Siebner, H.; Leduc, D.L.; Webb, S.M.; Millan, R.; Andrews, J.C.; Hernandez, L.E. Mercury localization and speciation in plants grown hydroponically or in a natural environment. Environ. Sci Technol 2013, 47, 3082–3090. [Google Scholar] [CrossRef]
- Wang, X.; Tam, N.F.-Y.; Fu, S.; Ametkhan, A.; Ouyang, Y.; Ye, Z. Selenium addition alters mercury uptake, bioavailability in the rhizosphere and root anatomy of rice (Oryza sativa). Ann. Bot. 2014, 114, 271–278. [Google Scholar] [CrossRef] [Green Version]
- Kabata-Pendias, A. Trace Elements in Soils and Plants; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Clemens, S.; Aarts, M.G.M.; Thomine, S.; Verbruggen, N. Plant science: The key to preventing slow cadmium poisoning. Trends Plant Sci. 2013, 18, 92–99. [Google Scholar] [CrossRef]
- Ishimaru, Y.; Takahashi, R.; Bashir, K.; Shimo, H.; Senoura, T.; Sugimoto, K.; Ono, K.; Yano, M.; Ishikawa, S.; Arao, T.; et al. Characterizing the role of rice NRAMP5 in manganese, iron and cadmium transport. Sci. Rep. 2012, 2, 286. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, R.; Ishimaru, Y.; Senoura, T.; Shimo, H.; Ishikawa, S.; Arao, T.; Nakanishi, H.; Nishizawa, N.K. The OsNRAMP1 iron transporter is involved in Cd accumulation in rice. J. Exp. Bot. 2011, 62, 4843–4850. [Google Scholar] [CrossRef] [Green Version]
- Esteban, E.; Deza, M.J.; Zornoza, P. Kinetics of mercury uptake by oilseed rape and white lupin: Influence of Mn and Cu. Acta Physiol. Plant. 2013, 35, 2339–2344. [Google Scholar] [CrossRef] [Green Version]
- Regier, N.; Larras, F.; Bravo, A.G.; Ungureanu, V.-G.; Amouroux, D.; Cosio, C. Mercury bioaccumulation in the aquatic plant Elodea nuttallii in the field and in microcosm: Accumulation in shoots from the water might involve copper transporters. Chemosphere 2013, 90, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Iglesia-Turiño, S.; Febrero, A.; Jauregui, O.; Caldelas, C.; Araus, J.L.; Bort, J. Detection and quantification of unbound Phytochelatin 2 in plant extracts of Brassica napusgrown with different levels of mercury. Plant Physiol. 2006, 142, 742–749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Yang, L.; Wang, Q. In vivo phytochelatins and Hg–phytochelatin complexes in Hg-stressed Brassica chinensis L. Metallomics 2009, 1, 101–106. [Google Scholar] [CrossRef] [Green Version]
- Gong, J.-M.; Lee, D.A.; Schroeder, J.I. Long-distance root-to-shoot transport of phytochelatins and cadmium in Arabidopsis. Proc. Natl. Acad. Sci. USA 2003, 100, 10118–10123. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Heaton, A.C.P.; Carreira, L.; Meagher, R.B. Enhanced tolerance to and accumulation of mercury, but not arsenic, in plants overexpressing two enzymes required for thiol peptide synthesis. Physiol. Plant. 2006, 128, 48–57. [Google Scholar] [CrossRef]
- Park, J.; Song, W.-Y.; Ko, D.; Eom, Y.; Hansen, T.H.; Schiller, M.; Lee, T.G.; Martinoia, E.; Lee, Y. The phytochelatin transporters AtABCC1 and AtABCC2 mediate tolerance to cadmium and mercury. Plant J. 2012, 69, 278–288. [Google Scholar] [CrossRef]
- Hall, J.L. Cellular mechanisms for heavy metal detoxification and tolerance. J. Exp. Bot. 2002, 53, 1–11. [Google Scholar] [CrossRef]
- Hossain, M.A.; Piyatida, P.; da Silva, J.A.T.; Fujita, M. Molecular mechanism of heavy metal toxicity and tolerance in plants: Central role of glutathione in detoxification of reactive oxygen species and methylglyoxal and in heavy metal chelation. J. Bot. 2012, 2012, 872875. [Google Scholar] [CrossRef]
- Ortega-Villasante, C.; Rellán-Álvarez, R.; Del Campo, F.F.; Carpena-Ruiz, R.O.; Hernández, L.E. Cellular damage induced by cadmium and mercury in Medicago sativa. J. Exp. Bot. 2005, 56, 2239–2251. [Google Scholar] [CrossRef]
- Zhou, Z.S.; Huang, S.Q.; Guo, K.; Mehta, S.K.; Zhang, P.C.; Yang, Z.M. Metabolic adaptations to mercury-induced oxidative stress in roots of Medicago sativa L. J. Inorg. Biochem. 2007, 101, 1–9. [Google Scholar] [CrossRef]
- Israr, M.; Sahi, S.; Datta, R.; Sarkar, D. Bioaccumulation and physiological effects of mercury in Sesbania drummondii. Chemosphere 2006, 65, 591–598. [Google Scholar] [CrossRef]
- Cho, U.-H.; Park, J.-O. Mercury-induced oxidative stress in tomato seedlings. Plant Sci. 2000, 156, 1–9. [Google Scholar] [CrossRef]
- Malar, S.; Sahi, S.V.; Favas, P.J.C.; Venkatachalam, P. Assessment of mercury heavy metal toxicity-induced physiochemical and molecular changes in Sesbania grandiflora L. Int. J. Environ. Sci. Technol. 2015, 12, 3273–3282. [Google Scholar] [CrossRef] [Green Version]
- Heidenreich, B.; Mayer, K.; Sandermann Jr, H.; Ernst, D. Mercury-induced genes in Arabidopsis thaliana: Identification of induced genes upon long-term mercuric ion exposure. Plant Cell Environ. 2001, 24, 1227–1234. [Google Scholar] [CrossRef]
- Sävenstrand, H.; Strid, Å. Six genes strongly regulated by mercury in Pisum sativum roots. Plant Physiol. Biochem. 2004, 42, 135–142. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, B.; Wang, L.-A.; Urbanovich, O.; Nagorskaya, L.; Li, X.; Tang, L. A review on phytoremediation of mercury contaminated soils. J. Hazard. Mater. 2020, 400, 123138. [Google Scholar] [CrossRef] [PubMed]
- Mench, M.; Schwitzguébel, J.-P.; Schroeder, P.; Bert, V.; Gawronski, S.; Gupta, S. Assessment of successful experiments and limitations of phytotechnologies: Contaminant uptake, detoxification and sequestration, and consequences for food safety. Environ. Sci. Pollut. Res. 2009, 16, 876. [Google Scholar] [CrossRef]
- Clemens, S.; Palmgren, M.G.; Krämer, U. A long way ahead: Understanding and engineering plant metal accumulation. Trends Plant Sci. 2002, 7, 309–315. [Google Scholar] [CrossRef]
- Meagher, R.B.; Heaton, A.C.P. Strategies for the engineered phytoremediation of toxic element pollution: Mercury and arsenic. J. Ind. Microbiol. Biotechnol. 2005, 32, 502–513. [Google Scholar] [CrossRef] [PubMed]
- Khalid, S.; Shahid, M.; Niazi, N.K.; Murtaza, B.; Bibi, I.; Dumat, C. A comparison of technologies for remediation of heavy metal contaminated soils. J. Geochem. Explor. 2017, 182, 247–268. [Google Scholar] [CrossRef] [Green Version]
- Krämer, U. Metal Hyperaccumulation in Plants. Annu. Rev. Plant Biol. 2010, 61, 517–534. [Google Scholar] [CrossRef] [PubMed]
- Kamal, M.; Ghaly, A.E.; Mahmoud, N.; Côté, R. Phytoaccumulation of heavy metals by aquatic plants. Environ. Int. 2004, 29, 1029–1039. [Google Scholar] [CrossRef]
- Skinner, K.; Wright, N.; Porter-Goff, E. Mercury uptake and accumulation by four species of aquatic plants. Environ. Pollut. 2007, 145, 234–237. [Google Scholar] [CrossRef] [PubMed]
- Marrugo-Negrete, J.; Durango-Hernández, J.; Díaz-Fernández, L.; Urango-Cárdenas, I.; Araméndiz-Tatis, H.; Vergara-Flórez, V.; Bravo, A.G.; Díez, S. Transfer and bioaccumulation of mercury from soil in cowpea in gold mining sites. Chemosphere 2020, 250, 126142. [Google Scholar] [CrossRef]
- Chamba, I.; Rosado, D.; Kalinhoff, C.; Thangaswamy, S.; Sánchez-Rodríguez, A.; Gazquez, M.J. Erato polymnioides—A novel Hg hyperaccumulator plant in ecuadorian rainforest acid soils with potential of microbe-associated phytoremediation. Chemosphere 2017, 188, 633–641. [Google Scholar] [CrossRef]
- Alcantara, H.J.P.; Doronila, A.I.; Kolev, S.D. Phytoextraction potential of Manihot esculenta Crantz. (cassava) grown in mercury- and gold-containing biosolids and mine tailings. Miner. Eng. 2017, 114, 57–63. [Google Scholar] [CrossRef]
- Marrugo-Negrete, J.; Durango-Hernández, J.; Pinedo-Hernández, J.; Olivero-Verbel, J.; Díez, S. Phytoremediation of mercury-contaminated soils by Jatropha curcas. Chemosphere 2015, 127, 58–63. [Google Scholar] [CrossRef]
- Smolinska, B.; Rowe, S. The potential of Lepidium sativum L. for phytoextraction of Hg-contaminated soil assisted by thiosulphate. J. Soils Sediments 2015, 15, 393–400. [Google Scholar] [CrossRef] [Green Version]
- Lomonte, C.; Doronila, A.I.; Gregory, D.; Baker, A.J.M.; Kolev, S.D. Phytotoxicity of biosolids and screening of selected plant species with potential for mercury phytoextraction. J. Hazard. Mater. 2010, 173, 494–501. [Google Scholar] [CrossRef]
- Rodríguez, E.; Peralta-Videa, J.R.; Israr, M.; Sahi, S.V.; Pelayo, H.; Sánchez-Salcido, B.; Gardea-Torresdey, J.L. Effect of mercury and gold on growth, nutrient uptake, and anatomical changes in Chilopsis linearis. Environ. Exp. Bot. 2009, 65, 253–262. [Google Scholar] [CrossRef]
- Moreno-Jiménez, E.; Gamarra, R.; Carpena-Ruiz, R.O.; Millán, R.; Peñalosa, J.M.; Esteban, E. Mercury bioaccumulation and phytotoxicity in two wild plant species of Almadén area. Chemosphere 2006, 63, 1969–1973. [Google Scholar] [CrossRef]
- Suszcynsky, E.M.; Shann, J.R. Phytotoxicity and accumulation of mercury in tobacco subjected to different exposure routes. Environ. Toxicol. Chem. 1995, 14, 61–67. [Google Scholar] [CrossRef]
- Shiyab, S.; Chen, J.; Han, F.X.; Monts, D.L.; Matta, F.B.; Gu, M.; Su, Y. Phytotoxicity of mercury in Indian mustard (Brassica juncea L.). Ecotoxicol. Environ. Saf. 2009, 72, 619–625. [Google Scholar] [CrossRef]
- Moreno, F.N.; Anderson, C.W.N.; Stewart, R.B.; Robinson, B.H. Phytofiltration of mercury-contaminated water: Volatilisation and plant-accumulation aspects. Environ. Exp. Bot. 2008, 62, 78–85. [Google Scholar] [CrossRef]
- Rodriguez, L.; Rincón, J.; Asencio, I.; Rodríguez-Castellanos, L. Capability of selected crop plants for shoot mercury accumulation from polluted soils: Phytoremediation perspectives. Int. J. Phytoremediat. 2007, 9, 1–13. [Google Scholar] [CrossRef]
- Cargnelutti, D.; Tabaldi, L.A.; Spanevello, R.M.; de Oliveira Jucoski, G.; Battisti, V.; Redin, M.; Linares, C.E.B.; Dressler, V.L.; de Moraes Flores, É.M.; Nicoloso, F.T.; et al. Mercury toxicity induces oxidative stress in growing cucumber seedlings. Chemosphere 2006, 65, 999–1006. [Google Scholar] [CrossRef]
- Du, X.; Zhu, Y.G.; Liu, W.J.; Zhao, X.S. Uptake of mercury (Hg) by seedlings of rice (Oryza sativa L.) grown in solution culture and interactions with arsenate uptake. Environ. Exp. Bot. 2005, 54, 1–7. [Google Scholar] [CrossRef]
- Beauford, W.; Barber, J.; Barringer, A.R. Uptake and distribution of mercury within higher plants. Physiol. Plant. 1977, 39, 261–265. [Google Scholar] [CrossRef]
- Horvat, M.; Nolde, N.; Fajon, V.; Jereb, V.; Logar, M.; Lojen, S.; Jacimovic, R.; Falnoga, I.; Liya, Q.; Faganeli, J.; et al. Total mercury, methylmercury and selenium in mercury polluted areas in the province Guizhou, China. Sci. Total Environ. 2003, 304, 231–256. [Google Scholar] [CrossRef]
- Qiu, G.; Feng, X.; Wang, S.; Shang, L. Mercury and methylmercury in riparian soil, sediments, mine-waste calcines, and moss from abandoned Hg mines in east Guizhou province, southwestern China. Appl. Geochem. 2005, 20, 627–638. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.-F.; Li, B.; Dong, Z.; Qu, L.; Gao, Y.; Chai, Z.; Chen, C. Multielemental contents of foodstuffs from the Wanshan (China) mercury mining area and the potential health risks. Appl. Geochem. 2011, 26, 182–187. [Google Scholar] [CrossRef]
- Rodrigues, S.M.; Henriques, B.; Reis, A.T.; Duarte, A.C.; Pereira, E.; Römkens, P.F.A.M. Hg transfer from contaminated soils to plants and animals. Environ. Chem. Lett. 2012, 10, 61–67. [Google Scholar] [CrossRef]
- Wang, Y.; Greger, M. Clonal differences in mercury tolerance, accumulation, and distribution in willow. J. Environ. Qual. 2004, 33, 1779–1785. [Google Scholar] [CrossRef]
- Wang, Y.; Stauffer, C.; Keller, C.; Greger, M. Changes in Hg fractionation in soil induced by willow. Plant Soil 2005, 275, 67–75. [Google Scholar] [CrossRef]
- Pérez-Sanz, A.; Millán, R.; Sierra, M.J.; Alarcón, R.; García, P.; Gil-Díaz, M.; Vazquez, S.; Lobo, M.C. Mercury uptake by Silene vulgaris grown on contaminated spiked soils. J. Environ. Manag. 2012, 95, S233–S237. [Google Scholar] [CrossRef] [PubMed]
- Sas-Nowosielska, A.; Galimska-Stypa, R.; Kucharski, R.; Zielonka, U.; Małkowski, E.; Gray, L. Remediation aspect of microbial changes of plant rhizosphere in mercury contaminated soil. Environ. Monit. Assess. 2008, 137, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Kerr, J. Phytoremediation of Metal-Contaminated Soils by Industrial Crops. Ph.D. Thesis, University of Glasgow, Glasgow, UK, 2003. [Google Scholar]
- Zgorelec, Z.; Bilandzija, N.; Knez, K.; Galic, M.; Zuzul, S. Cadmium and mercury phytostabilization from soil using Miscanthus × giganteus. Sci. Rep. 2020, 10, 6685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tangahu, B.V.; Sheikh Abdullah, S.R.; Basri, H.; Idris, M.; Anuar, N.; Mukhlisin, M. A review on heavy metals (As, Pb, and Hg) uptake by plants through phytoremediation. Int. J. Chem. Eng. 2011, 2011, 939161. [Google Scholar] [CrossRef]
- Barkay, T.; Turner, R.; Saouter, E.; Horn, J. Mercury biotransformations and their potential for remediation of mercury contamination. Biodegradation 1992, 3, 147–159. [Google Scholar] [CrossRef]
- Barkay, T.; Kritee, K.; Boyd, E.; Geesey, G. A thermophilic bacterial origin and subsequent constraints by redox, light and salinity on the evolution of the microbial mercuric reductase. Environ. Microbiol. 2010, 12, 2904–2917. [Google Scholar] [CrossRef]
- Lal, D.; Lal, R. Evolution of mercuric reductase (merA) gene: A case of horizontal gene transfer. Microbiology 2010, 79, 500–508. [Google Scholar] [CrossRef]
- Chang, J.; Shi, Y.; Si, G.; Yang, Q.; Dong, J.; Chen, J. The bioremediation potentials and mercury(II)-resistant mechanisms of a novel fungus Penicillium spp. DC-F11 isolated from contaminated soil. J. Hazard. Mater. 2020, 396, 122638. [Google Scholar] [CrossRef]
- Battke, F.; Ernst, D.; Halbach, S. Ascorbate promotes emission of mercury vapour from plants. Plant Cell Environ. 2005, 28, 1487–1495. [Google Scholar] [CrossRef]
- Moreno, F.N.; Anderson, C.W.N.; Stewart, R.B.; Robinson, B.H. Mercury volatilisation and phytoextraction from base-metal mine tailings. Environ. Pollut. 2005, 136, 341–352. [Google Scholar] [CrossRef]
- Gadd, G.M. Microbial influence on metal mobility and application for bioremediation. Geoderma 2004, 122, 109–119. [Google Scholar] [CrossRef]
- Gadd, G.M. Metals, minerals and microbes: Geomicrobiology and bioremediation. Microbiology 2010, 156, 609–643. [Google Scholar] [CrossRef]
- García-Sánchez, M.; Száková, J. Chapter 12—Biological Remediation of Mercury-Polluted Environments. In Plant Metal Interaction; Ahmad, P., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 311–334. [Google Scholar] [CrossRef]
- Camilios-Neto, D.; Bonato, P.; Wassem, R.; Tadra-Sfeir, M.Z.; Brusamarello-Santos, L.C.C.; Valdameri, G.; Donatti, L.; Faoro, H.; Weiss, V.A.; Chubatsu, L.S.; et al. Dual RNA-seq transcriptional analysis of wheat roots colonized by Azospirillum brasilense reveals up-regulation of nutrient acquisition and cell cycle genes. BMC Genom. 2014, 15, 378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Y.; Oliveira, R.S.; Freitas, H.; Zhang, C. Biochemical and molecular mechanisms of plant-microbe-metal interactions: Relevance for phytoremediation. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef]
- Romero, F.M.; Rossi, F.R.; Gárriz, A.; Carrasco, P.; Ruíz, O.A. A bacterial endophyte from apoplast fluids protects canola plants from different phytopathogens via antibiosis and induction of host resistance. Phytopatholog 2019, 109, 375–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glick, B.R. Plant growth-promoting bacteria: Mechanisms and applications. Scientifica 2012, 2012, 963401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naik, K.; Mishra, S.; Srichandan, H.; Singh, P.K.; Sarangi, P.K. Plant growth promoting microbes: Potential link to sustainable agriculture and environment. Biocatal. Agric. Biotechnol. 2019, 21, 101326. [Google Scholar] [CrossRef]
- Nonnoi, F.; Chinnaswamy, A.; García de la Torre, V.S.; Coba de la Peña, T.; Lucas, M.M.; Pueyo, J.J. Metal tolerance of rhizobial strains isolated from nodules of herbaceous legumes (Medicago spp. and Trifolium spp.) growing in mercury-contaminated soils. Appl. Soil Ecol. 2012, 61, 49–59. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Díez, B.; Quiñones, M.A.; Fajardo, S.; López, M.A.; Higueras, P.; Fernández-Pascual, M. Mercury-resistant rhizobial bacteria isolated from nodules of leguminous plants growing in high Hg-contaminated soils. Appl. Microbiol. Biotechnol. 2012, 96, 543–554. [Google Scholar] [CrossRef] [Green Version]
- Hamzah, A.; Sarmani, S.B.; Yatim, N.I. Phytoremediation of Pb and Hg by using Scirpus mucronatus with addition of bacterial inoculums. J. Radioanal. Nucl. Chem. 2015, 304, 151–155. [Google Scholar] [CrossRef]
- Sitarska, M.; Traczewska, T.; Filyarovskaya, V. Removal of mercury (II) from the aquatic environment by phytoremediation. Desalination Water Treat. 2016, 57, 1515–1524. [Google Scholar] [CrossRef]
- Gontia-Mishra, I.; Sapre, S.; Sharma, A.; Tiwari, S. Alleviation of mercury toxicity in wheat by the interaction of mercury-tolerant plant growth-promoting rhizobacteria. J. Plant Growth Regul. 2016, 35, 1000–1012. [Google Scholar] [CrossRef]
- Franchi, E.; Rolli, E.; Marasco, R.; Agazzi, G.; Borin, S.; Cosmina, P.; Pedron, F.; Rosellini, I.; Barbafieri, M.; Petruzzelli, G. Phytoremediation of a multi contaminated soil: Mercury and arsenic phytoextraction assisted by mobilizing agent and plant growth promoting bacteria. J. Soils Sediments 2017, 17, 1224–1236. [Google Scholar] [CrossRef]
- Idris, R.; Trifonova, R.; Puschenreiter, M.; Wenzel, W.W.; Sessitsch, A. Bacterial communities associated with flowering plants of the Ni hyperaccumulator Thlaspi goesingense. Appl. Environ. Microbiol. 2004, 70, 2667–2677. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Luo, S.; Li, X.; Wan, Y.; Chen, J.; Liu, C. Interaction of Cd-hyperaccumulator Solanum nigrum L. and functional endophyte Pseudomonas sp. Lk9 on soil heavy metals uptake. Soil Biol. Biochem. 2014, 68, 300–308. [Google Scholar] [CrossRef]
- Ahmed, E.; Holmström, S.J.M. Siderophores in environmental research: Roles and applications. Microb. Biotechnol. 2014, 7, 196–208. [Google Scholar] [CrossRef]
- Dimkpa, C.O.; Svatoš, A.; Dabrowska, P.; Schmidt, A.; Boland, W.; Kothe, E. Involvement of siderophores in the reduction of metal-induced inhibition of auxin synthesis in Streptomyces spp. Chemosphere 2008, 74, 19–25. [Google Scholar] [CrossRef] [Green Version]
- Dao, K.-H.T.; Hamer, K.E.; Clark, C.L.; Harshman, L.G. Pyoverdine production by Pseudomonas aeruginosa exposed to metals or an oxidative stress agent. Ecol. Appl. 1999, 9, 441–448. [Google Scholar] [CrossRef]
- Braud, A.; Hoegy, F.; Jezequel, K.; Lebeau, T.; Schalk, I.J. New insights into the metal specificity of the Pseudomonas aeruginosa pyoverdine–iron uptake pathway. Environ. Microbiol. 2009, 11, 1079–1091. [Google Scholar] [CrossRef]
- Manoj, S.R.; Karthik, C.; Kadirvelu, K.; Arulselvi, P.I.; Shanmugasundaram, T.; Bruno, B.; Rajkumar, M. Understanding the molecular mechanisms for the enhanced phytoremediation of heavy metals through plant growth promoting rhizobacteria: A review. J. Environ. Manag. 2020, 254, 109779. [Google Scholar] [CrossRef]
- Rajkumar, M.; Ae, N.; Prasad, M.N.V.; Freitas, H. Potential of siderophore-producing bacteria for improving heavy metal phytoextraction. Trends Biotechnol. 2010, 28, 142–149. [Google Scholar] [CrossRef]
- Trivedi, P.; Leach, J.E.; Tringe, S.G.; Sa, T.; Singh, B.K. Plant–microbiome interactions: From community assembly to plant health. Nat. Rev. Microbiol. 2020, 18, 607–621. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive tree of life (iTOL) v3: An online tool for the display and annotation of phylogenetic and other trees. Nucleic Acids Res. 2016, 44, W242–W245. [Google Scholar] [CrossRef]
- Patten, C.L.; Glick, B.R. Bacterial biosynthesis of indole-3-acetic acid. Can. J. Microbiol. 1996, 42, 207–220. [Google Scholar] [CrossRef]
- Gamalero, E.; Glick, B.R. Bacterial modulation of plant ethylene levels. Plant Physiol. 2015, 169, 13–22. [Google Scholar] [CrossRef] [Green Version]
- Christena, V.-T.; Murthy, B.N.S.; Joseph, O.; Saxena, P.K. Modulation of somatic embryogenesis in hypocotyl-derived cultures of Geranium (Pelargonium x hortorum Bailey) cv Ringo Rose by a Bacterium. In Vitro Cell. Dev. Biol. Plant 1994, 30P, 140–143. [Google Scholar]
- Spaepen, S.; Vanderleyden, J.; Remans, R. Indole-3-acetic acid in microbial and microorganism-plant signaling. FEMS Microbiol. Rev. 2007, 31, 425–448. [Google Scholar] [CrossRef] [Green Version]
- Spaepen, S.; Vanderleyden, J. Auxin and plant-microbe interactions. Cold Spring Harb. Perspect. Biol. 2011, 3. [Google Scholar] [CrossRef] [Green Version]
- Soundar Raju, C.; Aslam, A.; Thangadurai, D.; Sangeetha, J.; Kathiravan, K.; Shajahan, A. Indole acetic acid (IAA) producing endophytic bacteria on direct somatic embryogenesis and plant regeneration of Exacum travancoricum Bedd. Vegetos 2020, 33, 690–702. [Google Scholar] [CrossRef]
- Kong, Z.; Wu, Z.; Glick, B.R.; He, S.; Huang, C.; Wu, L. Co-occurrence patterns of microbial communities affected by inoculants of plant growth-promoting bacteria during phytoremediation of heavy metal-contaminated soils. Ecotoxicol. Environ. Saf. 2019, 183, 109504. [Google Scholar] [CrossRef]
- van der Heijden, M.G.A.; Martin, F.M.; Selosse, M.-A.; Sanders, I.R. Mycorrhizal ecology and evolution: The past, the present, and the future. New Phytol. 2015, 205, 1406–1423. [Google Scholar] [CrossRef]
- Averill, C.; Bhatnagar, J.M.; Dietze, M.C.; Pearse, W.D.; Kivlin, S.N. Global imprint of mycorrhizal fungi on whole-plant nutrient economics. Proc. Natl. Acad. Sci. USA 2019, 116, 23163–23168. [Google Scholar] [CrossRef]
- Genre, A.; Lanfranco, L.; Perotto, S.; Bonfante, P. Unique and common traits in mycorrhizal symbioses. Nat. Rev. Microbiol. 2020, 18, 649–660. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, S.; Huang, H. Behavior of mercury in a soil–plant system as affected by inoculation with the arbuscular mycorrhizal fungus Glomus mosseae. Mycorrhiza 2010, 20, 407–414. [Google Scholar] [CrossRef]
- Cozzolino, V.; De Martino, A.; Nebbioso, A.; Di Meo, V.; Salluzzo, A.; Piccolo, A. Plant tolerance to mercury in a contaminated soil is enhanced by the combined effects of humic matter addition and inoculation with arbuscular mycorrhizal fungi. Environ. Sci. Pollut. Res. 2016, 23, 11312–11322. [Google Scholar] [CrossRef]
- Vargas Aguirre, C.F.; Rivera Páez, F.A.; Escobar Vargas, S. Effect of arbuscular mycorrhizae and mercury on Lactuca sativa (Asteraceae) seedling morpho—histology. Environ. Exp. Bot. 2018, 156, 197–202. [Google Scholar] [CrossRef]
- Leudo, A.M.; Cruz, Y.; Montoya-Ruiz, C.; Delgado, M.d.P.; Saldarriaga, J.F. Mercury phytoremediation with Lolium perenne-mycorrhizae in contaminated soils. Sustainability 2020, 12, 3795. [Google Scholar] [CrossRef]
- Debeljak, M.; van Elteren, J.T.; Špruk, A.; Izmer, A.; Vanhaecke, F.; Vogel-Mikuš, K. The role of arbuscular mycorrhiza in mercury and mineral nutrient uptake in maize. Chemosphere 2018, 212, 1076–1084. [Google Scholar] [CrossRef]
- Bretaña, B.L.; Salcedo, S.; Casim, L.; Manceras, R. Growth performance and inorganic mercury uptake of Vetiver (Chrysopogon zizanoides Nash) inoculated with arbuscular mycorrhiza fungi (AMF): Its implication to phytoremediation. J. Agric. Res. Dev. Ext. Technol. 2019, 1, 39–47. [Google Scholar]
- Pietro-Souza, W.; de Campos Pereira, F.; Mello, I.S.; Stachack, F.F.F.; Terezo, A.J.; Cunha, C.N.D.; White, J.F.; Li, H.; Soares, M.A. Mercury resistance and bioremediation mediated by endophytic fungi. Chemosphere 2020, 240, 124874. [Google Scholar] [CrossRef]
- Kneer, R.; Kutchan, T.M.; Hochberger, A.; Zenk, M.H. Saccharomyces cerevisiae and Neurospora crassa contain heavy metal sequestering phytochelatin. Arch. Microbiol. 1992, 157, 305–310. [Google Scholar] [CrossRef]
- Cobine, P.A.; McKay, R.T.; Zangger, K.; Dameron, C.T.; Armitage, I.M. Solution structure of Cu6 metallothionein from the fungus Neurospora crassa. Eur. J. Biochem. 2004, 271, 4213–4221. [Google Scholar] [CrossRef]
- Shine, A.M.; Shakya, V.P.S.; Idnurm, A. Phytochelatin synthase is required for tolerating metal toxicity in a basidiomycete yeast and is a conserved factor involved in metal homeostasis in fungi. Fungal Biol. Biotechnol. 2015, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorenzo-Gutiérrez, D.; Gómez-Gil, L.; Guarro, J.; Roncero, M.I.G.; Fernández-Bravo, A.; Capilla, J.; López-Fernández, L. Role of the Fusarium oxysporum metallothionein Mt1 in resistance to metal toxicity and virulence. Metallomics 2019, 11, 1230–1240. [Google Scholar] [CrossRef]
- Bayry, J.; Aimanianda, V.; Guijarro, J.I.; Sunde, M.; Latgé, J.-P. Hydrophobins—Unique Fungal Proteins. PLoS Pathog. 2012, 8, e1002700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puglisi, I.; Faedda, R.; Sanzaro, V.; Lo Piero, A.R.; Petrone, G.; Cacciola, S.O. Identification of differentially expressed genes in response to mercury I and II stress in Trichoderma harzianum. Gene 2012, 506, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Shafiul, H.; Md, Z.; Gowher, N.; Srivastava, P.S. Transgenic tobacco plant expressing environmental E. coli merA gene for enhanced volatilization of ionic mercury. J. Microbiol. Biotechnol. 2010, 20, 917–924. [Google Scholar] [CrossRef] [Green Version]
- Rugh, C.L.; Wilde, H.D.; Stack, N.M.; Thompson, D.M.; Summers, A.O.; Meagher, R.B. Mercuric ion reduction and resistance in transgenic Arabidopsis thaliana plants expressing a modified bacterial merA gene. Proc. Natl. Acad. Sci. USA 1996, 93, 3182–3187. [Google Scholar] [CrossRef] [Green Version]
- Rugh, C.L.; Senecoff, J.F.; Meagher, R.B.; Merkle, S.A. Development of transgenic yellow poplar for mercury phytoremediation. Nat. Biotechnol. 1998, 16, 925–928. [Google Scholar] [CrossRef] [PubMed]
- He, Y.K.; Sun, J.G.; Feng, X.Z.; CzakÓ, M.; MÁRton, L. Differential mercury volatilization by tobacco organs expressing a modified bacterial merA gene. Cell Res. 2001, 11, 231–236. [Google Scholar] [CrossRef]
- Heaton, A.C.P.; Rugh, C.L.; Wang, N.-J.; Meagher, R.B. Physiological responses of transgenic merA-TOBACCO (Nicotiana tabacum) to foliar and root mercury exposure. Water Air Soil Pollut. 2005, 161, 137–155. [Google Scholar] [CrossRef]
- Yang, H.; Nairn, J.O.E.; Ozias-Akins, P. Transformation of peanut using a modified bacterial mercuric ion reductase gene driven by an actin promoter from Arabidopsis thaliana. J. Plant Physiol. 2003, 160, 945–952. [Google Scholar] [CrossRef]
- Che, D.; Meagher, R.B.; Heaton, A.C.P.; Lima, A.; Rugh, C.L.; Merkle, S.A. Expression of mercuric ion reductase in Eastern cottonwood (Populus deltoides) confers mercuric ion reduction and resistance. Plant Biotechnol. J. 2003, 1, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Heaton, A.C.P.; Rugh, C.L.; Kim, T.; Wang, N.J.; Meagher, R.B. Toward detoxifying mercury-polluted aquatic sediments with rice genetically engineered for mercury resistance. Environ. Toxicol. Chem. 2003, 22, 2940–2947. [Google Scholar] [CrossRef]
- Bizily, S.P.; Rugh, C.L.; Summers, A.O.; Meagher, R.B. Phytoremediation of methylmercury pollution: merB expression in Arabidopsis thaliana confers resistance to organomercurials. Proc. Natl. Acad. Sci. USA 1999, 96, 6808–6813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bizily, S.P.; Rugh, C.L.; Meagher, R.B. Phytodetoxification of hazardous organomercurials by genetically engineered plants. Nat. Biotechnol. 2000, 18, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Bizily, S.P.; Kim, T.; Kandasamy, M.K.; Meagher, R.B. Subcellular targeting of methylmercury lyase enhances its specific activity for organic mercury detoxification in Plants. Plant Physiol. 2003, 131, 463–471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyyra, S.; Meagher, R.B.; Kim, T.; Heaton, A.; Montello, P.; Balish, R.S.; Merkle, S.A. Coupling two mercury resistance genes in Eastern cottonwood enhances the processing of organomercury. Plant Biotechnol. J. 2007, 5, 254–262. [Google Scholar] [CrossRef]
- Hussein, H.S.; Ruiz, O.N.; Terry, N.; Daniell, H. Phytoremediation of Mercury and Organomercurials in Chloroplast Transgenic Plants: Enhanced Root Uptake, Translocation to Shoots, and Volatilization. Environ. Sci. Technol. 2007, 41, 8439–8446. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Wu, H.; Ding, J.; Li, N.; Fu, W.; Gan, L.; Li, Y. Transgenic merA and merB expression reduces mercury contamination in vegetables and grains grown in mercury-contaminated soil. Plant Cell Rep. 2020, 39, 1369–1380. [Google Scholar] [CrossRef]
- Sasaki, Y.; Hayakawa, T.; Inoue, C.; Miyazaki, A.; Silver, S.; Kusano, T. Generation of mercury-hyperaccumulating plants through transgenic expression of the bacterial mercury membrane transport protein MerC. Transgenic Res. 2006, 15, 615. [Google Scholar] [CrossRef]
- Hsieh, J.-L.; Chen, C.-Y.; Chiu, M.-H.; Chein, M.-f.; Chang, J.-S.; Endo, G.; Huang, C.-C. Expressing a bacterial mercuric ion binding protein in plant for phytoremediation of heavy metals. J. Hazard. Mater. 2009, 161, 920–925. [Google Scholar] [CrossRef]
- Kiyono, M.; Oka, Y.; Sone, Y.; Nakamura, R.; Sato, M.H.; Sakabe, K.; Pan-Hou, H. Bacterial heavy metal transporter MerC increases mercury accumulation in Arabidopsis thaliana. Biochem. Eng. J. 2013, 71, 19–24. [Google Scholar] [CrossRef]
- Sone, Y.; Nakamura, R.; Pan-Hou, H.; Sato, M.H.; Itoh, T.; Kiyono, M. Increase methylmercury accumulation in Arabidopsis thaliana expressing bacterial broad-spectrum mercury transporter MerE. AMB Express 2013, 3, 52. [Google Scholar] [CrossRef] [Green Version]
- Xu, S.; Sun, B.; Wang, R.; He, J.; Xia, B.; Xue, Y.; Wang, R. Overexpression of a bacterial mercury transporter MerT in Arabidopsis enhances mercury tolerance. Biochem. Biophys. Res. Commun. 2017, 490, 528–534. [Google Scholar] [CrossRef]
- Uraguchi, S.; Sone, Y.; Kamezawa, M.; Tanabe, M.; Hirakawa, M.; Nakamura, R.; Takanezawa, Y.; Kiyono, M. Ectopic expression of a bacterial mercury transporter MerC in root epidermis for efficient mercury accumulation in shoots of Arabidopsis plants. Sci. Rep. 2019, 9, 4347. [Google Scholar] [CrossRef]
- Uraguchi, S.; Sone, Y.; Yoshikawa, A.; Tanabe, M.; Sato, H.; Otsuka, Y.; Nakamura, R.; Takanezawa, Y.; Kiyono, M. SCARECROW promoter-driven expression of a bacterial mercury transporter MerC in root endodermal cells enhances mercury accumulation in Arabidopsis shoots. Planta 2019, 250, 667–674. [Google Scholar] [CrossRef]
- Nagata, T.; Kiyono, M.; Pan-Hou, H. Engineering expression of bacterial polyphosphate kinase in tobacco for mercury remediation. Appl. Microbiol. Biotechnol. 2006, 72, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Nagata, T.; Ishikawa, C.; Kiyono, M.; Pan-Hou, H. Accumulation of mercury in transgenic tobacco expressing bacterial polyphosphate. Biol. Pharm. Bull. 2006, 29, 2350–2353. [Google Scholar] [CrossRef] [Green Version]
- Nagata, T.; Nakamura, A.; Akizawa, T.; Pan-Hou, H. Genetic engineering of transgenic tobacco for enhanced uptake and bioaccumulation of mercury. Biol. Pharm. Bull. 2009, 32, 1491–1495. [Google Scholar] [CrossRef] [Green Version]
- Nagata, T.; Morita, H.; Akizawa, T.; Pan-Hou, H. Development of a transgenic tobacco plant for phytoremediation of methylmercury pollution. Appl. Microbiol. Biotechnol. 2010, 87, 781–786. [Google Scholar] [CrossRef]
- Ruiz, O.N.; Alvarez, D.; Torres, C.; Roman, L.; Daniell, H. Metallothionein expression in chloroplasts enhances mercury accumulation and phytoremediation capability. Plant Biotechnol. J. 2011, 9, 609–617. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Ma, Y.; Wang, H.; Huang, W.; Wang, X.; Han, L.; Sun, W.; Han, E.; Wang, B. Overexpression of PtABCC1 contributes to mercury tolerance and accumulation in Arabidopsis and poplar. Biochem. Biophys. Res. Commun. 2018, 497, 997–1002. [Google Scholar] [CrossRef]
- De Temmerman, L.; Claeys, N.; Roekens, E.; Guns, M. Biomonitoring of airborne mercury with perennial ryegrass cultures. Environ. Pollut. 2007, 146, 458–462. [Google Scholar] [CrossRef]
- De Temmerman, L.; Waegeneers, N.; Claeys, N.; Roekens, E. Comparison of concentrations of mercury in ambient air to its accumulation by leafy vegetables: An important step in terrestrial food chain analysis. Environ. Pollut. 2009, 157, 1337–1341. [Google Scholar] [CrossRef]
- Niu, Z.; Zhang, X.; Wang, Z.; Ci, Z. Field controlled experiments of mercury accumulation in crops from air and soil. Environ. Pollut. 2011, 159, 2684–2689. [Google Scholar] [CrossRef]
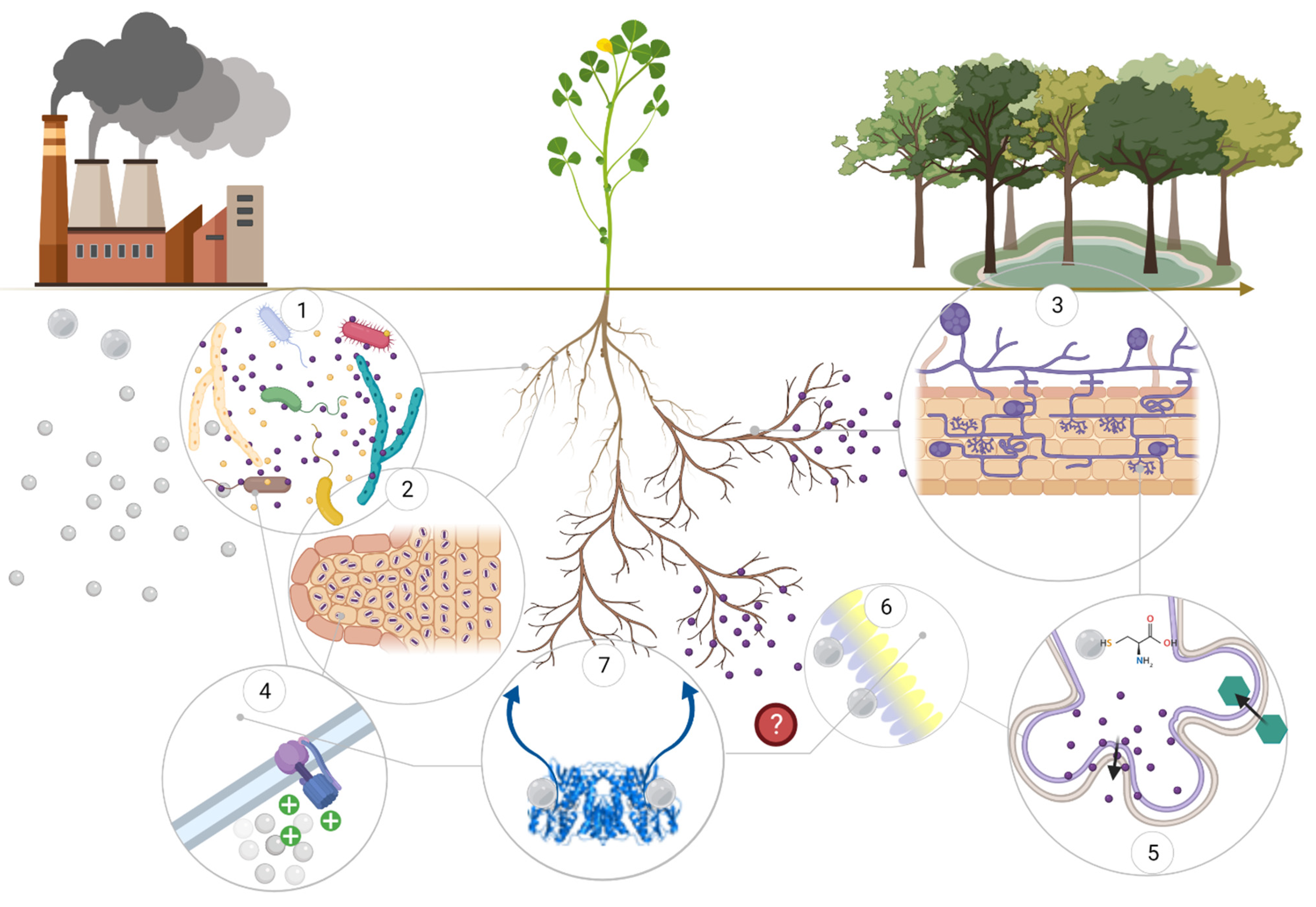

| Type | Plant sp. | Growth Conditions | Phytotoxic Concentration | Growth Parameters (Phytotox. Conc.) * | Hg Accumulation (BAF, BCF and TF) | References |
|---|---|---|---|---|---|---|
| potential Hg (hyper)accumulator native species | Vigna unguiculata L. Walp | Soil pots—3 m old ecotypes: 1. native genotype 2. commercial line L-019 3. commercial line L-042 | 5 and 8 mg kg−1 Hg(NO3)2 (added to 0.2 mg Hg kg−1 contaminated soil) | Negligible biomass decrease with ^ Hg | root > leaf > stem; BCF < 1 (all genotypes); BAFstem/soil < 0.5, BAFseed/soil < 0.5; 1. TF < 1 for native genotype 2. TF~1.5 (for 0.2 mg Hg kg−1 dw) for both commercial lines | [102] |
| Phragmites australis | Plant samples were taken from gold mine contaminated wetland (wet and dry season) | - | - | root[Hg]—806 µg kg−1 dw stem[Hg]—495 µg kg−1 dw leaves[Hg]—833 µg kg−1 dw BAF—0.73/0.22 TF—0.57/1.99 | [55] | |
| Cyperus eragrostis | BAF—0.22/0.35 TF—1.99/3/60 | |||||
| Datura stramonium | BAF—0.20/0.61 TF—4.26/8.30 | |||||
| Panicum coloratum | BAF—0.11/0.13 TF—3.70/10.94 | |||||
| Persicaria lapathifolia | BAF—0.11/0.20 TF—3.10/3.07 | |||||
| Melilotus alba | BAF—0.13/0.21 TF—0.54/0.60 | |||||
| Lathyrus pratensis | Aerial parts of plants growing in the area of an abandoned gold mine in the Czech Republic were collected (0.207–15.0 mg total Hg kg−1 soil) | - | - | Shoot[Hg]—0.108 mg kg−1 dw | [54] | |
| Epipactis sp. | Shoot[Hg]—0.152 mg kg−1 dw | |||||
| Axonopus compressus | Plant samples were taken from soil contaminated by artisanal small-scale gold mines (arbuscular mycorrhizal fungi (AMF) colonization was aslo determined | - | - | root[Hg]—0.15 mg kg−1 dw shoot[Hg]—0.33 mg kg−1 dw BAFroot/leaves—0.03/0.06 TF—2.16 | [103] | |
| Erato polymnioides | root[Hg]—3.56 mg kg−1 dw shoot[Hg]—1.48 mg kg−1 dw BAFroot—0.80; TF—0.42 | |||||
| Miconia zamorensis | root[Hg]—2.06 mg kg−1 dw shoot[Hg]—0.98 mg kg−1 dw BAFroot—0.47; TF—0.47 | |||||
| Cyrtomium macrophyllum | 60 d old seedlings from uncontaminated sites (grown 1st hydroponically) 1. 225.73 mg total Hg kg−1 soil or 2. 0, 5, 10, 20, 50, 100, 200, 500 and 1000 mg HgCl2 kg−1 soil | 500 and 1000 mg kg−1 HgCl2 | 20.2% biomass reduction | 1. shoot[Hg]—36.44 mg kg−1 dw root[Hg]—13.90 mg kg−1 dw BCF—0.061; TF—2.62 2. for treatments up to 200 mg kg−1: BCF > 1; TF > 1 | [53] | |
| Manihot esculenta Crantz | 1. soil pots with mixtures of mine tailings and biosolids; 4 w old cuttings ( 11.67 mg total Hg kg−1 mine tailings); 2. hydroponic solution with 50 or 100 µM HgCl2; 5 w old plants | mixtures with 50, 75, or 100% mine tailings | significant root biomass decrease | 1. Hg not determined in plants 2. root[Hg]—6.836 and 12.13 g kg−1 dw (50 and 100 µM Hg) | [104] | |
| Dillenia suffruticosa | Plants were cultivated on 2 ex-gold mine tailings areas: (i) tailings site where last mining activity was 2 years prior (0.5 mg Hg kg−1) (ii) tailings site where last mining activity was 10 years prior (0.02 mg Hg kg−1) | none observed | no significant decrease in plant growth (height and diameter) | BCF—15.5; TF—3.0 | [50] | |
| Vitex pinnata | BCF—40; TF—0.6 | |||||
| Archidendron pauciflorum | BCF—11.0; TF—0.1 | |||||
| Anacardium occidentale | BCF—6.5; TF—0.3 | |||||
| Shorea leprosula | BCF—7.5; TF—0.5 | |||||
| Alstonia scholaris | BCF—45.0; TF—1.3 | |||||
| Hevea brasiliensis | BCF—13.5; TF—0.1 | |||||
| Alyssum saxatile L. | Plant samples were collected from 41 sites in an active mining district in Western Turkey (mean 6.609 µg Hg kg−1 soil) | - | - | root[Hg]/soil[Hg]—0.10 shoot[Hg]/soil[Hg]—0.04 Mean TF—0.85 | [52] | |
| Anchusa arvensis L. | root[Hg]/soil[Hg]—0.06 shoot[Hg]/soil[Hg]—0.06 Mean TF—1.03 | |||||
| Centaurea cyanus L. | root[Hg]/soil[Hg] < 0.5 shoot[Hg]/soil[Hg] < 0.5 Mean TF > 1 | |||||
| Cynoglossum officinale | root[Hg]/soil[Hg] < 1 shoot[Hg]/soil[Hg] < 1 Mean TF < 1 | |||||
| Glaucium flavum | root[Hg]/soil[Hg]—0.09 shoot[Hg]/soil[Hg]—0.02 Mean TF—0.25 | |||||
| Isatis sp. L. | root[Hg]/soil[Hg]—0.02 shoot[Hg]/soil[Hg]—0.02 Mean TF—0.63 | |||||
| Onosma sp. | root[Hg]/soil[Hg] < 0.5 shoot[Hg]/soil[Hg] < 0.5 Mean TF > 1 | |||||
| Phlomis sp. | root[Hg]/soil[Hg]—0.21 shoot[Hg]/soil[Hg]—0.56 Mean TF—2.05 | |||||
| Silene compacta | root[Hg]/soil[Hg] < 0.5 shoot[Hg]/soil[Hg] < 0.5 Mean TF—1.66 | |||||
| Tripleurospermum maritimum | root[Hg]/soil[Hg]—0.02 shoot[Hg]/soil[Hg]—0.01 Mean TF—0.59 | |||||
| Verbascum thapsus L. | root[Hg]/soil[Hg]—0.03 shoot[Hg]/soil[Hg]—0.06 Mean TF—2.47 | |||||
| Sesbania grandiflora | 17 d old seedlings in hydroponic solution | 50 and 60 mg L−1 HgCl2 | 56% growth decrease 19% biomass reduction (60 mg Hg L−1) | mostly in roots; TF—low. | [91] | |
| Jatropha curcas | Pots with Hg-contaminated soil (1.76 mg kg−1) spiked with 1, 5 or 10 mg Hg(NO3)2 kg−1; 1, 2, 3 or 4 m old seedlings (seeds of plants from uncontaminated soil) | none observed | - | plant[Hg]—max. 7.25 mg kg−1 dw (for 10 mg Hg kg−1 soil) BCF—good, with increased exposure (4th month); TF~1 (after 2 months, then decreased) | [105] | |
| Lepidium sativum L. | Soil pots (spiked with 10 or 100 mg HgCl2 kg-1 dw) with/without different fractions of uncontaminated compost; 10 d seedlings | (a) 10 and 100 mg kg−1 HgCl2; (b) none observed for compost amended soil | (a) 27% decrease in shoot length; 53% decrease in root (10 mg Hg kg−1) | mostly in roots; add. compost—^ accumulation; BCF—high for 10 mg Hg kg−1 dw in 2/1 compost | [106] | |
| Flueggea tinctoria (L.) G.L. Webster | Aerial plant parts were collected from a riparian area in the mining district of Almadén (122—385 mg total Hg kg−1 soil) | - | - | BCF—5.9 | [49] | |
| Tamarix canariensis Willd. | BCF—10.72 | |||||
| Nerium oleander L. | BCF—6.2 | |||||
| Typha domingensis Pers. | BCF—4.3 | |||||
| Phragmites australis Cav. | BCF—32.2 | |||||
| Atriplex conodocarpa | 25 seeds/species were sown in pots with Hg spiked potting mix (17.3 mg Hg kg−1 soil) | no phytotoxic symptoms were observed | Biomass, leaf area and number remained unchanged (in regards to unspiked soil) | shoot[Hg]—1.09 mg kg−1 dw translocation %—19% | [107] | |
| Australodanthonia caespitose | shoot[Hg]—1.20 mg kg−1 dw translocation—15.9% | |||||
| Chilopsis linearis | 2 w old seedlings in Hoagland solution | 50, 100, 200 µM (CH3COO)2Hg | 49% decrease in root length | root[Hg]—^ with Hg conc. TF—low | [108] | |
| Medicago sativa | 4 d old seedlings in 1/4 Hoagland solution | 20 µM HgCl2 | 54% decrease in root biomass | - | [88] | |
| Eichornia crassipes | 30 d old plants in spring water tanks (0, 0.5, 2 mg L−1 HgSO4) | - | - | root[Hg]—26.2 mg kg−1 dw (for 2 mg Hg L−1) | [101] | |
| Pistia stratiotes | root[Hg]—83.2 mg kg−1 dw | |||||
| Scirpus tabernaemontani | root[Hg]—3.88 mg kg−1 dw | |||||
| Colocasia esculenta | root[Hg]—6.99 mg kg−1 dw | |||||
| Sesbania drummondii | 15 d old seedlings in 1/2 Hoagland solution | 50 and 100 mg L−1 HgCl2 | 36.8% biomass reduction (100 mg Hg L−1) | root[Hg] > shoot[Hg] | [89] | |
| Rumex induratus | Field experiment; Whole plants were collected from sites with: 122.4 mg total Hg kg−1 dw (0.006% available Hg) | - | root[Hg]—8.3 mg kg−1 dw shoot[Hg]—7.3 mg kg−1 dw TF—0.96 Phytoextraction efficiency 12.9 g Hg ha−1 year−1 | [109] | ||
| Marrubium vulgare | 550.1 mg total Hg kg−1 dw (0.032% available) | root[Hg]—67.2 mg kg−1 dw shoot[Hg]—23.0 mg kg−1 dw TF—0.34 Phytoextraction efficiency 27.6 g Hg ha−1 year−1 | ||||
| Medicago sativa | 12 d old seedlings in a beaker-size hydroponic system | 30 µM HgCl2 | abrupt 30–40% growth inhibition (first 24 h) | - | [87] | |
| Myriophylhum aquaticum Ludwigina palustris Mentha aquatica | 21 d old plants in water solution with hydroponic fertilizer | - | - | average removal efficiency—99.8% (all 3 plants); removal rate—0.0787–0.0002 mg Hg L−1 d−1 | [100] | |
| Nicotiana miersii | 5 w old plants in 1/4 Hoagland | 1. 1.0 mg Hg0 m3 2. 1.0 µg HgCl2 mL−1 | 1. Visible signs of stress 2. Inhibition of root and shoot | 1. only in shoots 2. mostly in roots | [110] | |
| broad-spectrum heavy metal (hyper)accumulator species | Brassica juncea Long-standing and Florida Broad Leaf cultivars | 2 and 4 w old plants grown hydroponically | 1.96, 4.11, 12.2, and 16.7 mg L−1 Hg(NO3)2 | 25% biomass decrease | BCFroot—750–1100; BCFshoots—82–104; roots[Hg]/shoot[Hg]—8–100 | [111] |
| Brassica juncea | 36 d old seedlings grown hydroponically | 5 and 10 mg L−1 HgCl2 | 5.1-fold reduced transpiration rates | BCFroot—100–270; BCFshoot—0.31–1.07; shoots[Hg]/root[Hg]–0.3–0.76 | [112] | |
| crop plant species | Hordeum vulgare | Soil pots—3 soil compositions: 1. 8.35 mg HgCl2 kg−1 dw; 2. 32.16 mg total Hg kg−1 dw; 3. 32.16 mg total Hg kg−1 dw + 1 mg HgCl2 kg−1; 150 d old plants | - | - | 1. shoot[Hg]—1.51–5.13 mg kg−1 dw; (L. esculenta and L. albus the highest); 2. shoot[Hg]—0.16–1.13 mg kg−1 dw; 3. shoot[Hg]—6× L. albus, 5× C. aretinum, 3.5× H. vulgare and L. esculenta (* regards to 2nd treatment) | [113] |
| Lupinus albus | ||||||
| Lens esculenta | ||||||
| Cicer aretinum | ||||||
| Cucumis sativus | 10 and 15 d old seedlings in 10% MS media | 250–500 µM HgCl2 | 96% root length reduction (10 d old seedlings) 98% root length reduction (15 d old seedlings) | root[Hg]—7-fold and 5.6-fold > cotyledons (after 10 and 15 d) | [114] | |
| Oryza sativa | 3 w old seedlings in Long Ashton modified nutrient solution | 0.5 mg L−1 HgCl2 | 50% shoot biomass reduction | root[Hg] 2× > shoot[Hg] BCF~1900 (for higher Hg conc.) | [115] | |
| Lycopersicon esculentum | 30 d old seedlings in modified Hoagland | 50 µM HgCl2 | suppressed biomass production (roots and shoots) | root[Hg]—27-fold > shoot; uptake ^ linearly with concentration | [90] | |
| Pisum sativum | seedlings in solution culture | 5 and 10 mg L−1 HgCl2 or 203HgCl2 | growth inhibition: 50% shoot and root length decrease (10 mg Hg L−1) | mostly in roots; linearly increase with [Hg]; TF—low | [116] | |
| Mentha spicata | cuttings in solution culture |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tiodar, E.D.; Văcar, C.L.; Podar, D. Phytoremediation and Microorganisms-Assisted Phytoremediation of Mercury-Contaminated Soils: Challenges and Perspectives. Int. J. Environ. Res. Public Health 2021, 18, 2435. https://doi.org/10.3390/ijerph18052435
Tiodar ED, Văcar CL, Podar D. Phytoremediation and Microorganisms-Assisted Phytoremediation of Mercury-Contaminated Soils: Challenges and Perspectives. International Journal of Environmental Research and Public Health. 2021; 18(5):2435. https://doi.org/10.3390/ijerph18052435
Chicago/Turabian StyleTiodar, Emanuela D., Cristina L. Văcar, and Dorina Podar. 2021. "Phytoremediation and Microorganisms-Assisted Phytoremediation of Mercury-Contaminated Soils: Challenges and Perspectives" International Journal of Environmental Research and Public Health 18, no. 5: 2435. https://doi.org/10.3390/ijerph18052435
APA StyleTiodar, E. D., Văcar, C. L., & Podar, D. (2021). Phytoremediation and Microorganisms-Assisted Phytoremediation of Mercury-Contaminated Soils: Challenges and Perspectives. International Journal of Environmental Research and Public Health, 18(5), 2435. https://doi.org/10.3390/ijerph18052435






