Characterization of Sleep Disturbances in Children and Adolescents with Down Syndrome and Their Relation with Cognitive and Behavioral Features
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedure
2.3. Measures
2.3.1. Screening for Sleep Disorders
2.3.2. Neuropsychological Evaluation
2.3.3. Evaluation of Adaptive Abilities
2.3.4. Evaluation of Psychopathological Symptoms and Behavioral Problems
2.3.5. Evaluation of Maternal Stress
2.4. Statistical Analysis
3. Results
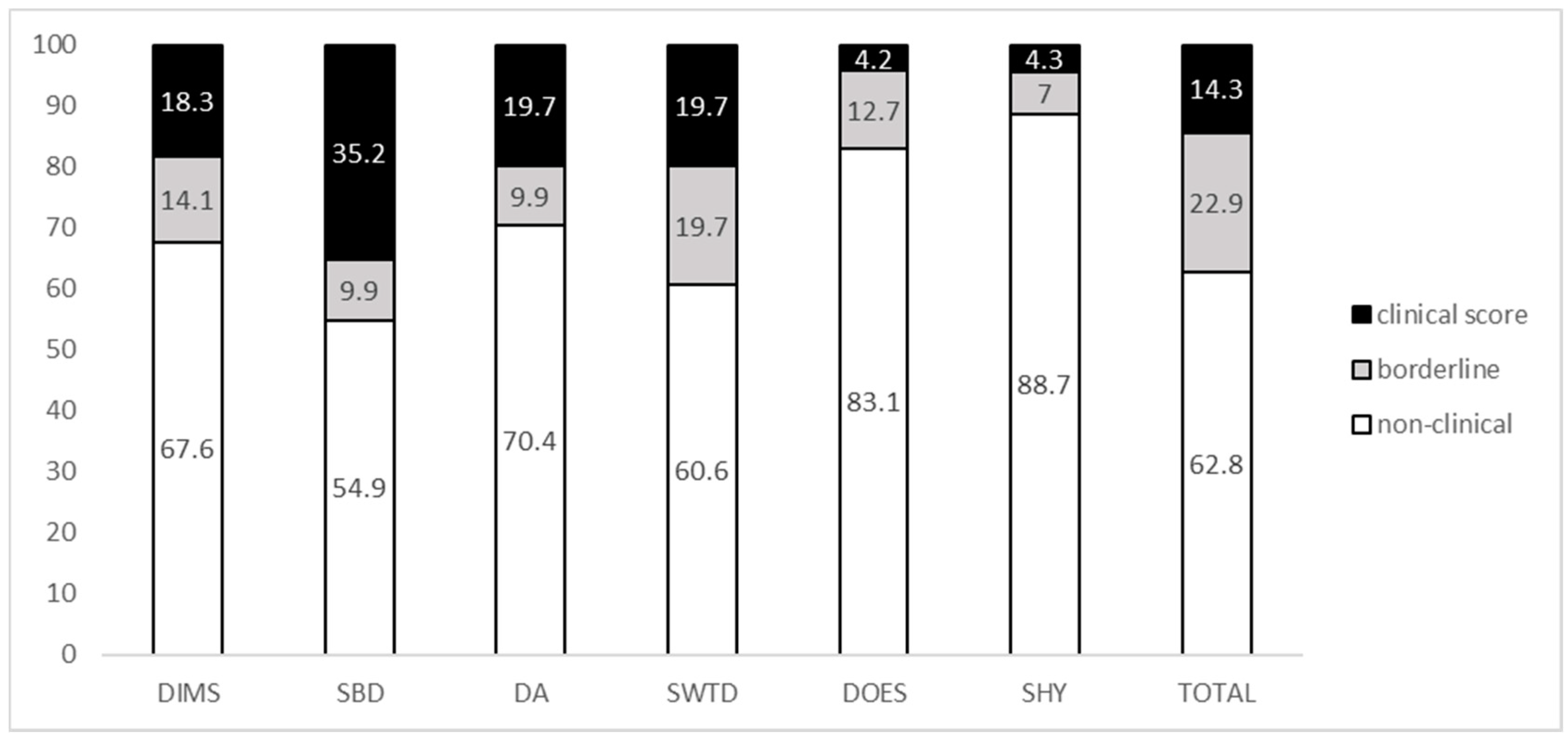
3.1. Distribution of Sleep Disturbances
3.2. Association with Individual Features, Neuropsychological Variables, and Adaptive Profile
3.3. Association with Psychopathological, Behavioral Features, and Maternal Stress
4. Discussion
4.1. Prevalence of Sleep Disturbances and the Role of Individual and Neuropsychological Features
4.2. Association with Psychopathology and Behavioral Problems
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Canfield, M.A.; Honein, M.A.; Yuskiv, N.; Xing, J.; Mai, C.T.; Collins, J.S.; Devine, O.; Petrini, J.; Ramadhani, T.A.; Hobbs, C.A.; et al. National estimates and race/ethnic-specific variation of selected birth defects in the United States, 1999–2001. Birth Defects Res. A Clin. Mol. Teratol. 2006, 76, 747–756. [Google Scholar] [CrossRef]
- Parker, S.E.; Mai, C.T.; Canfield, M.A.; Rickard, R.; Wang, Y.; Meyer, R.E.; Anderson, P.; Mason, C.A.; Collins, J.S.; Kirby, R.S.; et al. National Birth Defects Prevention Network. Updated National Birth Prevalence estimates for selected birth defects in the United States, 2004–2006. Birth Defects Res. A Clin. Mol. Teratol. 2010, 88, 1008–1016. [Google Scholar] [CrossRef] [PubMed]
- Weijerman, M.E.; de Winter, J.P. Clinical practice. The care of children with Down syndrome. Eur. J. Pediatr. 2010, 169, 1445e52. [Google Scholar] [CrossRef]
- Subramanyam, R.; Fleck, R.; McAuliffe, J.; Radhakrishnan, R.; Jung, D.; Patino, M.; Mahmoud, M. Upper airway morphology in Down Syndrome patients under dexmedetomidine sedation. Braz. J. Anesthesiol. 2016, 66, 388. [Google Scholar] [CrossRef]
- Bull, M.J. Down Syndrome. N. Engl. J. Med. 2020, 382, 2344–2352. [Google Scholar] [CrossRef] [PubMed]
- Dyken, M.E.; Lin-Dyken, D.C.; Poulton, S.; Zimmerman, M.B.; Sedars, E. Prospective polysomnographic analysis of obstructive sleep apnea in down syndrome. Arch. Pediatr. Adolesc. Med. 2003, 157, 655–660. [Google Scholar] [CrossRef]
- Bassell, J.L.; Phan, H.; Leu, R.; Kronk, R.; Visootsak, J. Sleep profiles in children with Down syndrome. Am. J. Med. Genet. A 2015, 167, 1830–1835. [Google Scholar] [CrossRef]
- Maris, M.; Verhulst, S.; Wojciechowski, M.; Van de Heyning, P.; Boudewyns, A. Prevalence of obstructive sleep apnea in children with Down syndrome. Sleep 2016, 39, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.F.; Lee, C.H.; Hsueh, W.Y.; Lin, M.T.; Kang, K.T. Prevalence of Obstructive Sleep Apnea in Children with Down Syndrome: A Meta-Analysis. J. Clin. Sleep Med. 2018, 14, 867–875. [Google Scholar] [CrossRef]
- DelRosso, L.M. Epidemiology and Diagnosis of pediatric obstructive sleep apnea. Curr. Probl. Pediatr. Adolesc. Health Care 2016, 46, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Carter, M.; McCaughey, E.; Annaz, D.; Hill, C.M. Sleep problems in a Down syndrome population. Arch. Dis. Childhood 2009, 94, 308–310. [Google Scholar] [CrossRef]
- MacCrosain, A.M.; Byrne, M.C. Are we ignoring the problem of sleep disorder in children with intellectual disabilities? Ir. J. Med. Sci. 2009, 178, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Kelmanson, I.A. Sleep disturbances, behavioural problems and adaptive skills in children with down’s syndrome. Early Child Dev. Care 2017, 187, 1679–1693. [Google Scholar] [CrossRef]
- Ashworth, A.; Hill, C.M.; Karmiloff-Smith, A.; Dimitriou, D. The importance of sleep: Attentional problems in school-aged children with down syndrome and Williams syndrome. Behav. Sleep Med. 2015, 13, 455–471. [Google Scholar] [CrossRef]
- Edgin, J.O.; Tooley, U.; Demara, B.; Nyhuis, C.; Anand, P.; Spano, G. Sleep disturbance and expressive language development in preschool-age children with down syndrome. Child Dev. 2015, 86, 1984–1998. [Google Scholar] [CrossRef] [PubMed]
- Nisbet, L.C.; Phillips, N.N.; Hoban, T.F.; O’Brien, L.M. Characterization of a sleep architectural phenotype in children with Down syndrome. Sleep Breath Schlaf Atmung 2015, 19, 1065–1071. [Google Scholar] [CrossRef]
- Joyce, A.; Dimitriou, D. Sleep-disordered breathing and cognitive functioning in preschool children with and without Down syndrome. J. Intellect Disabil. Res. 2017, 61, 778–791. [Google Scholar] [CrossRef]
- Horne, R.S.; Wijayaratne, P.; Nixon, G.M.; Walter, L.M. Sleep and sleep disordered breathing in children with down syndrome: Effects on behaviour, neurocognition and the cardiovascular system. Sleep Med. Rev. 2019, 44, 1–11. [Google Scholar] [CrossRef]
- Fitzgerald, D.A.; Paul, A.; Richmond, C. Severity of obstructive apnoea in children with Down syndrome who snore. Arch. Dis. Child. 2007, 92, 423–425. [Google Scholar] [CrossRef]
- Shires, C.B.; Anold, S.L.; Schoumacher, R.A.; Dehoff, G.W.; Donepudi, S.K.; Stocks, R.M. Body mass index as an indicator of obstructive sleep apnea in pediatric Down syndrome. Int. J. Pediatr. Otorhinolaryngol. 2010, 74, 768–772. [Google Scholar] [CrossRef] [PubMed]
- Breslin, J.; Spano, G.; Bootzin, R.; Anand, P.; Nadel, L.; Edgin, J. Obstructive sleep apnea syndrome and cognition in Down syndrome. Dev. Med. Child Neurol. 2014, 56, 657–664. [Google Scholar] [CrossRef]
- Short, M.A.; Blunden, S.; Rigney, G.; Matricciani, L.; Coussens, S.; MReynolds, C.; Galland, B. Cognition and objectively measured sleep duration in children: A systematic review and meta-analysis. Sleep Health 2018, 4, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Spano, G.; Edgin, J.O. The impact of sleep disruption on executive function in Down syndrome. Res. Dev. Disabil. 2013, 34, 2033–2039. [Google Scholar] [CrossRef]
- Arias-Trejo, N.; Angulo-Chavira, A.Q.; Demara, B.; Figueroa, C.; Edgin, J. The influence of sleep on language production modalities in preschool children with Down syndrome. J. Sleep Res. 2020, e13120. [Google Scholar] [CrossRef]
- Esbensen, A.J.; Hoffman, E.K. Impact of sleep on executive functioning in school-age children with Down syndrome. J. Intellect. Disabil. Res. 2018, 62, 569–580. [Google Scholar] [CrossRef] [PubMed]
- Stores, R.; Stores, G.; Fellows, B.; Buckley, S. A factor analysis of sleep problems and their psychological associations in children with Down’s syndrome. J. Appl. Res. Intellect. Disabil. 1998, 17, 345–354. [Google Scholar] [CrossRef]
- Ivanenko, A.; Johnson, K. Sleep disturbances in children with psychiatric disorders. Semin. Pediatr. Neurol. 2008, 15, 70–78. [Google Scholar] [CrossRef] [PubMed]
- McConnell, E.J.; Hill, E.A.; Celmiņa, M.; Kotoulas, S.C.; Riha, R.L. Behavioural and emotional disturbances associated with sleep-disordered breathing symptomatology in children with Down’s syndrome. J. Intellect. Disabil. Res. 2020. [Google Scholar] [CrossRef]
- Fallone, G.; Owens, J.A.; Deane, J. Sleepiness in children and adolescents: Clinical implications. Sleep Med. Rev. 2002, 6, 287–306. [Google Scholar] [CrossRef]
- Vermeulen, M.C.M.; Van der Heijden, K.B.; Swaab, H.; Van Someren, E.J.W. Sleep spindle characteristics and sleep architecture are associated with learning of executive functions in school-age children. J. Sleep Res. 2019, 28, e12779. [Google Scholar] [CrossRef]
- Churchill, S.S.; Kieckhefer, G.M.; Bjornson, K.F.; Herting, J.R. Relationship between sleep disturbance and functional outcomes in daily life habits of children with Down syndrome. Sleep 2015, 38, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Lukowski, A.F.; Milojevich, H.M. Sleep problems and temperament in young children with Down syndrome and typically developing controls. J. Intellect. Disabil. Res. 2017, 61, 221–232. [Google Scholar] [CrossRef]
- Esbensen, A.J.; Hoffman, E.K.; Beebe, D.W.; Byars, K.C.; Epstein, J. Links between sleep and daytime behaviour problems in children with Down syndrome. J. Intellect. Disabil. Res. 2018, 62, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.K.; Jung, E.; Van Riper, M.; Lee, Y.J. Sleep problems in Korean children with Down syndrome and parental quality of life. J. Intellect. Disabil. Res. 2019, 63, 1346–1358. [Google Scholar] [CrossRef] [PubMed]
- Chawla, J.K.; Burgess, S.; Heussler, H. The impact of sleep problems on functional and cognitive outcomes in children with Down syndrome: A review of the literature. J. Clin. Sleep Med. 2020. [Google Scholar] [CrossRef]
- Bruni, O.; Ottaviano, S.; Guidetti, V.; Romoli, M.; Innocenzi, M.; Cortesi, F.; Giannotti, F. The Sleep Disturbance Scale for Children (SDSC). Construction and validation of an instrument to evaluate sleep disturbances in childhood and adolescence. J. Sleep Res. 1996, 5, 251–261. [Google Scholar] [CrossRef]
- Cohen, R.; Halevy, A.; Shuper, A. Children’s sleep disturbance scale in differentiating neurological disorders. Pediatr. Neurol. 2013, 49, 465–468. [Google Scholar] [CrossRef]
- Romeo, D.M.; Leo, G.; Lapenta, L.; Leone, D.; Turrini, I.; Brogna, C.; Gallini, F.; Cota, F.; Vento, G.; Mercuri, E. Sleep disorders in low-risk preschool very preterm children. Sleep Med. 2019, 63, 137–141. [Google Scholar] [CrossRef]
- Roid, G.H.; Miller, L.J.; Pomplun, M.; Koch, C. Leiter International Performance Scale, 3rd ed.; Western Psychological Services: Los Angeles, CA, USA, 2013. [Google Scholar]
- Green, E.; Stroud, L.; Bloomfield, S.; Cronje, J.; Foxcroft, C.; Hunter, K.; Lane, H.; Marais, R.; McAlinden, P.; O’Connell, R.; et al. Griffith III: Griffiths Scales of Child Development, 3rd ed.; Association for Research in Infant and Child Development (ARCID): Oxford, UK, 2016. [Google Scholar]
- Beery, K.E.; Beery, N.A. The Beery-Buktenica Developmental Test of Visual Motor Integration: Administration, Scoring, and Teaching Manual, 5th ed.; Modern Curriculum Press: Cleveland, OH, USA, 2004. [Google Scholar]
- Harrison, P.L.; Oakland, T. Adaptive Behavior Assessment System®, 2nd ed.; ABAS®-II; Harcourt: San Antonio, CA, USA, 2003. [Google Scholar]
- Achenbach, T.M.; Rescorla, L.A. Manual for the ASEBA Preschool Forms & Profiles; University of Vermont: Burlington, VT, USA, 2000. [Google Scholar]
- Achenbach, T.M.; Rescorla, L.A. Manual for ASEBA School-Age Forms and Profiles; University of Vermont: Burlington, VT, USA, 2001. [Google Scholar]
- Conners C., K. Conners’ Rating Scales—Revised: User’s Manual. Multi-Health Systems, Incorporated, Italian edition; Nobile, M., Alberti, B., Zuddas, A., Eds.; Giunti Organizzazioni Speciali: Firenze, Italy, 1997. [Google Scholar]
- Aman, M.G.; Singh, N.N.; Stewart, A.W.; Field, C.J. The aberrant behavior checklist: A behavior rating scale for the assessment of treatment effects. Am. J. Ment. Defic. 1985, 89, 485–491. [Google Scholar] [CrossRef]
- Capone, G.T.; Grados, M.A.; Kaufmann, W.E.; Bernad-Ripoll, S.; Jewell, A. Down syndrome and comorbid autism-spectrum disorder: Characterization using the aberrant behavior checklist. Am. J. Med. Genet. A 2005, 134, 373–380. [Google Scholar] [CrossRef]
- Sansone, S.M.; Widaman, K.F.; Hall, S.S.; Reiss, A.L.; Lightbody, A.; Kaufmann, W.E.; Berry-Kravis, E.; Lachiewicz, A.; Brown, E.C.; Hessl, D. Psychometric study of the Aberrant Behavior Checklist in Fragile X Syndrome and implications for targeted treatment. J. Autism Dev. Disord. 2012, 42, 1377–1392. [Google Scholar] [CrossRef] [PubMed]
- Bodfish, J.W.; Symons, F.J.; Parker, D.E.; Lewis, M.H. Varieties of repetitive behavior in autism: Comparisons to mental retardation. J. Autism Dev. Disord. 2000, 30, 237–243. [Google Scholar] [CrossRef]
- Abidin, R.R. Parenting Stress Index; PAR: San Francisco, CA, USA, 2012. [Google Scholar]
- Grieco, J.; Pulsifer, M.; Seligsohn, K.; Skotko, B.; Schwartz, A. Down syndrome: Cognitive and behavioral functioning across the lifespan. Am. J. Med. Genet. C Semin. Med. Genet. 2015, 169, 135–149. [Google Scholar] [CrossRef]
- Esbensen, A.J.; Hoffman, E.K. Reliability of parent report measures of sleep in children with Down syndrome. J. Intellect. Disabil. Res. 2017, 61, 210–220. [Google Scholar] [CrossRef]
- Bloomfield, E.R.; Shatkin, J.P. Parasomnias and movement disorders in children and adolescents. Child Adolesc. Psychiatr. Clin. N. Am. 2009, 18, 947–965. [Google Scholar] [CrossRef] [PubMed]
- Calhoun, S.L.; Vgontzas, A.N.; Fernandez-Mendoza, J.; Mayes, S.D.; Tsaoussoglou, M.; Basta, M.; Bixler, E.O. Prevalence and risk factors of excessive daytime sleepiness in a community sample of young children: The role of obesity, asthma, anxiety/depression, and sleep. Sleep 2011, 34, 503–507. [Google Scholar] [CrossRef]
- Fernandez-Mendoza, J.; Vgontzas, A.N.; Kritikou, I.; Calhoun, S.L.; Liao, D.; Bixler, E.O. Natural history of excessive daytime sleepiness: Role of obesity, weight loss, depression, and sleep propensity. Sleep 2015, 38, 351–360. [Google Scholar] [CrossRef]
- Stickgold, R. Parsing the role of sleep in memory processing. Curr. Opin. Neurobiol. 2013, 23, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Ma, L.; Yang, G.; Gan, W.B. REM sleep selectively prunes and maintains new synapses in development and learning. Nat. Neurosci. 2017, 20, 427–437. [Google Scholar] [CrossRef]
- Spano, M.; Mercuri, E.; Rando, T.; Panto, T.; Gagliano, A.; Henderson, S.; Guqqetta, F. Motor and perception-motor competence in children with Down syndrome: Variation in performance with age. Eur. J. Paediatr. Neurol. 1999, 3, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Perfect, M.M.; Archbold, K.; Goodwin, J.L.; Levine-Donnerstein, D.; Quan, S.F. Risk of behavioral and adaptive functioning difficulties in youth with previous and current sleep disordered breathing. Sleep 2013, 36, 517–525B. [Google Scholar] [CrossRef]
- Taylor, M.A.; Schreck, K.A.; Mulick, J.A. Sleep disruption as a correlate to cognitive and adaptive behavior problems in autism spectrum disorders. Res. Dev. Disabil. 2012, 33, 1408–1417. [Google Scholar] [CrossRef]
- Nixon, G.M.; Biggs, S.N.; Jitpiriyaroj, S.; Horne, R.S. The Relationship between Sleep-Disordered Breathing Severity and Daytime Adaptive Functioning in Children with Down Syndrome. CNS Neurosci. Ther. 2016, 22, 936–937. [Google Scholar] [CrossRef] [PubMed]
- Brooks, L.J.; Olsen, M.N.; Bacevice, A.M.; Beebe, A.; Konstantinopoulou, S.; Taylor, H.G. Relationship between sleep, sleep apnea, and neuropsychological function in children with Down syndrome. Sleep Breath 2015, 19, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Corkum, P.; Tannock, R.; Moldofsky, H. Sleep disturbances in children with attention-deficit/hyperactivity disorder. J. Am. Acad. Child Adolesc. Psychiatry 1998, 37, 637e46. [Google Scholar] [CrossRef]
- Hodgkins, P.; Setyawan, J.; Mitra, D.; Davis, K.; Quintero, J.; Fridman, M.; Shaw, M.; Harpin, V. Management of ADHD in children across Europe: Patient demographics, physician characteristics and treatment patterns. Eur. J. Pediatr. 2013, 172, 895–906. [Google Scholar] [CrossRef] [PubMed]
- Owens, J.A. The ADHD and sleep conundrum: A review. J. Dev. Behav. Pediatr. 2005, 26, 312–322. [Google Scholar] [CrossRef]
- Sung, V.; Hiscock, H.; Sciberras, E.; Efron, D. Sleep problems in children with attention-deficit/hyperactivity disorder: Prevalence and the effect on the child and family. Arch. Pediatr. Adolesc. Med. 2008, 162, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Cortese, S.; Faraone, S.V.; Konofal, E.; Lecendreux, M. Sleep in children with attention-deficit/hyperactivity disorder: Meta-analysis of subjective and objective studies. J. Am. Acad. Child Adolesc. Psychiatry 2009, 48, 894e908. [Google Scholar] [CrossRef]
- Kalil Neto, F.; Nunes, M.L. Evaluation of sleep organization in patients with attention deficit hyperactivity disorder (ADHD) and ADHD as a comorbidity of epilepsy. Sleep Med. 2017, 33, 91–96. [Google Scholar] [CrossRef]
- Mayes, S.D.; Calhoun, S.L. Variables related to sleep problems in children with autism. Res. Autism Spectr. Disord. 2009, 3, 931–941. [Google Scholar] [CrossRef]
- Stormark, K.M.; Fosse, H.E.; Pallesen, S.; Hysing, M. The association between sleep problems and academic performance in primary school-aged children: Findings from a Norwegian longitudinal population-based study. PLoS ONE 2019, 14, e0224139. [Google Scholar] [CrossRef] [PubMed]
- Um, Y.H.; Hong, S.C.; Jeong, J.H. Sleep Problems as Predictors in Attention-Deficit Hyperactivity Disorder: Causal Mechanisms, Consequences and Treatment. Clin. Psychopharmacol. Neurosci. 2017, 15, 9–18. [Google Scholar] [CrossRef]
- Owens, J.A.; Spirito, A.; McGuinn, M. The Children’s Sleep Habits Questionnaire (CSHQ): Psychometric properties of a survey instrument for school-aged children. Sleep 2000, 23, 1043–1051. [Google Scholar] [CrossRef]
- Dekker, M.C.; Koot, H.M. DSM-IV disorders in children with borderline to moderate intellectual disability. I: Prevalence and impact. J. Am. Acad. Child Adolesc. Psychiatry 2003, 42, 915–922. [Google Scholar] [CrossRef]
- Ekstein, S.; Glick, B.; Weill, M.; Kay, B.; Berger, I. Down syndrome and Attention-Deficit/Hyperactivity Disorder. J. Child Neurol. 2011, 26, 1290–1295. [Google Scholar] [CrossRef] [PubMed]
- Froehlich, T.E.; Lanphear, B.P.; Epstein, J.N.; Barbaresi, W.J.; Katusic, S.K.; Kahn, R.S. Prevalence, recognition, and treatment of attention-deficit/hyperactivity disorder in a national sample of US children. Arch. Pediatr. Adolesc. Med. 2007, 161, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Spanò, G.; Gómez, R.L.; Demara, B.I.; Alt, M.; Cowen, S.L.; Edgin, J.O. REM sleep in naps differentially relates to memory consolidation in typical preschoolers and children with Down syndrome. Proc. Natl. Acad. Sci. USA 2018, 115, 11844–11849. [Google Scholar] [CrossRef]
- Thorsteinsson, E.B.; Brown, R.F.; Owens, M.T. Modeling the Effects of Stress, Anxiety, and Depression on Rumination, Sleep, and Fatigue in a Nonclinical Sample. J. Nerv. Ment. Dis. 2019, 207, 355–359. [Google Scholar] [CrossRef] [PubMed]

| DIMS r (p) | SBD r (p) | DA r (p) | SWTD r (p) | DOES r (p) | SHY r (p) | TOTAL r (p) | |
|---|---|---|---|---|---|---|---|
| Anx./Dep. | 0.182 (0.137) | 0.060 (0.629) | 0.109 (0.375) | 0.052 (0.675) | 0.420 ** (0.000) | −0.059 (0.634) | 0.199 (0.105) |
| Som. Compl. | 0.246 * (0.043) | 0.201 (0.086) | 0.330 * (0.006) | 0.336 * (0.005) | 0.364 * (0.002) | −0.001 (0.991) | 0.376 * (0.002) |
| Attention | 0.486 ** (0.000) | 0.183 (0.135) | 0.124 (0.080) | 0.384 ** (0.001) | 0.207 (0.090) | 0.166 (0.177) | 0.467 ** (0.000) |
| Aggress. | 0.186 (0.129) | 0.037 (0.767) | −0.054 (0.661) | 0.163 (0.183) | 0.074 (0.546) | 0.137 (0.267) | 0.191 (0.118) |
| Intern. | 0.230 (0.060) | 0.160 (0.193) | 0.159 (0.195) | 0.107 (0.384) | 0.351 * (0.003) | 0.011 (0.929) | 0.291 * (0.016) |
| Extern. | 0.346 * (0.004) | 0.159 (0.195) | 0.119 (0.335) | 0.309 * (0.010) | 0.334 * (0.005) | 0.084 (0.493) | 0.368 * (0.002) |
| Total Problems | 0.373 * (0.002) | 0.163 (0.184) | 0.180 (0.143) | 0.247 * (0.043) | 0.403 ** (0.001) | 0.040 (0.749) | 0.380 ** (0.001) |
| DIMS | SBD | DA | SWTD | DOES | SHY | TOTAL | |
|---|---|---|---|---|---|---|---|
| r (p) | r (p) | r (p) | r (p) | r (p) | r (p) | r (p) | |
| Oppositional | 0.539 ** | 0.085 | 0.261 | 0.376 * | 0.424 * | 0.265 | 0.527 ** |
| 0 | −0.548 | (0–061) | −0.006 | −0.002 | −0.057 | 0 | |
| Cognitive problems/inattention | 0.519 ** | 0.096 | 0.279 * | 0.320 * | 0.309 * | 0.122 | 0.460 * |
| 0 | −0.5 | −0.045 | −0.021 | −0.026 | −0.388 | −0.001 | |
| Hyperactive | 0.476 ** | 0.02 | 0.264 | 0.400 * | 0.281 * | 0.266 | 0.427 * |
| 0 | −0.886 | −0.058 | −0.003 | −0.044 | −0.057 | −0.002 | |
| Anxious | 0.499 ** | −0.068 | 0.251 | 0.141 | 0.448 * | −0.072 | 0.389 * |
| 0 | −0.633 | −0.072 | −0.32 | −0.001 | −0.61 | −0.004 | |
| Perfectionism | 0.409 * | 0.053 | 0.319 * | 0.161 | 0.329 * | 0.17 | 0.380 * |
| −0.003 | −0.708 | −0.021 | −0.255 | −0.017 | −0.228 | −0.005 | |
| Social problems | 0.492 ** | −0.104 | 0.149 | 0.177 | 0.263 | 0.1 | 0.371 * |
| 0 | −0.464 | −0.291 | −0.408 | −0.06 | −0.48 | −0.007 | |
| Psychosomatic | 0.231 | 0.167 | 0.323 * | 0.117 | 0.27 | −0.068 | 0.291 * |
| 0.1 | −0.236 | −0.019 | −0.408 | −0.053 | −0.633 | −0.036 | |
| ADHD index | 0.497 ** | 0.096 | 0.278 * | 0.370 * | 0.306 * | 0.157 | 0.453 * |
| 0 | −0.498 | −0.046 | −0.007 | −0.027 | −0.267 | −0.001 | |
| Global index- Restless-impulsive | 0.572 ** | 0.049 | 0.301 * | 0.474 ** | 0.329 * | 0.181 | 0.497 ** |
| 0 | −0.73 | −0.03 | 0 | −0.017 | −0.199 | 0 | |
| Emotional lability | 0.532 ** | 0.214 | 0.256 | 0.421 ** | 0.391 * | 0.348 * | 0.557 ** |
| 0 | −0.128 | −0.066 | 0 | −0.004 | −0.012 | 0 | |
| CPRS | 0.588 ** | 0.104 | 0.323 * | 0.477 ** | 0.356 * | 0.249 | 0.542 ** |
| Global index | 0 | −0.462 | −0.019 | 0 | −0.01 | −0.075 | 0 |
| DSM-IV Inattentive | 0.489 ** | −0.041 | 0.227 | 0.305 * | 0.295 * | 0.195 | 0.421 * |
| 0 | −0.772 | −0.106 | −0.028 | −0.034 | −0.166 | −0.002 | |
| DSM-IV Hyperactive/impulsive | 0.526 ** | 0.066 | 0.343 * | 0.402 * | 0.451 * | 0.196 | 0.492 ** |
| 0 | −0.641 | −0.013 | −0.003 | −0.001 | −0.163 | 0 | |
| DSM-IV Total | 0.551 ** | 0.014 | 0.299 * | 0.375 * | 0.375 * | 0.189 | 0.482 ** |
| 0 | −0.919 | −0.031 | −0.006 | −0.006 | −0.178 | 0 |
| DIMS | SBD | DA | SWTD | DOES | SHY | TOTAL | |
|---|---|---|---|---|---|---|---|
| r (p) | r (p) | r (p) | r (p) | r (p) | r (p) | r (p) | |
| Irritability/Agitation/Crying | 0.468 ** | 0 | 0.163 | 0.184 | 0.262 | 0.173 | 0.342 * |
| 0 | −0.998 | −0.238 | −0.182 | −0.055 | −0.21 | −0.011 | |
| Lethargy/ | 0.234 | −0.070 | 0.304 * | 0.018 | 0.121 | −0.078 | 0.127 |
| Social Withdrawal | −0.088 | −0.617 | −0.026 | −0.9 | −0.383 | −0.574 | −0.36 |
| Stereotypic | 0.393 * | −0.057 | 0.362 * | 0.167 | 0.241 | 0.05 | 0.332 * |
| Behavior | −0.003 | −0.681 | −0.007 | −0.227 | −0.079 | −0.72 | −0.014 |
| Hyperactivity/ | 0.428 ** | 0.021 | 0.076 | 0.193 | 0.2 | 0.074 | 0.269 * |
| Noncompliance | −0.001 | −0.88 | −0.585 | −0.162 | −0.147 | −0.594 | −0.049 |
| Inappropriate Speech | 0.288 * | 0.026 | 0.053 | 0.027 | 0.048 | −0.129 | 0.129 |
| −0.035 | −0.851 | −0.701 | −0.846 | −0.732 | −0.352 | −0.353 | |
| ABC Total Score | 0.478 ** | −0.027 | 0.246 | 0.165 | 0.244 | 0.042 | 0.317 * |
| 0 | −0.847 | −0.073 | −0.233 | −0.075 | −0.761 | −0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fucà, E.; Costanzo, F.; Celestini, L.; Mandarino, A.; Vicari, S. Characterization of Sleep Disturbances in Children and Adolescents with Down Syndrome and Their Relation with Cognitive and Behavioral Features. Int. J. Environ. Res. Public Health 2021, 18, 5001. https://doi.org/10.3390/ijerph18095001
Fucà E, Costanzo F, Celestini L, Mandarino A, Vicari S. Characterization of Sleep Disturbances in Children and Adolescents with Down Syndrome and Their Relation with Cognitive and Behavioral Features. International Journal of Environmental Research and Public Health. 2021; 18(9):5001. https://doi.org/10.3390/ijerph18095001
Chicago/Turabian StyleFucà, Elisa, Floriana Costanzo, Laura Celestini, Alessandra Mandarino, and Stefano Vicari. 2021. "Characterization of Sleep Disturbances in Children and Adolescents with Down Syndrome and Their Relation with Cognitive and Behavioral Features" International Journal of Environmental Research and Public Health 18, no. 9: 5001. https://doi.org/10.3390/ijerph18095001
APA StyleFucà, E., Costanzo, F., Celestini, L., Mandarino, A., & Vicari, S. (2021). Characterization of Sleep Disturbances in Children and Adolescents with Down Syndrome and Their Relation with Cognitive and Behavioral Features. International Journal of Environmental Research and Public Health, 18(9), 5001. https://doi.org/10.3390/ijerph18095001







