Earlier Is Better? Timing of Adductor Canal Block for Arthroscopic Knee Surgery under General Anesthesia: A Retrospective Cohort Study
Abstract
:1. Introduction
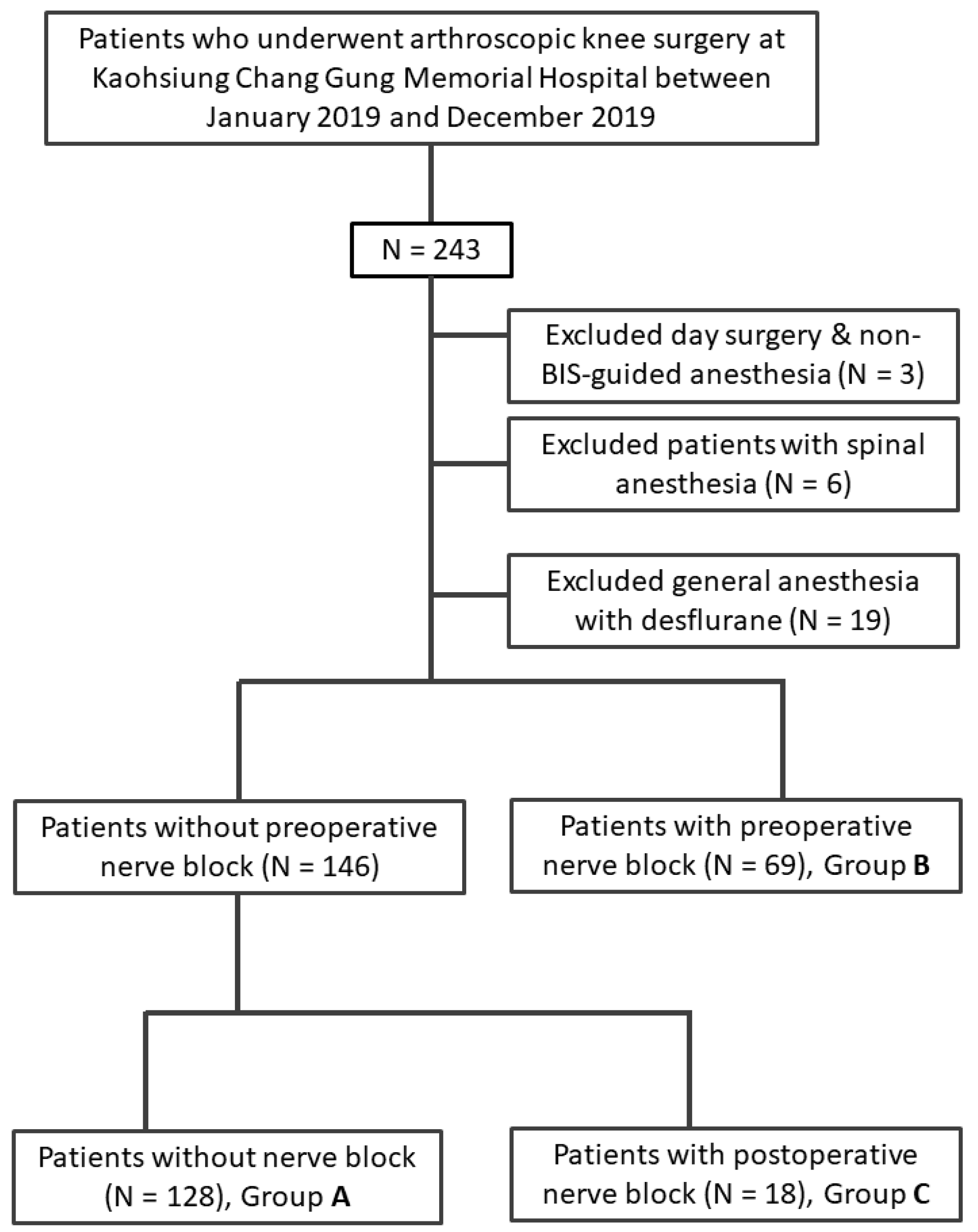
2. Materials and Methods
Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jackson, R.W.; Dandy, D.J. Arthroscopy of the Knee; Grune & Stratton: London, UK, 1976. [Google Scholar]
- Bircher, E. Die Arthroendoskopie. Zentralbl. Chir; Georg Thieme Verlag: Stuttgart, Germany, 1921; Volume 48, pp. 1460–1461. [Google Scholar]
- De Sire, A.; Stagno, D.; Minetto, M.A.; Cisari, C.; Baricich, A.; Invernizzi, M. Long-term effects of intra-articular oxygen-ozone therapy versus hyaluronic acid in older people affected by knee osteoarthritis: A randomized single-blind extension study. J. Back Musculoskelet. Rehabil. 2020, 33, 347–354. [Google Scholar] [CrossRef]
- Paolucci, T.; Agostini, F.; Bernetti, A.; Paoloni, M.; Mangone, M.; Santilli, V.; Pezzi, L.; Bellomo, R.G.; Saggini, R. Integration of focal vibration and intra-articular oxygen–ozone therapy in rehabilitation of painful knee osteoarthritis. J. Int. Med. Res. 2021, 49, 300060520986705. [Google Scholar] [CrossRef] [PubMed]
- Rabini, A.; De Sire, A.; Marzetti, E.; Gimigliano, R.; Ferriero, G.; Piazzini, D.B.; Iolascon, G.; Gimigliano, F. Effects of focal muscle vibration on physical functioning in patients with knee osteoarthritis: A randomized controlled trial. Eur. J. Phys. Rehabil. Med. 2015, 51, 513–520. [Google Scholar]
- Chhabra, A.; Ashikyan, O.; Hlis, R.; Cai, A.; Planchard, K.; Xi, Y.; McCrum, C.; Shah, J. The International Society of Arthroscopy, Knee Surgery and Orthopaedic Sports Medicine classification of knee meniscus tears: Three-dimensional MRI and arthroscopy correlation. Eur. Radiol. 2019, 29, 6372–6384. [Google Scholar] [CrossRef] [PubMed]
- Bert, J.M. First, Do No Harm: Protect the Articular Cartilage When Performing Arthroscopic Knee Surgery! Arthroscopy 2016, 32, 2169–2174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaquero-Picado, A.; Rodriguez-Merchan, E.C. Arthroscopic repair of the meniscus: Surgical management and clinical outcomes. EFORT Open Rev. 2018, 3, 584–594. [Google Scholar] [CrossRef]
- Shin, C.S.; Lee, J.H. Arthroscopic Treatment for Osteoarthritic Knee. Knee Surg. Relat. Res. 2012, 24, 187–192. [Google Scholar] [CrossRef] [Green Version]
- McGrath, B.; Elgendy, H.; Chung, F.; Kamming, D.; Curti, B.; King, S. Thirty percent of patients have moderate to severe pain 24 hr after ambulatory surgery: A survey of 5,703 patients. Can. J. Anaesth. 2004, 51, 886–891. [Google Scholar] [CrossRef] [PubMed]
- Pavlin, D.; Chen, C.; A Penaloza, D.; Buckley, F. A survey of pain and other symptoms that affect the recovery process after discharge from an ambulatory surgery unit. J. Clin. Anesth. 2004, 16, 200–206. [Google Scholar] [CrossRef]
- Castrodad, I.M.D.; Recai, T.M.; Abraham, M.M.; Etcheson, J.I.; Mohamed, N.S.; Edalatpour, A.; Delanois, R.E. Rehabilitation protocols following total knee arthroplasty: A review of study designs and outcome measures. Ann. Transl. Med. 2019, 7, S255. [Google Scholar] [CrossRef]
- Manchikanti, L.; Singh, A. Therapeutic opioids: A ten-year perspective on the complexities and complications of the escalating use, abuse, and nonmedical use of opioids. Pain Phys. 2008, 11, 63–88. [Google Scholar] [CrossRef]
- Chirwa, S.; MacLeod, B.; Day, B. Intraarticular bupivacaine (Marcaine) after arthroscopic meniscectomy: A randomized double-blind controlled study. Arthrosc. J. Arthrosc. Relat. Surg. 1989, 5, 33–35. [Google Scholar] [CrossRef]
- Drosos, G.I.; Vlachonikolis, I.G.; Papoutsidakis, A.N.; Gavalas, N.S.; Anthopoulos, G. Intra-articular morphine and postoperative analgesia after knee arthroscopy. Knee 2002, 9, 335–340. [Google Scholar] [CrossRef]
- Rosseland, L.A.; Stubhaug, A.; Skoglund, A.; Breivik, H. Intra-articular morphine for pain relief after knee arthroscopy. Acta Anaesthesiol. Scand. 1999, 43, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Ruwe, P.A.; Klein, I.; Shields, C.L. The Effect of Intraarticular Injection of Morphine and Bupivacaine on Postarthroscopic Pain Control. Am. J. Sports Med. 1995, 23, 59–64. [Google Scholar] [CrossRef]
- Aasbø, V.; Raeder, J.C.; Grøgaard, B.; Røise, O. No additional analgesic effect of intra-articular morphine or bupivacaine compared with placebo after elective knee arthroscopy. Acta Anaesthesiol. Scand. 1996, 40, 585–588. [Google Scholar] [CrossRef]
- Franceschi, F.; Rizzello, G.; Cataldo, R.; Denaro, V. Comparison of morphine and ropivacaine following knee arthroscopy. Arthrosc. J. Arthrosc. Relat. Surg. 2001, 17, 477–480. [Google Scholar] [CrossRef]
- Møiniche, S.; Mikkelsen, S.; Wetterslev, J.; Dahl, J.B. A systematic review of intra-articular local anesthesia for postoperative pain relief after arthroscopic knee surgery. Reg. Anesth. Pain Med. 1999, 24, 430–437. [Google Scholar] [CrossRef]
- Rautoma, P.; Santanen, U.; Avela, R.; Luurila, H.; Perhoniemi, V.; Erkola, O. Diclofenac premedication but not intra-articular ropivacaine alleviates pain following day-case knee arthroscopy. Can. J. Anaesth. 2000, 47, 220–224. [Google Scholar] [CrossRef] [Green Version]
- Albrecht, E.; Chin, K.J. Advances in regional anaesthesia and acute pain management: A narrative review. Anaesthesia 2020, 75, e101–e110. [Google Scholar] [CrossRef]
- Manickam, B.; Perlas, A.; Duggan, E.; Brull, R.; Chan, V.W.; Ramlogan, R. Feasibility and Efficacy of Ultrasound-Guided Block of the Saphenous Nerve in the Adductor Canal. Reg. Anesth. Pain Med. 2009, 34, 578–580. [Google Scholar] [CrossRef]
- Vora, M.U.; Nicholas, T.A.; Kassel, C.A.; Grant, S.A. Adductor canal block for knee surgical procedures: Review article. J. Clin. Anesth. 2016, 35, 295–303. [Google Scholar] [CrossRef]
- Grevstad, U.; Mathiesen, O.; Valentiner, L.S.; Jaeger, P.; Hilsted, K.L.; Dahl, J.B. Effect of Adductor Canal Block Versus Femoral Nerve Block on Quadriceps Strength, Mobilization, and Pain After Total Knee Arthroplasty. Reg. Anesth. Pain Med. 2015, 40, 3–10. [Google Scholar] [CrossRef] [Green Version]
- Jæger, P.; Zaric, D.; Fomsgaard, J.S.; Hilsted, K.L.; Bjerregaard, J.; Gyrn, J.; Mathiesen, O.; Larsen, T.K.; Dahl, J.B. Adductor canal block versus femoral nerve block for analgesia after total knee arthroplasty: A randomized, double-blind study. Reg. Anesth. Pain Med. 2013, 38, 526–532. [Google Scholar] [CrossRef] [Green Version]
- Sehmbi, H.; Brull, R.; Shah, U.J.; El-Boghdadly, K.; Nguyen, D.; Joshi, G.P.; Abdallah, F.W. Evidence Basis for Regional Anesthesia in Ambulatory Arthroscopic Knee Surgery and Anterior Cruciate Ligament Reconstruction: Part II: Adductor Canal Nerve Block-A Systematic Review and Meta-analysis. Anesth. Analg. 2019, 128, 223–238. [Google Scholar] [CrossRef] [PubMed]
- Joshi, G.; Gandhi, K.; Shah, N.; Gadsden, J.; Corman, S.L. Peripheral nerve blocks in the management of postoperative pain: Challenges and opportunities. J. Clin. Anesth. 2016, 35, 524–529. [Google Scholar] [CrossRef] [Green Version]
- Calatayud, J.; González, Á. History of the Development and Evolution of Local Anesthesia Since the Coca Leaf. J. Am. Soc. Anesthesiol. 2003, 98, 1503–1508. [Google Scholar] [CrossRef] [PubMed]
- Bowness, J.; Taylor, A. Ultrasound-Guided Regional Anaesthesia: Visualising the Nerve and Needle. Adv. Exp. Med. Biol. 2020, 1235, 19–34. [Google Scholar] [CrossRef]
- Sites, B.D.; Taenzer, A.H.; Herrick, M.D.; Gilloon, C.; Antonakakis, J.; Richins, J.; Beach, M.L. Incidence of Local Anesthetic Systemic Toxicity and Postoperative Neurologic Symptoms Associated With 12,668 Ultrasound-Guided Nerve Blocks. Reg. Anesth. Pain Med. 2012, 37, 478–482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernetti, A.; Mangone, M.; Paolucci, T.; Santillis, V.; Verna, S.; Agostini, F.; Paoloni, M. Evaluation of the efficacy of intra-articular injective treatment with reticular hyaluronic acid (Mo.Re. Technology) in amateur athletes with over-use gonarthrosis. Med. Dello Sport 2020, 73, 127–139. [Google Scholar]
- Poon, Y.-Y.; Chang, H.-C.; Chiang, M.-H.; Hung, K.-C.; Lu, H.-F.; Wang, C.-H.; Chin, J.-C.; Wu, S.-C. “A real-world evidence” in reduction of volatile anesthetics by BIS-guided anesthesia. Sci. Rep. 2020, 10, 11245. [Google Scholar] [CrossRef] [PubMed]
- Apfel, C.; Heidrich, F.; Jukar-Rao, S.; Jalota, L.; Hornuss, C.; Whelan, R.; Zhang, K.; Cakmakkaya, O. Evidence-based analysis of risk factors for postoperative nausea and vomiting †. Br. J. Anaesth. 2012, 109, 742–753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bijker, J.B.; Gelb, A.W. Review article: The role of hypotension in perioperative stroke. Can. J. Anaesth. 2012, 60, 159–167. [Google Scholar] [CrossRef] [Green Version]
- Bijker, J.B.; Persoon, S.; Peelen, L.M.; Moons, K.G.; Kalkman, C.J.; Kappelle, L.J.; Van Klei, W.A. Intraoperative hypotension and perioperative ischemic stroke after general surgery: A nested case-control study. Anesthesiology 2012, 116, 658–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Group A (n = 128) | Group B (n = 69) | Group C (n = 18) | p Value | |
|---|---|---|---|---|
| Gender (Male/Female) | 65/63 | 32/37 | 7/11 | 0.59 |
| Age (years) | 44.0 (30.5–57.5) | 44.5 (27.0–58.8) | 47.5 (38.8–52.0) | 0.767 |
| Body weight | 69.0 (61.0–81.0) | 69.5 (58.3–81.5) | 70.0 (59.5–82.0) | 0.997 |
| ASA | ||||
| I | 18 (14.1%) | 12 (17.4%) | 1 (5.6%) | 0.23 |
| II | 97 (75.8%) | 54 (78.2%) | 17 (94.4%) | |
| III | 13 (10.1%) | 3 (4.4%) | 0 (0%) | |
| Anesthesia time (hour) | 1.88 (1.57–2.50) | 2.18 (1.67–2.49) | 2.35 (1.85–2.81) | 0.177 |
| PONV risk | ||||
| Low risk | 34 (26.4%) | 18 (26.5%) | 5 (27.8%) | 0.984 |
| High risk | 95 (73.6%) | 50 (73.5%) | 13 (72.2%) | |
| Arthroscopic surgery | 0.368 | |||
| Cruciate ligament | 18 (14.1%) | 17 (24.6%) | 5 (27.8%) | |
| Medial/Lateral ligament | 1 (0.8%) | 1 (1.4%) | 1 (5.6%) | |
| Meniscus | 49 (38.3%) | 22 (31.9%) | 6 (33.3%) | |
| Synovial/Articular shaving | 60 (46.9%) | 29 (42.0%) | 6 (33.3%) | |
| Comorbidity | ||||
| Hypertension | 26 (20.3%) | 12 (17.4%) | 4 (22.2%) | 0.874 |
| Diabetes Mellitus | 7 (5.5%) | 5 (7.2%) | 2 (11.1%) | 0.621 |
| COPD | 2 (1.6%) | 0 (0.0%) | 0 (0.0%) | 0.51 |
| CAD | 1 (0.8%) | 1 (1.1%) | 0 (0.0%) | 0.811 |
| CHF | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | - |
| CVD | 1 (0.8%) | 0(0.0%) | 0 (0.0%) | 0.715 |
| ESRD | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | - |
| Group A (n = 128) | Group B (n = 69) | Group C (n = 18) | p Value | |
|---|---|---|---|---|
| Mean sevoflurane concentration (%) in 1st hour | 2.50 (2.20–2.90) | 2.20 (1.9–2.7) | 2.65(2.50–2.80) | 0.004 |
| Sevoflurane consumption (ml/kg/h) | 0.22 (0.18–0.27) | 0.19 (0.15–0.25) | 0.22 (0.21–0.26) | 0.005 |
| Intraoperative fluid given (ml/kg) | 2.26 (1.74–2.80) | 2.08 (1.74–2.47) | 1.97 (1.71–2.40) | 0.175 |
| Baseline systolic blood pressure (mmHg) | 136 (124–153) | 142 (130–155) | 134 (119–146) | 0.477 |
| Patients with intraoperative hypertensive response | 33 (25.8%) | 8 (11.6%) | 5 (27.8%) | 0.042 |
| Intraoperative opioid consumption (MME) | 0.10 (0.08–0.13) | 0.08 (0.06–0.11) | 0.09 (0.07–0.12) | 0.001 |
| Group A (n = 128) | Group B (n = 69) | Group C (n = 18) | p Value | |
|---|---|---|---|---|
| VAS at PACU | 3.0 (2.0–4.0) | 1.0 (1.0–2.0) | 1.0 (1.0–2.0) | <0.001 |
| VAS at ward | ||||
| Rest | 1.00 (1.00–2.00) | 1.00 (0.00–1.00) | 1.0 (0.00–1.00) | 0.932 |
| Dynamic | 3.00 (2.00–4.00) | 2.00 (1.00–2.00) | 1.50 (1.00–2.00) | <0.001 |
| Number of patients required analgesics at ward | ||||
| Parecoxib (intravenous) | 45 (35.1%) | 9 (13.0%) | 3 (16.7%) | 0.001 |
| NSAID (oral) | 64 (50%) | 27 (39.1%) | 10 (55.6%) | 0.258 |
| Opioid consumption at ward | ||||
| MME | 0.00 (0.00–0.38) | 0.00 (0.00–0.13) | 0.00 (0.00–0.09) | 0.137 |
| Number of patients with postoperative side effects | ||||
| Headache | 1 (0.8%) | 1 (1.4%) | 0 (0.0%) | 0.763 |
| Nausea | 5 (3.9%) | 2 (2.9%) | 1 (5.6%) | 0.861 |
| Vomiting | 10 (7.8%) | 4 (5.8%) | 1 (5.6%) | 0.840 |
| Dizziness | 9 (7.0%) | 5 (5.6%) | 1 (5.6%) | 0.966 |
| Length of stay after surgery (day) | 3.00 (2.00–4.38) | 3.00 (2.00–4.00) | 3.50 (2.50–4.38) | 0.505 |
| Patient satisfaction (1–5) | 5.00 (4.00–5.00) | 5.00 (4.00–5.00) | 5.00 (4.00–5.00) | 0.955 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, S.-C.; Hsu, C.-Y.; Lu, H.-F.; Chen, C.-C.; Hou, S.-Y.; Poon, Y.-Y. Earlier Is Better? Timing of Adductor Canal Block for Arthroscopic Knee Surgery under General Anesthesia: A Retrospective Cohort Study. Int. J. Environ. Res. Public Health 2021, 18, 3945. https://doi.org/10.3390/ijerph18083945
Wu S-C, Hsu C-Y, Lu H-F, Chen C-C, Hou S-Y, Poon Y-Y. Earlier Is Better? Timing of Adductor Canal Block for Arthroscopic Knee Surgery under General Anesthesia: A Retrospective Cohort Study. International Journal of Environmental Research and Public Health. 2021; 18(8):3945. https://doi.org/10.3390/ijerph18083945
Chicago/Turabian StyleWu, Shao-Chun, Chih-Yi Hsu, Hsiao-Feng Lu, Chih-Chun Chen, Shao-Yun Hou, and Yan-Yuen Poon. 2021. "Earlier Is Better? Timing of Adductor Canal Block for Arthroscopic Knee Surgery under General Anesthesia: A Retrospective Cohort Study" International Journal of Environmental Research and Public Health 18, no. 8: 3945. https://doi.org/10.3390/ijerph18083945
APA StyleWu, S. -C., Hsu, C. -Y., Lu, H. -F., Chen, C. -C., Hou, S. -Y., & Poon, Y. -Y. (2021). Earlier Is Better? Timing of Adductor Canal Block for Arthroscopic Knee Surgery under General Anesthesia: A Retrospective Cohort Study. International Journal of Environmental Research and Public Health, 18(8), 3945. https://doi.org/10.3390/ijerph18083945







