Toxicological Assessment of Oral Co-Exposure to Bisphenol A (BPA) and Bis(2-ethylhexyl) Phthalate (DEHP) in Juvenile Rats at Environmentally Relevant Dose Levels: Evaluation of the Synergic, Additive or Antagonistic Effects
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
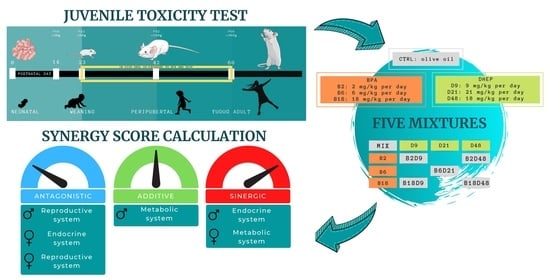
2.2. Definition of the Mixtures
- BPA low (2 mg/kg body weight bw per day) + DEHP low (9 mg/kg bw per day) = Mix B2D9
- BPA middle (6 mg/kg bw per day) + DEHP middle (21 mg/kg bw per day) = Mix B6D21
- BPA high (18 mg/kg bw per day) + DEHP high (48 mg/kg bw per day) = Mix B18D48
- BPA low (2 mg/kg bw per day) + DEHP high (48 mg/kg bw per day) = Mix B2D48
- BPA high (18 mg/kg bw per day) + DEHP low (9 mg/kg bw per day) = Mix B18D9
2.3. Study Design
2.4. Hormone Serum Levels
2.5. Histopathological Analysis
2.6. Gene Expression Analysis
2.7. Statistical Analysis
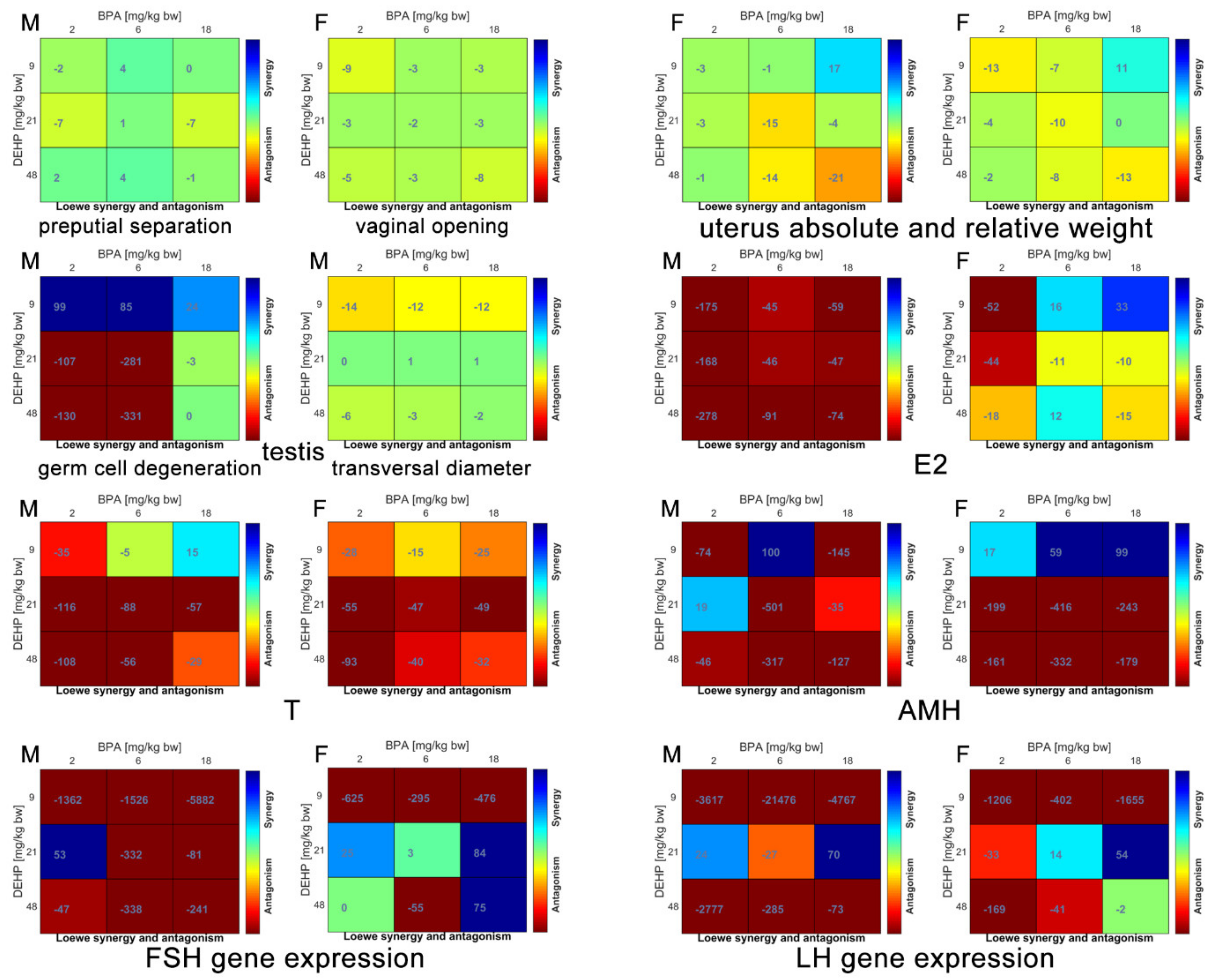
2.8. Synergy Score Calculation
3. Results
3.1. Metabolic System
3.2. Endocrine System
3.3. Reproductive Systems
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Commission Staff Working Document. Progress Report on the Assessment and Management of Combined Exposures to Multiple Chemicals (Chemical Mixtures) and Associated Risks Accompanying the Document. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions Chemicals Strategy for Sustainability towards a Toxic-Free Environment 2020, SWD/2020/250 final. Available online: https://op.europa.eu/en/publication-detail/-/publication/2e7f5564-0f02-11eb-bc07-01aa75ed71a1/language-de (accessed on 20 January 2021).
- Taylor, K.W.; Joubert, B.R.; Braun, J.M.; Dilworth, C.; Gennings, C.; Hauser, R.; Heindel, J.J.; Rider, C.V.; Webster, T.F.; Carlin, D.J. Statistical Approaches for Assessing Health Effects of Environmental Chemical Mixtures in Epidemiology: Lessons from an Innovative Workshop. Environ. Health Perspect. 2016, 124, A227–A229. [Google Scholar] [CrossRef]
- Tsatsakis, A.M.; Docea, A.O.; Tsitsimpikou, C. New Challenges in Risk Assessment of Chemicals when Simulating Real Exposure Scenarios; Simultaneous Multi-Chemicals’ Low Dose Exposure. Food Chem Toxicol. 2016, 96, 174–176. [Google Scholar] [CrossRef]
- European Commission. European Union Risk Assessment. Report Bis (2-Ethylhexyl) Phthalate (DEHP). EUR—Scientific and Technical Research Series; European Commission: Ispra (Varese), Italy, 2008. [Google Scholar]
- Rochester, J.R. Bisphenol A and Human Health: A Review of the Literature. Reprod. Toxicol. 2013, 42, 132–155. [Google Scholar] [CrossRef]
- Rowdhwal, S.S.S.; Chen, J. Toxic Effects of Di-2-Ethylhexyl Phthalate: An Overview. BioMed Res. Int. 2018, 2018, 1750368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathieu-Denoncourt, J.; Wallace, S.J.; de Solla, S.R.; Langlois, V.S. Plasticizer Endocrine Disruption: Highlighting Developmental and Reproductive Effects in Mammals and Non-Mammalian Aquatic Species. Gen. Comp. Endocrinol. 2015, 219, 74–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubin, B.S. Bisphenol A: An Endocrine Disruptor with Widespread Exposure and Multiple Effects. J. Steroid Biochem. Mol. Biol. 2011, 127, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Li, S.S. Epigenetic Effects of Environmental Chemicals Bisphenol A and Phthalates. Int. J. Mol. Sci. 2012, 13, 10143–10153. [Google Scholar] [CrossRef] [PubMed]
- Tassinari, R.; Narciso, L.; Tait, S.; Busani, L.; Martinelli, A.; Virgilio, A.D.; Carli, F.; Deodati, A.; Rocca, C.; Maranghi, F.; et al. Juvenile Toxicity Rodent Model to Study Toxicological Effects of Bisphenol A (BPA) at Dose Levels Derived from Italian Children Biomonitoring Study. Toxicol. Sci. 2020, 173, 387–401. [Google Scholar] [CrossRef]
- Tassinari, R.; Tait, S.; Busani, L.; Martinelli, A.; Narciso, L.; Valeri, M.; Gastaldelli, A.; Deodati, A.; La Rocca, C.; Maranghi, F.; et al. Metabolic, Reproductive and Thyroid Effects of Bis(2-Ethylhexyl) Phthalate (DEHP) Orally Administered to Male and Female Juvenile Rats at Dose Levels Derived from Children Biomonitoring Study. Toxicology 2021, 449, 152653. [Google Scholar] [CrossRef]
- Baralic, K.; Buha Djordjevic, A.; Zivancevic, K.; Antonijevic, E.; Andelkovic, M.; Javorac, D.; Curcic, M.; Bulat, Z.; Antonijevic, B.; Dukic-Cosic, D. Toxic Effects of the Mixture of Phthalates and Bisphenol A-Subacute Oral Toxicity Study in Wistar Rats. Int. J. Environ. Res. Public. Health 2020, 17, 746. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Zhao, Y.; Cheng, C.; Li, L.; Xiao, M.; Zhang, G.; Lu, X. Combined Effects of Di (2-Ethylhexyl) Phthalate and Bisphenol A on Thyroid Hormone Homeostasis in Adolescent Female Rats. Environ. Sci. Pollut. Res. Int. 2020, 27, 40882–40892. [Google Scholar] [CrossRef] [PubMed]
- Tait, S.; Carli, F.; Busani, L.; Buzzigoli, E.; Della Latta, V.; Deodati, A.; Fabbrizi, E.; Gaggini, M.; Maranghi, F.; Tassinari, R.; et al. Biomonitoring of Bis(2-Ethylhexyl)Phthalate (DEHP) in Italian Children and Adolescents: Data from LIFE PERSUADED Project. Environ. Res. 2020, 185, 109428. [Google Scholar] [CrossRef] [PubMed]
- Tait, S.; Carli, F.; Busani, L.; Buzzigoli, E.; Della Latta, V.; Deodati, A.; Fabbrizi, E.; Gaggini, M.; Maranghi, F.; Tassinari, R.; et al. Biomonitoring of Bisphenol A in Italian Children: Reference Values and Daily Intake Evaluation from LIFE PERSUADED Project. 2021; Unpublished work. [Google Scholar]
- European Food Safety Authority (EFSA). Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food (AFC) Related to Bis(2-Ethylhexyl)Phthalate (DEHP) for use in Food Contact Materials. EFSA J. 2005, 3, 243. [Google Scholar] [CrossRef]
- SCENIHR (Scientific Committee on Emerging and Newly-Identified Health Risks). Opinion on the Safety of Medical Devices Containing DEHP-Plasticized PVC or Other Plasticizers on Neonates and Other Groups Possibly at Risk; European Commission: Luxembourg, 2015. [Google Scholar]
- EFSA. Panel on Food Contact Materials, Enzymes, Flavourings and, Processing Aids. Scientific Opinion on the Risks to Public Health Related to the Presence of Bisphenol A (BPA) in Foodstuffs. EFSA J. 2015, 13, 3978. [Google Scholar] [CrossRef]
- La Rocca, C.; Maranghi, F.; Tait, S.; Tassinari, R.; Baldi, F.; Bottaro, G.; Buzzigoli, E.; Carli, F.; Cianfarani, S.; Conte, R.; et al. The LIFE PERSUADED Project Approach on Phthalates and Bisphenol A Biomonitoring in Italian Mother-Child Pairs Linking Exposure and Juvenile Diseases. Environ. Sci. Pollut. Res. Int. 2018, 25, 25618–25625. [Google Scholar] [CrossRef] [Green Version]
- Narciso, L.; Catone, T.; Aquilina, G.; Attias, L.; De Angelis, I.; Iuliano, M.G.; Tassinari, R.; Mantovani, A.; Maranghi, F. The Juvenile Toxicity Study as a Tool for a Science-Based Risk Assessment in the Children Population Group. Reprod Toxicol. 2017, 72, 136–141. [Google Scholar] [CrossRef]
- Maranghi, F.; Tassinari, R.; Mantovani, A. Toxicological Assessment of Drugs that Affect the Endocrine System in Puberty-Related Disorders. Expert Opin. Drug Metab. Toxicol. 2013, 9, 1309–1316. [Google Scholar] [CrossRef]
- EFSA, S.C.; More, S.J.; Bampidis, V.; Benford, D.; Bennekou, S.H.; Bragard, C.; Halldorsson, T.I.; Hernández-Jerez, A.F.; Koutsoumanis, K.; Naegeli, H.; et al. Guidance on Harmonised Methodologies for Human Health, Animal Health and Ecological Risk Assessment of Combined Exposure to Multiple Chemicals. EFSA J. 2019, 17, e05634. [Google Scholar]
- Shackelford, C.; Long, G.; Wolf, J.; Okerberg, C.; Herbert, R. Qualitative and Quantitative Analysis of Nonneoplastic Lesions in Toxicology Studies. Toxicol. Pathol. 2002, 30, 93–96. [Google Scholar] [CrossRef] [Green Version]
- Rasinger, J.D.; Carroll, T.S.; Maranghi, F.; Tassinari, R.; Moracci, G.; Altieri, I.; Mantovani, A.; Lundebye, A.; Hogstrand, C. Low Dose Exposure to HBCD, CB-153 Or TCDD Induces Histopathological and Hormonal Effects and Changes in Brain Protein and Gene Expression in Juvenile Female BALB/C Mice. Reprod. Toxicol. 2018, 80, 105–116. [Google Scholar] [CrossRef] [Green Version]
- Maranghi, F.; De Angelis, S.; Tassinari, R.; Chiarotti, F.; Lorenzetti, S.; Moracci, G.; Marcoccia, D.; Gilardi, E.; Di Virgilio, A.; Eusepi, A.; et al. Reproductive Toxicity and Thyroid Effects in Sprague Dawley Rats Exposed to Low Doses of Ethylenethiourea. Food Chem. Toxicol. 2013, 59, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Maranghi, F.; Tassinari, R.; Lagatta, V.; Moracci, G.; Macrì, C.; Eusepi, A.; Di Virgilio, A.; Scattoni, M.L.; Calamandrei, G. Effects of the Food Contaminant Semicarbazide Following Oral Administration in Juvenile Sprague-Dawley Rats. Food Chem. Toxicol. 2009, 47, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Ianevski, A.; Giri, A.K.; Gautam, P.; Kononov, A.; Potdar, S.; Saarela, J.; Wennerberg, K.; Aittokallio, T. Prediction of Drug Combination Effects with a Minimal Set of Experiments. Nat. Mach. Intell. 2019, 1, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Di Veroli, G.Y.; Fornari, C.; Wang, D.; Mollard, S.; Bramhall, J.L.; Richards, F.M.; Jodrell, D.I. Combenefit: An Interactive Platform for the Analysis and Visualization of Drug Combinations. Bioinformatics 2016, 32, 2866–2868. [Google Scholar] [CrossRef]
- Loewe, S. The Problem of Synergism and Antagonism of Combined Drugs. Arzneimittelforschung 1953, 3, 285–290. [Google Scholar]
- Bliss, C.I. The Calculation of Microbial Assays. Bacteriol. Rev. 1956, 20, 243–258. [Google Scholar] [CrossRef] [PubMed]
- Feron, V.J.; Cassee, F.R.; Groten, J.P. Toxicology of Chemical Mixtures: International Perspective. Environ. Health Perspect. 1998, 106 (Suppl. 6), 1281–1289. [Google Scholar] [CrossRef] [Green Version]
- Robinson, A.G.J.; Kanaan, Y.M.; Copeland, R.L. Combinatorial Cytotoxic Effects of 2,3-Dichloro-5,8-Dimethoxy-1,4-Naphthoquinone and 4-Hydroxytamoxifen in Triple-Negative Breast Cancer Cell Lines. Anticancer Res. 2020, 40, 6623–6635. [Google Scholar] [CrossRef]
- Kim, D.; Yoo, E.R.; Li, A.A.; Cholankeril, G.; Tighe, S.P.; Kim, W.; Harrison, S.A.; Ahmed, A. Elevated Urinary Bisphenol A Levels are Associated with Non-Alcoholic Fatty Liver Disease among Adults in the United States. Liver Int. 2019, 39, 1335–1342. [Google Scholar] [CrossRef]
- Lin, R.; Wu, D.; Wu, F.J.; Meng, Y.; Zhang, J.H.; Wang, X.G.; Jia, L.H. Non-Alcoholic Fatty Liver Disease Induced by Perinatal Exposure to Bisphenol A is Associated with Activated mTOR and TLR4/NF-kappaB Signaling Pathways in Offspring Rats. Front. Endocrinol. (Lausanne) 2019, 10, 620. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, J.C. DEHP: Genotoxicity and Potential Carcinogenic Mechanisms—A Review. Mutat. Res. 2012, 751, 82–157. [Google Scholar] [CrossRef]
- Forny-Germano, L.; De Felice, F.G.; Vieira, M.N.D.N. The Role of Leptin and Adiponectin in Obesity-Associated Cognitive Decline and Alzheimer’s Disease. Front. Neurosci. 2019, 12, 1027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, C.; Gao, L.; Flaws, J.A. Prenatal Exposure to an Environmentally Relevant Phthalate Mixture Disrupts Reproduction in F1 Female Mice. Toxicol. Appl. Pharmacol. 2017, 318, 49–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baralic, K.; Jorgovanovic, D.; Zivancevic, K.; Buha Djordjevic, A.; Antonijevic Miljakovic, E.; Miljkovic, M.; Kotur-Stevuljevic, J.; Antonijevic, B.; Dukic-Cosic, D. Combining in Vivo Pathohistological and Redox Status Analysis with in Silico Toxicogenomic Study to Explore the Phthalates and Bisphenol A Mixture-Induced Testicular Toxicity. Chemosphere 2021, 267, 129296. [Google Scholar] [CrossRef]
- Balci, A.; Ozkemahli, G.; Erkekoglu, P.; Zeybek, N.D.; Yersal, N.; Kocer-Gumusel, B. Histopathologic, Apoptotic and Autophagic, Effects of Prenatal Bisphenol A and/Or Di(2-Ethylhexyl) Phthalate Exposure on Prepubertal Rat Testis. Environ. Sci. Pollut. Res. Int. 2020, 27, 20104–20116. [Google Scholar] [CrossRef]
- Acconcia, F.; Pallottini, V.; Marino, M. Molecular Mechanisms of Action of BPA. Dose Response 2015, 13, 1559325815610582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beg, M.A.; Sheikh, I.A. Endocrine Disruption: Structural Interactions of Androgen Receptor Against Di(2-Ethylhexyl) Phthalate and its Metabolites. Toxics 2020, 8, 115. [Google Scholar] [CrossRef] [PubMed]


| Endpoint | Sex | C | B2D9 | B6D21 | B18D48 | B2D48 | B18D9 |
|---|---|---|---|---|---|---|---|
| Body weight gain (g; M ± SEM) | Male | 296 ± 5 (n = 9) | 311 ± 16 (n = 8) | 331 ± 10 * (n = 8) | 300 ± 11 n = 8) | 304 ± 9 (n = 7) | 292 ± 10 (n = 8) |
| Female | 162 ± 4 (n = 9) | 165 ± 10 (n = 7) | 176 ± 4 (n = 7) | 167 ± 10 (n = 8) | 176 ± 6 (n = 8) | 157 ± 6 (n = 8) | |
| Feed consumption (g; M ± SEM) | Male | 19.66 ± 0.38 (n = 9) | 19.25 ± 0.49 (n = 8) | 21.60 ± 1.14 (n = 8) | 20.42 ± 0.84 (n = 8) | 19.51 ± 0.8 (n = 7) | 18.47 ± 0.82 (n = 8) |
| Female | 14.85 ± 0.85 (n = 9) | 13.03 ± 0.26 * (n = 7) | 15.52 ± 0.32 (n = 7) | 14.66 ± 0.52 (n = 8) | 16.511.18 (n = 8) | 12.57 ± 0.78 * (n = 8) | |
| Liver absolute weight (g; M ± SEM) | Male | 10.72 ± 1.60 (n = 9) | 15.36 ± 1.16 *(n = 8) | 15.98 ± 0.55 **(n = 8) | 16.81 ± 0.81 **(n = 7) | 13.82 ± 0.75 (n = 6) | 15.28 ± 0.88 (n = 7) |
| Female | 7.20 ± 0.83 (n = 9) | 8.49 ± 0.59 (n = 7) | 8.22± 0.26 (n = 7) | 7.40 ± 0.27 (n = 8) | 8.88 ± 0.26 (n = 8) | 8.88 ± 0.68 (n = 8) | |
| Liver relative weight (×100; M ± SEM) | Male | 4.05 ± 0.15 (n = 9) | 4.23 ± 0.36 (n = 8) | 4.41 ± 0.08 (n = 8) | 4.63 ± 0.13 * (n = 7) | 3.69 ± 0.54 (n = 6) | 4.06 ± 0.21 (n = 7) |
| Female | 4.00 ± 0.12 (n = 9) | 3.78 ± 0.15 (n = 7) | 3.62 ± 0.12 (n = 7) | 3.95 ± 0.13 (n = 8) | 3.84 ± 0.10 (n = 8) | 3.53 ± 0.08 * (n = 8) | |
| Liver extramedullary hematopoiesis | Male | 0/9 | 0/8 | 3/8 § | 0/7 | 0/8 | 0/8 |
| Female | 0/9 | 0/7 | 3/7 § | 0/5 | 1/8 | 0/8 | |
| Liver inflammatory cell foci | Male | 5/9 | 7/8 | 5/8 | 7/7 § | 6/8 | 5/8 |
| Female | 3/9 | 7/7 § | 4/7 | 4/5 | 5/8 | 6/8 | |
| Liver hepatocytic vacuolation | Male | 5/9 | 1/8 | 2/8 | 0/7 § | 3/8 | 0/8 § |
| Female | 1/9 | 0/7 | 0/7 | 0/5 | 0/8 | 1/8 | |
| Adiponectin serum levels (ng/mL) | Male | 474 ± 116 (n = 6) | 531 ± 121 (n = 5) | 772 ± 130 (n = 5) | 485 ± 85 (n = 5) | 1714 ± 144 ** (n = 7) | 667 ± 168 (n = 5) |
| Female | 838 ± 173 (n = 5) | 499 ± 166 (n = 6) | 367 ± 92 (n = 5) | 2127 ± 184 ** (n = 8) | 1004 ± 390 * (n = 6) | 838 ± 173 (n = 5) | |
| Leptin serum levels (ng/mL) | Male | 7.65 ± 0.62 (n = 5) | 6.12 ± 1.27 (n = 6) | 6.45 ± 1.12 (n = 6) | 6.50 ± 1.34 * (n = 6) | 0.15 ± 0.02 * (n = 7) | 5.03 ± 0.64 (n = 6) |
| Female | 4.44 ± 0.84 (n = 5) | 3.24 ± 0.53 (n = 6) | 1.90 ± 0.60 (n = 6) | 1.47 ±0.44 ** (n = 6) | 0.14 ± 0.02 ** (n = 7) | 1.86 ± 0.46 * (n = 6) |
| Endpoint | Sex | C | B2D9 | B6D21 | B18D48 | B2D48 | B18D9 |
|---|---|---|---|---|---|---|---|
| Adrenal absolute weight (g; M ± SEM) | Male | 0.07 ± 0.02 (n = 9) | 0.12 ± 0.02 (n = 8) | 0.12 ± 0.07 (n = 8) | 0.11 ± 0.01 (n = 8) | 0.11 ± 0.03 (n = 7) | 0.12 ± 0.01 (n = 8) |
| Female | 0.07 ± 0.01 (n = 9) | 0.09 ± 0.04 (n = 7) | 0.09 ± 0.02 (n = 6) | 0.10 ± 0.01* (n = 8) | 0.12 ± 0.03 ** (n = 8) | 0.09 ± 0.02 (n = 8) | |
| Adrenal relative weight (X100; M ± SEM) | Male | 0.03 ± 0.01 (n = 9) | 0.03 ± 0.01 (n = 8) | 0.03 ± 0.02 (n = 8) | 0.03 ± 0.01 (n = 8) | 0.03 ± 0.01 (n = 7) | 0.04 ± 0.01 (n = 7) |
| Female | 0.04 ± 0.00 (n = 9) | 0.04 ± 0.01 (n = 7) | 0.04 ± 0.01 (n = 6) | 0.05 ± 0.01 (n = 8) | 0.05 ± 0.01 * (n = 8) | 0.04 ± 0.01 (n = 8) | |
| Adrenal cortex area (mm2; M ± SEM) | Male | 4.07 ± 0.97 (n = 5) | 4.60± 0.29 (n = 8) | 5.35 ± 0.43 (n = 8) | 4.98 ± 0.29 (n = 7) | 5.50 ± 0.58 (n = 6) | 4.14 ± 0.17 (n = 6) |
| Female | 3.75 ± 1.08 (n = 5) | 4.21± 0.44 (n = 7) | 4.51 ± 0.46 (n = 6) | 6.28 ± 0.29 * (n = 6) | 5.94 ± 0.57 (n = 6) | 4.93 ± 0.67 (n = 6) | |
| Adrenal medulla area (mm2; M ± SEM) | Male | 0.53 ± 0.14 (n = 5) | 1.02 ± 0.18 (n = 8) | 0.88 ± 0.17 (n = 8) | 0.61 ± 0.07 (n = 7) | 0.73 ± 0.12 (n = 6) | 0.67 ± 0.15 (n = 6) |
| Female | 0.59 ± 0.16 (n = 5) | 0.84 ± 0.12 (n = 7) | 0.54 ± 0.10 (n = 6) | 0.91 ± 0.04 * (n = 6) | 0.81 ± 0.21 (n = 6) | 0.58 ± 0.08 (n = 6) | |
| Thyroid follicular epithelium areas (mm2; M ± SEM) | Male | 435 ± 32 (n = 7) | 397 ± 100 (n = 6) | 504 ± 28 (n = 7) | 305 ± 17 ** (n = 8) | 415 ± 62 (n = 5) | 355 ± 101 (n = 6) |
| Female | 326 ± 19 (n = 7) | 746 ± 237 (n = 6) | 660 ± 235 (n = 7) | 1854 ± 536 (n = 8) | 470± 103 (n = 5) | 285 ± 65 (n = 5) | |
| Thyroid follicular epithelium areas/number of cells (mm2/n; M ± SEM) | Male | 19.10 ± 1.91 (n = 7) | 34.23 ± 11.86 (n = 6) | 22.06 ± 1.46 (n = 7) | 18.16 ± 1.26 (n = 8) | 21.41 ± 1.58 (n = 5) | 21.08 ± 4.61 (n = 6) |
| Female | 17.42 ± 1.51 (n = 7) | 38.36 ± 12.10 (n = 6) | 26.25 ± 6.97 (n = 7) | 47.04 ± 17.44 * (n = 8) | 24.43 ± 2.44 * (n = 5) | 11.53 ± 2.20 (n = 5) |
| Endpoint | Sex | C | B2D9 | B6D21 | B18D48 | B2D48 | B18D9 |
|---|---|---|---|---|---|---|---|
| Timing of preputial separation/vaginal opening (day; M ± SEM) | Male | 41.11 ± 0.48 (n = 9) | 43.13 ± 0.99 (n = 8) | 41.13 ± 0.61 (n = 8) | 41.25 ± 0.31 (n = 8) | 40.50 ± 0.38 (n = 8) | 41.43 ± 0.57 (n = 7) |
| Female | 34.78 ± 0.52 (n = 9) | 36.29 ± 0.89 (n = 7) | 34.86 ± 0.77 (n = 7) | 36.75 ± 0.77 * (n = 8) | 35.88 ± 0.58 (n = 8) | 34.88 ± 0.30 (n = 8) | |
| Uterus absolute weight (g; M ± SEM) | Female | 0.58 ± 0.14 (n = 9) | 0.70 ± 0.24 (n = 7) | 0.63 ± 0.12 (n = 7) | 0.64 ± 0.26 (n = 8) | 0.46 ± 0.15 (n = 8) | 0.58 ± 0.14 (n = 8) |
| Uterus relative weight (X100; M ± SEM) | Female | 0.27 ± 0.06 (n = 9) | 0.30 ± 0.10 (n = 7) | 0.29 ± 0.08 (n = 7) | 0.28 ± 0.10 (n = 8) | 0.22 ± 0.07 (n = 8) | 0.27 ± 0.06 * (n = 8) |
| Estradiol (pg/mL; M ± SEM) | Male | 2.69 ± 0.43 (n = 6) | 5.83 ± 0.46 ** (n = 5) | 2.26 ± 0.29 (n = 5) | 3.51 ± 0.98 (n = 5) | 8.47 ± 1.08 ** (n = 6) | 3.40 ± 0.58 (n = 5) |
| Female | 20.49 ± 3.74 (n = 7) | 28.50 ± 8.05 (n = 5) | 16.96 ± 5.79 (n = 5) | 11.38 ± 3.79 (n = 5) | 14.16 ± 3.05 (n = 6) | 3.75 ± 1.01 * (n = 5) | |
| Testosterone (ng/mL; M ± SEM) | Male | 2.50 ± 0.59 (n = 6) | 3.09 ± 0.82 (n = 6) | 4.61 ± 1.14 (n = 6) | 3.14 ± 0.94 (n = 6) | 4.04 ± 0.48 (n = 7) | 1.84 ± 0.45 (n = 6) |
| Female | 0.92 ± 0.11 (n = 6) | 0.89 ± 0.19 (n = 6) | 1.25 ± 0.17 (n = 6) | 0.96 ± 0.12 (n = 6) | 1.51 ± 0.09 ** (n = 7) | 0.85 ± 0.11 (n = 6) | |
| Anti-Mullerian Hormone (ng/mL; M ± SEM) | Male | 1.92 ± 0.42 (n = 5) | 3.18 ± 0.51 (n = 5) | 7.63 ± 1.74 * (n = 5) | 3.94 ± 0.84 (n = 5) | 2.26 ± 0.21 (n = 7) | 3.90 ± 0.28 * (n = 7) |
| Female | 8.61 ± 1.25 (n = 6) | 9.04 ± 3.92 (n = 5) | 39.71 ± 11.69 ** (n = 6) | 21.45 ± 8.23 (n = 5) | 24.95 ± 4.73 ** (n = 7) | 23.53 ± 20.39 (n = 5) | |
| Testis germ cell degeneration | Male | 1/9 | 0/6 | 6/8§ | 1/6 | 2/7 | 0/7 |
| Testis transversal diameter (mμ; M ± SEM) | Male | 165.21 ± 4.65 (n = 7) | 189.86 ± 4.45 ** (n = 6) | 162.67 ± 3.41 (n = 8) | 164.44 ± 10.25 (n = 8) | 172.39 ± 2.57 (n = 7) | 186.88 ± 6.73 * (n = 6) |
| Follicle- stimulating hormone gene expression (ΔΔct; M ± SEM) | Male | 0 ± 0.30 (n = 5) | 3.38 ± 1.64 (n = 5) | 1.81 ± 0.58 (n = 5) | 1.50 ± 0.45 (n = 5) | −0.35 ± 0.79 (n = 5) | 5.95 ± 0.34 (n = 5) |
| Female | 0 ± 0.30 (n = 5) | 2.62 ± 1.01 (n = 5) | −0.21 ± 0.44 (n = 5) | −3.33 ± 0.97 * (n = 5) | 0.09 ± 0.18 (n = 5) | 0.38 ± 2.81 (n = 5) | |
| Luteinizing hormone gene expression (ΔΔct; M ± SEM) | Male | 0 ± 0.15 (n = 5) | 5.24 ± 0.57 * (n = 5) | 0.26 ± 0.20 (n = 5) | 0.64 ± 0.31 (n = 5) | 4.88 ± 0.54 * (n = 5) | 5.03 ± 0.72 (n = 5) |
| Female | 0 ± 0.28 (n = 5) | 4.26 ± 0.61 * (n = 5) | −0.45 ± 0.27 (n = 5) | −0.36 ± 0.21 (n = 5) | 1.23 ± 0.33 * (n = 5) | 4.04 ± 0.88 * (n = 5) |
| Endpoint | Sex | Synergism | Additive | Antagonism |
|---|---|---|---|---|
| Metabolic system | Male | 33% | 42% | 25% |
| Female | 50% | 30% | 20% | |
| Endocrine system | Male | 100% | ||
| Female | 43% | 57% | ||
| Reproductive system | Male | 100% | ||
| Female | 30% | 10% | 60% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tassinari, R.; Tait, S.; Busani, L.; Martinelli, A.; Valeri, M.; Gastaldelli, A.; Deodati, A.; La Rocca, C.; Maranghi, F.; the LIFE PERSUADED Project Group. Toxicological Assessment of Oral Co-Exposure to Bisphenol A (BPA) and Bis(2-ethylhexyl) Phthalate (DEHP) in Juvenile Rats at Environmentally Relevant Dose Levels: Evaluation of the Synergic, Additive or Antagonistic Effects. Int. J. Environ. Res. Public Health 2021, 18, 4584. https://doi.org/10.3390/ijerph18094584
Tassinari R, Tait S, Busani L, Martinelli A, Valeri M, Gastaldelli A, Deodati A, La Rocca C, Maranghi F, the LIFE PERSUADED Project Group. Toxicological Assessment of Oral Co-Exposure to Bisphenol A (BPA) and Bis(2-ethylhexyl) Phthalate (DEHP) in Juvenile Rats at Environmentally Relevant Dose Levels: Evaluation of the Synergic, Additive or Antagonistic Effects. International Journal of Environmental Research and Public Health. 2021; 18(9):4584. https://doi.org/10.3390/ijerph18094584
Chicago/Turabian StyleTassinari, Roberta, Sabrina Tait, Luca Busani, Andrea Martinelli, Mauro Valeri, Amalia Gastaldelli, Annalisa Deodati, Cinzia La Rocca, Francesca Maranghi, and the LIFE PERSUADED Project Group. 2021. "Toxicological Assessment of Oral Co-Exposure to Bisphenol A (BPA) and Bis(2-ethylhexyl) Phthalate (DEHP) in Juvenile Rats at Environmentally Relevant Dose Levels: Evaluation of the Synergic, Additive or Antagonistic Effects" International Journal of Environmental Research and Public Health 18, no. 9: 4584. https://doi.org/10.3390/ijerph18094584