The Dynamic Response of Nitrogen Transformation to the Dissolved Oxygen Variations in the Simulated Biofilm Reactor
Abstract
:1. Introduction
2. Materials and Methods
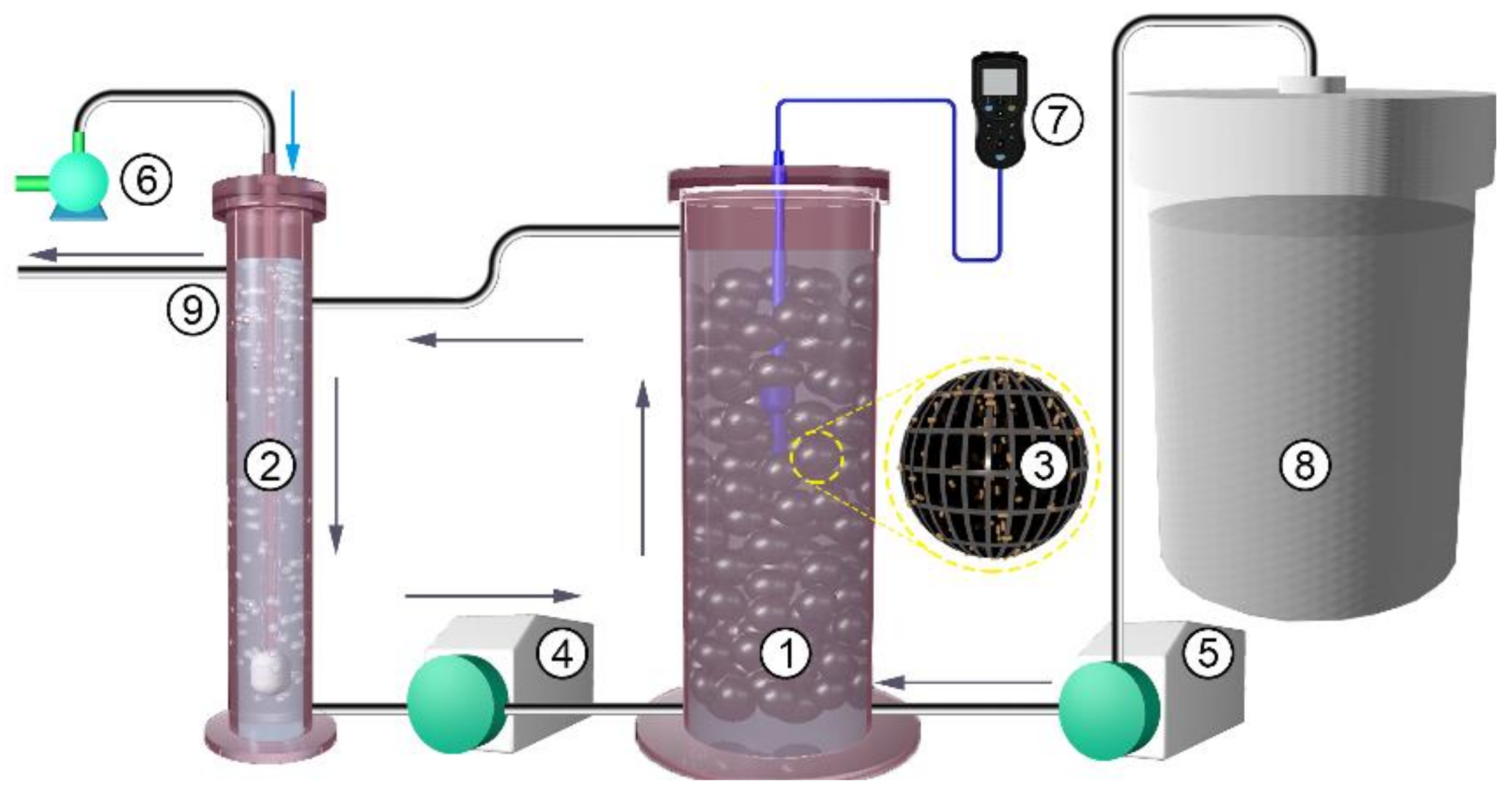
2.1. The Simulated BR System
2.2. Experimental Design and Operation Conditions
2.3. Sampling and Measurement
3. Results and Discussion
3.1. The Transformation between the SSs in Terms of the DO
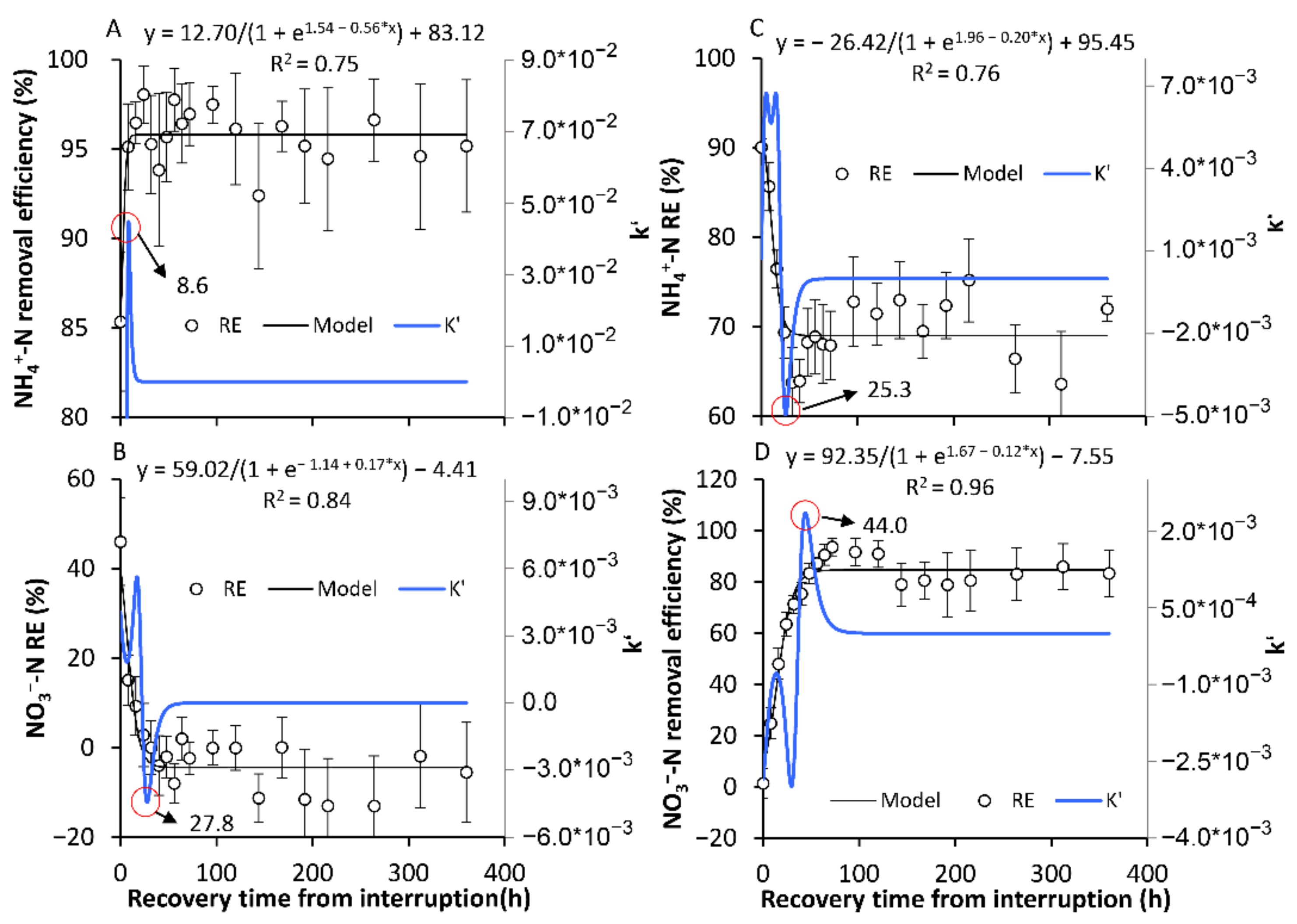
3.2. The Transformation between the SSs in Terms of the NH4+-N and NO3−-N REs
3.2.1. The Formation of a New Temporary SS
3.2.2. The Recovery of the Original SS in Terms of the NH4+-N and NO3−-N REs
3.3. The Transformation between SSs in Terms of the Key Nitrification and Denitrification Genes
3.3.1. The Formation of a New SS in Terms of the amoA and nirS Activities
3.3.2. The Recovery of the Original SS in Terms of the amoA and nirS Activities
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| Ae | Aeration |
| AE-D | Aeration disturbed by a short-term aeration interruption |
| As | Aeration stop |
| AN-D | No aeration disturbed by short-term aeration |
| COD | Chemical oxygen demand |
| SBR | Simulated biofilm reactor |
| DO | Dissolved oxygen |
| NH4+-N | Ammoniacal nitrogen |
| NO3−-N | Nitrate nitrogen |
| RE | Removal efficiency |
| SS | Stable state |
| SS-AE | Aerobic stable state |
| SS-AEamoA | Aerobic stable state of the amoA activity |
| SS-AEDO | Aerobic stable state of the DO |
| SS-AENH4+ | Aerobic stable state of the NH4+-N removal efficiency |
| SS-AEnirS | Aerobic stable state of the nirS activity |
| SS-AENO3− | Aerobic stable state of the NO3−-N removal efficiency |
| SS-AN | Anaerobic stable state |
| SS-ANamoA | Anaerobic stable state of the amoA activity |
| SS-ANDO | Anaerobic stable state of the DO |
| SS-ANNH4+ | Anaerobic stable state of the NH4+-N removal efficiency |
| SS-ANnirS | Anaerobic stable state of the nirS activity |
| SS-ANNO3− | Anaerobic stable state of the NO3−-N removal efficiency |
| SS-ANDO | Anaerobic stable state of the DO |
| WWTS | Wastewater treatment system |
References
- Wu, H.; Zhang, J.; Guo, W.; Liang, S.; Fan, J. Secondary effluent purification by a large-scale multi-stage surface-flow constructed wetland: A case study in northern China. Bioresour. Technol. 2018, 249, 1092–1096. [Google Scholar] [CrossRef]
- Juan-García, P.; Butler, D.; Comas, J.; Darch, G.; Sweetapple, C.; Thornton, A.; Corominas, L. Resilience theory incorporated into urban wastewater systems management. State of the art. Water Res. 2017, 115, 149–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sukias, J.P.S.; Park, J.B.K.; Stott, R.; Tanner, C.C. Quantifying treatment system resilience to shock loadings in constructed wetlands and denitrification bioreactors. Water Res. 2018, 139, 450–461. [Google Scholar] [CrossRef] [PubMed]
- Schoen, M.; Hawkins, T.; Xue, X.; Ma, C.; Garland, J.; Ashbolt, N.J. Technologic resilience assessment of coastal community water and wastewater service options. Sustain. Water Qual. Ecol. 2015, 6, 75–87. [Google Scholar] [CrossRef]
- Galvão, A.; Matos, J. Response of horizontal sub-surface flow constructed wetlands to sudden organic load changes. Ecol. Eng. 2012, 49, 123–129. [Google Scholar] [CrossRef]
- Song, S.; Liu, B.; Zhang, W.; Wang, P.; Qiao, Y.; Zhao, D.; Yang, T.; An, S.; Leng, X. Performance of a large-scale wetland treatment system in treating tailwater from a sewage treatment plant. Mar. Freshw. Res. 2018, 69, 833–839. [Google Scholar] [CrossRef]
- Erisman, J.W.; Galloway, J.N.; Seitzinger, S.; Bleeker, A.; Dise, N.B.; Petrescu, A.M.R.; Leach, A.M.; de Vries, W. Consequences of human modification of the global nitrogen cycle. Philos. Trans. R. Soc. B-Biol. Sci. 2013, 368. [Google Scholar] [CrossRef] [Green Version]
- Finlay, J.C.; Small, G.E.; Sterner, R.W. Human Influences on Nitrogen Removal in Lakes. Science 2013, 342, 247–250. [Google Scholar] [CrossRef]
- Zhang, X.W.; Hu, Z.; Ngo, H.H.; Zhang, J.; Guo, W.S.; Liang, S.; Xie, H.J. Simultaneous improvement of waste gas purification and nitrogen removal using a novel aerated vertical flow constructed wetland. Water Res. 2018, 130, 79–87. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.Q.; Hu, Z.; Zhang, J.; Ngo, H.H.; Guo, W.S.; Liang, S.; Fan, J.L.; Lu, S.Y.; Wu, H.M. Optimizations on supply and distribution of dissolved oxygen in constructed wetlands: A review. Bioresour. Technol. 2016, 214, 797–805. [Google Scholar] [CrossRef]
- Feng, L.K.; Liu, Y.; Zhang, J.Y.; Li, C.; Wu, H.M. Dynamic variation in nitrogen removal of constructed wetlands modified by biochar for treating secondary livestock effluent under varying oxygen supplying conditions. J. Environ. Manag. 2020, 260, 110152. [Google Scholar] [CrossRef]
- Wang, J.; Hou, J.; Xia, L.; Jia, Z.M.; He, X.G.; Li, D.P.; Zhou, Y.Y. The combined effect of dissolved oxygen and COD/N on nitrogen removal and the corresponding mechanisms in intermittent aeration constructed wetlands. Biochem. Eng. J. 2020, 153, 107400. [Google Scholar] [CrossRef]
- Zhang, P.F.; Peng, Y.K.; Lu, J.L.; Li, J.; Chen, H.P.; Xiao, L. Microbial communities and functional genes of nitrogen cycling in an electrolysis augmented constructed wetland treating wastewater treatment plant effluent. Chemosphere 2018, 211, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Kuypers, M.M.M.; Marchant, H.K.; Kartal, B. The microbial nitrogen-cycling network. Nat. Rev. Microbiol. 2018, 16, 263–276. [Google Scholar] [CrossRef]
- Stein, O.R.; Hook, P.B.; Biederman, J.A.; Allen, W.C.; Borden, D.J. Does batch operation enhance oxidation in subsurface constructed wetlands? Water Sci. Technol. 2003, 48, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Xia, L.; Ma, T.; Zhang, Y.; Zhou, Y.; He, X. Achieving short-cut nitrification and denitrification in modified intermittently aerated constructed wetland. Bioresour. Technol. 2017, 232, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Kuschk, P.; Brix, H.; Vymazal, J.; Dong, R. Development of constructed wetlands in performance intensifications for wastewater treatment: A nitrogen and organic matter targeted review. Water Res. 2014, 57, 40–55. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, J.; Ngo, H.H.; Guo, W.; Hu, Z.; Liang, S.; Fan, J.; Liu, H. A review on the sustainability of constructed wetlands for wastewater treatment: Design and operation. Bioresour. Technol. 2015, 175, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Boog, J.; Nivala, J.; Aubron, T.; Mothes, S.; van Afferden, M.; Müller, R.A. Resilience of carbon and nitrogen removal due to aeration interruption in aerated treatment wetlands. Sci. Total Environ. 2018, 621, 960–969. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Qi, S.; Sun, Y.; Jiang, Y.; Zhao, N.; Huang, L.; Sun, Y. Nitrogen removal and nitrogen functional gene abundances in three subsurface wastewater infiltration systems under different modes of aeration and influent C/N ratios. Bioresour. Technol. 2017, 241, 1162–1167. [Google Scholar] [CrossRef] [PubMed]
- Faulwetter, J.L.; Gagnon, V.; Sundberg, C.; Chazarenc, F.; Burr, M.D.; Brisson, J.; Camper, A.K.; Stein, O.R. Microbial processes influencing performance of treatment wetlands: A review. Ecol. Eng. 2009, 35, 987–1004. [Google Scholar] [CrossRef]
- Truu, M.; Juhanson, J.; Truu, J. Microbial biomass, activity and community composition in constructed wetlands. Sci. Total Environ. 2009, 407, 3958–3971. [Google Scholar] [CrossRef] [PubMed]
- Samsó, R.; García, J. Bacteria distribution and dynamics in constructed wetlands based on modelling results. Sci. Total Environ. 2013, 461–462, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.; Rajabzadeh, A.R.; Weber, K.P.; Nivala, J.; Wallace, S.D.; Cooper, D.J. Nitrification cessation and recovery in an aerated saturated vertical subsurface flow treatment wetland: Field studies and microscale biofilm modeling. Bioresour. Technol. 2016, 209, 125–132. [Google Scholar] [CrossRef]
- Chen, Y.; Vymazal, J. Comment on “Enhanced Long-Term Nitrogen Removal and Its Quantitative Molecular Mechanism in Tidal Flow Constructed Wetlands”. Environ. Sci. Technol. 2015, 49, 11241–11242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhi, W.; Ji, G. Quantitative response relationships between nitrogen transformation rates and nitrogen functional genes in a tidal flow constructed wetland under C/N ratio constraints. Water Res. 2014, 64, 32–41. [Google Scholar] [CrossRef]
- Zhuang, L.-L.; Yang, T.; Zhang, J.; Li, X. The configuration, purification effect and mechanism of intensified constructed wetland for wastewater treatment from the aspect of nitrogen removal: A review. Bioresour. Technol. 2019, 293, 122086. [Google Scholar] [CrossRef]
- Zhang, X.; Friedl, M.A.; Schaaf, C.B.; Strahler, A.H.; Hodges, J.C.F.; Gao, F.; Reed, B.C.; Huete, A. Monitoring vegetation phenology using MODIS. Remote Sens. Environ. 2003, 84, 471–475. [Google Scholar] [CrossRef]
- Arnon, D.I.; Hoagland, D.R. Crop production in artificial culture solutions and in soils with special reference to factors influencing yields and absorption of inorganic nutrients. Soil Sci. 1940, 50, 463–485. [Google Scholar]
- Dionisi, H.M.; Layton, A.C.; Harms, G.; Gregory, I.R.; Robinson, K.G.; Sayler, G.S. Quantification of Nitrosomonas oligotropha-like ammonia-oxidizing bacteria and Nitrospira spp. from full-scale wastewater treatment plants by competitive PCR. Appl. Environ. Microbiol. 2002, 68, 245–253. [Google Scholar] [CrossRef] [Green Version]
- Throbäck, I.N.; Enwall, K.; Jarvis, Å.; Hallin, S. Reassessing PCR primers targeting nirS, nirK and nosZ genes for community surveys of denitrifying bacteria with DGGE. Fems Microbiol. Ecol. 2004, 49, 401–417. [Google Scholar] [CrossRef]
- Zhao, D.; Zhang, M.; Liu, Z.; Sheng, J.; An, S. Can cold-season macrophytes at the senescence stage improve nitrogen removal in integrated constructed wetland systems treating low carbon/nitrogen effluent? Bioresour. Technol. 2018, 265, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y. The Treatment of Nitrogen and Phosphorus in Wastewater; The Press of Normal University in Eastern China: Shanghai, China, 2006. [Google Scholar]
- Sun, H.; Yang, Z.; Wei, C.; Wu, W. Nitrogen removal performance and functional genes distribution patterns in solid-phase denitrification sub-surface constructed wetland with micro aeration. Bioresour. Technol. 2018, 263, 223–231. [Google Scholar] [CrossRef]
- Levy-Booth, D.J.; Prescott, C.E.; Grayston, S.J. Microbial functional genes involved in nitrogen fixation, nitrification and denitrification in forest ecosystems. Soil Biol. Biochem. 2014, 75, 11–25. [Google Scholar] [CrossRef]
- Li, W.; Zheng, T.; Ma, Y.; Liu, J. Current status and future prospects of sewer biofilms: Their structure, influencing factors, and substance transformations. Sci. Total Environ. 2019, 695, 133815. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, J.; Gao, R.; Wang, M.; Yang, L.; Wang, X.; Zhang, L.; Peng, Y. A critical review of one-stage anammox processes for treating industrial wastewater: Optimization strategies based on key functional microorganisms. Bioresour. Technol. 2018, 265, 498–505. [Google Scholar] [CrossRef]
- Du, R.; Peng, Y.; Ji, J.; Shi, L.; Gao, R.; Li, X. Partial denitrification providing nitrite: Opportunities of extending application for anammox. Environ. Int. 2019, 131, 105001. [Google Scholar] [CrossRef]
- Braker, G.; Zhou, J.; Wu, L.; Devol, A.H.; Tiedje, J.M. Nitrite reductase genes (nirK and nirS) as functional markers to investigate diversity of denitrifying bacteria in pacific northwest marine sediment communities. Appl. Environ. Microbiol. 2000, 66, 2096–2104. [Google Scholar] [CrossRef] [Green Version]
- Kraiem, K.; Kallali, H.; Wahab, M.A.; Fra-vazquez, A.; Mosquera-Corral, A.; Jedidi, N. Comparative study on pilots between Anammox favored conditions in a partially saturated vertical flow constructed wetland and a hybrid system for rural wastewater treatment. Sci. Total Environ. 2019, 670, 644–653. [Google Scholar] [CrossRef]
- Jaramillo, F.; Orchard, M.; Muñoz, C.; Zamorano, M.; Antileo, C. Advanced strategies to improve nitrification process in sequencing batch reactors—A review. J. Environ. Manag. 2018, 218, 154–164. [Google Scholar] [CrossRef]
- Saggar, S.; Jha, N.; Deslippe, J.; Bolan, N.S.; Luo, J.; Giltrap, D.L.; Kim, D.G.; Zaman, M.; Tillman, R.W. Denitrification and N2O:N2 production in temperate grasslands: Processes, measurements, modelling and mitigating negative impacts. Sci. Total Environ. 2013, 465, 173–195. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, Y.; Chen, Y. Recent advances in partial denitrification in biological nitrogen removal: From enrichment to application. Bioresour. Technol. 2019, 298, 122444. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chapman, S.J.; Nicol, G.W.; Yao, H. Nitrification and nitrifiers in acidic soils. Soil Biol. Biochem. 2018, 116, 290–301. [Google Scholar] [CrossRef]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Q.; Zhang, N.; Chen, C.; Zhang, M.; Zhao, D.; An, S. The Dynamic Response of Nitrogen Transformation to the Dissolved Oxygen Variations in the Simulated Biofilm Reactor. Int. J. Environ. Res. Public Health 2021, 18, 3633. https://doi.org/10.3390/ijerph18073633
Lu Q, Zhang N, Chen C, Zhang M, Zhao D, An S. The Dynamic Response of Nitrogen Transformation to the Dissolved Oxygen Variations in the Simulated Biofilm Reactor. International Journal of Environmental Research and Public Health. 2021; 18(7):3633. https://doi.org/10.3390/ijerph18073633
Chicago/Turabian StyleLu, Qianqian, Nannan Zhang, Chen Chen, Miao Zhang, Dehua Zhao, and Shuqing An. 2021. "The Dynamic Response of Nitrogen Transformation to the Dissolved Oxygen Variations in the Simulated Biofilm Reactor" International Journal of Environmental Research and Public Health 18, no. 7: 3633. https://doi.org/10.3390/ijerph18073633
APA StyleLu, Q., Zhang, N., Chen, C., Zhang, M., Zhao, D., & An, S. (2021). The Dynamic Response of Nitrogen Transformation to the Dissolved Oxygen Variations in the Simulated Biofilm Reactor. International Journal of Environmental Research and Public Health, 18(7), 3633. https://doi.org/10.3390/ijerph18073633





