Effects of Nutritional Interventions on Accuracy and Reaction Time with Relevance to Mental Fatigue in Sporting, Military, and Aerospace Populations: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Information Sources and Search Strategy
2.2. Eligibility Criteria
2.3. Participants
2.4. Interventions
2.5. Comparators
Main Outcome(s)
2.6. Study Design
2.7. Study Selection and Data Extraction
2.8. Study Quality Assessment
2.9. Meta-Analysis
2.10. Data Synthesis
2.11. Analysis of Subgroups
3. Results
3.1. Included Studies
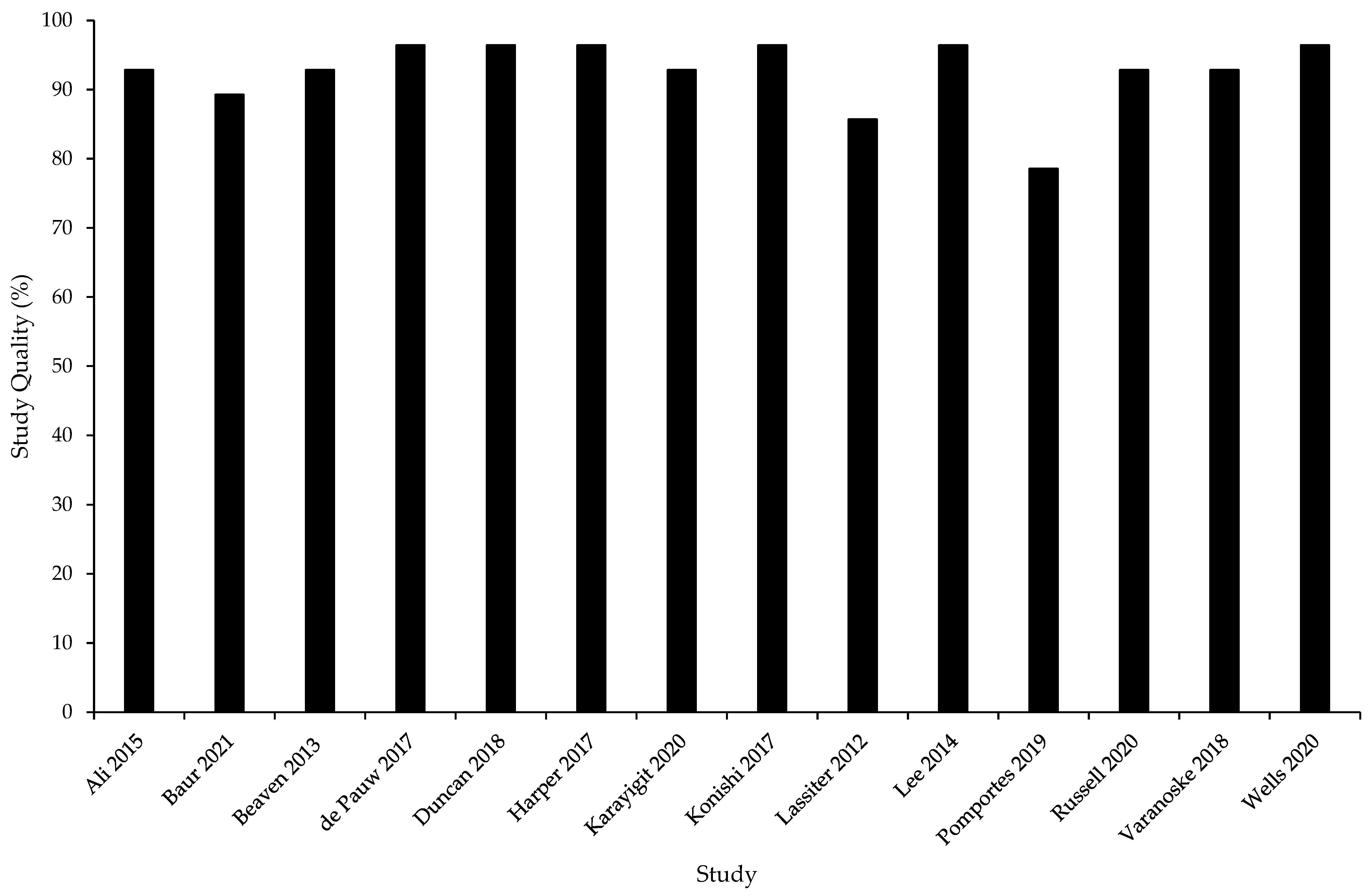
3.2. Study Quality
3.3. Meta-Analysis
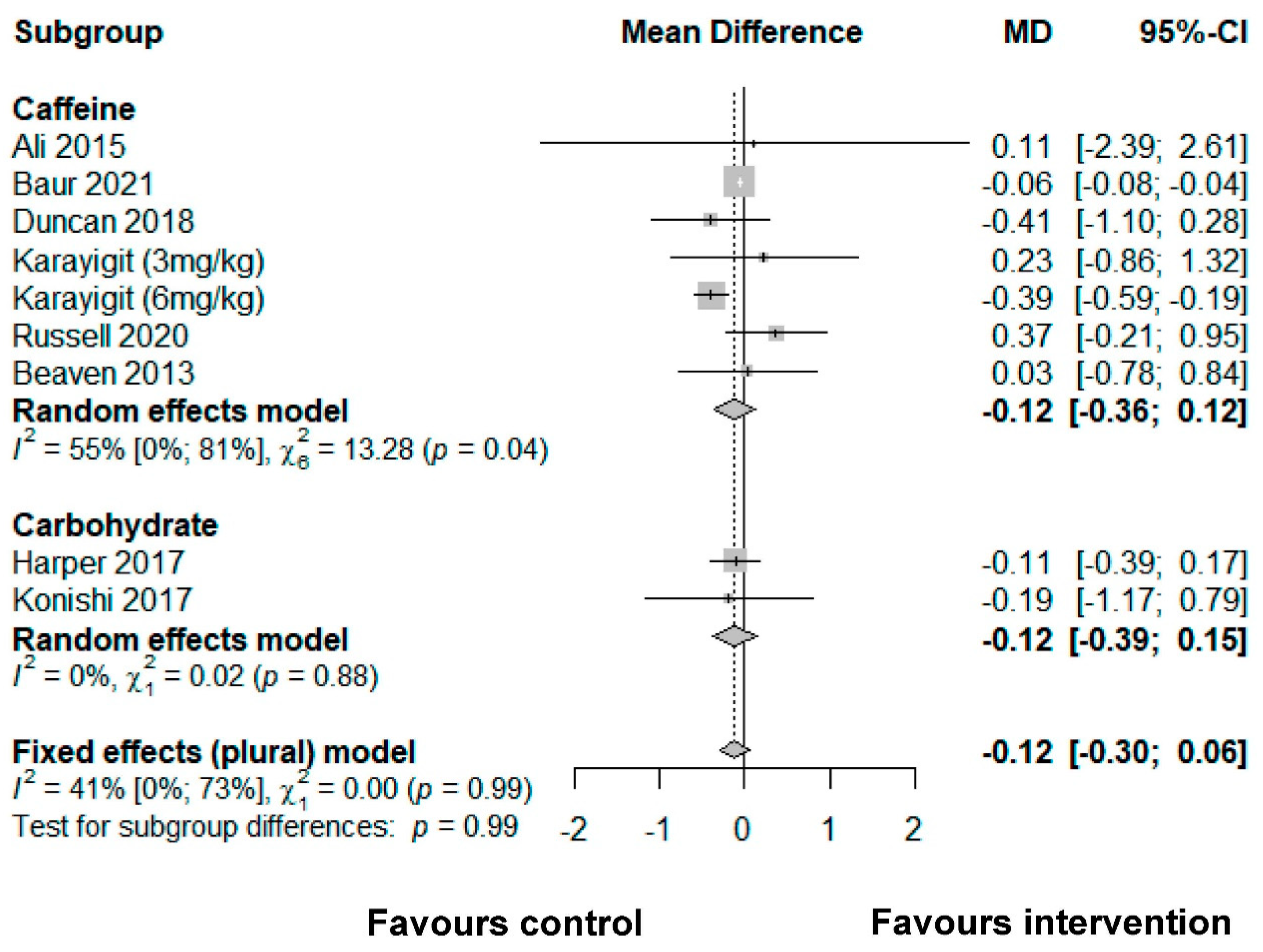
3.4. Accuracy
3.4.1. Caffeine
3.4.2. Carbohydrate
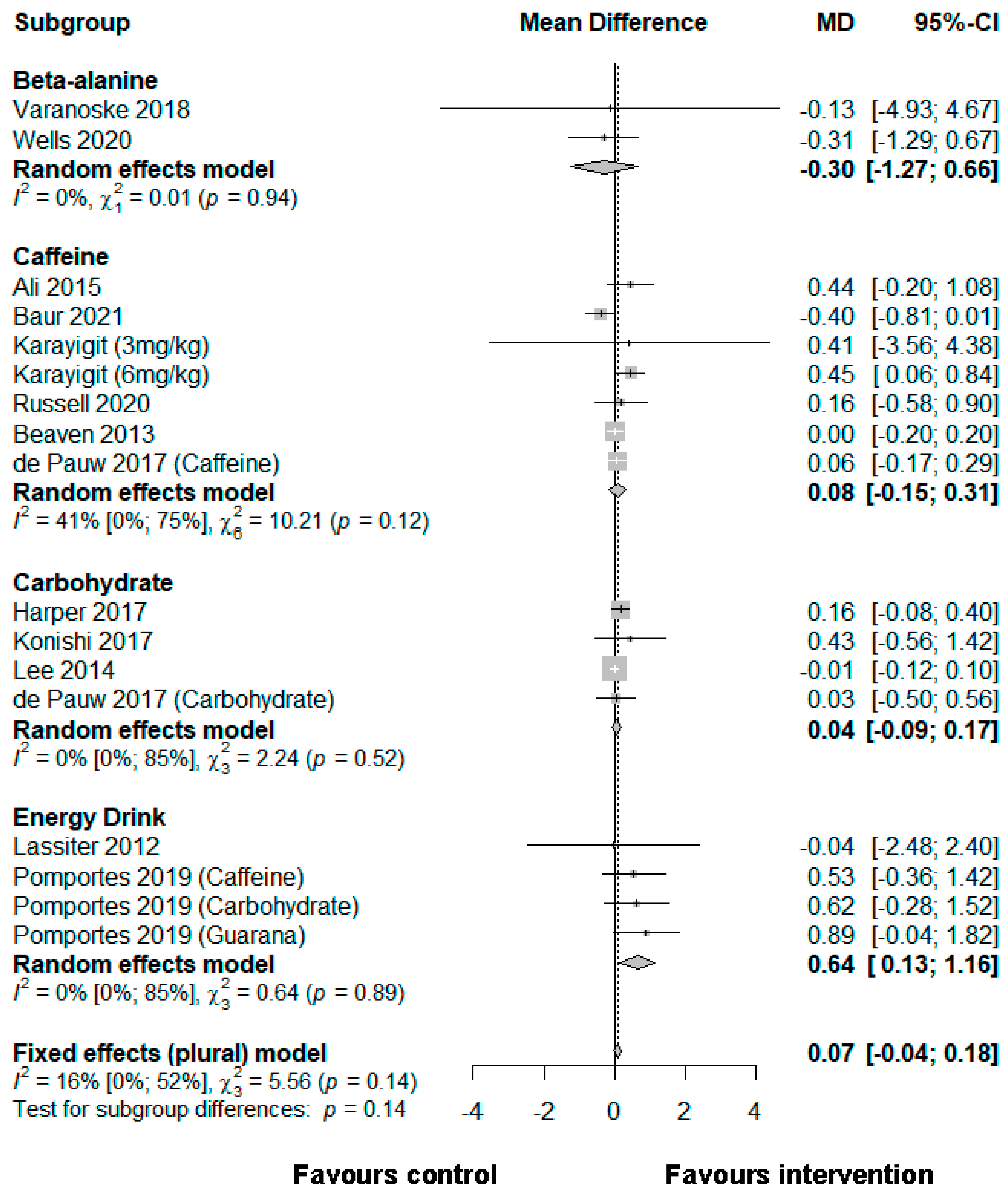
3.5. Reaction Time
3.5.1. Beta-Alanine
3.5.2. Caffeine
3.5.3. Carbohydrate
3.5.4. Energy Drinks
| Subgroup | Study | Methodology | Sample Demographics | Nutritional Intervention | Placebo/Control Comparison | Relevant Outcome/s | Cognitive Domain | Effects Size/s (95% CI) |
|---|---|---|---|---|---|---|---|---|
| Beta-alanine | Wells et al. [61] | Parallel groups | PLA: 10 SUP: 9 | 12 g sustained release beta-alanine × 14 days | 12 g rice powder × 14 days | ANAM: SRT Code substitution Mathematical processing Code substitution—delayed | RT | 0.31 (−1.29; 0.67) |
| (Varanoske et al. [62] | Parallel groups | PLA: 10 SUP: 9 | 12 g sustained release beta-alanine × 14 days | 12 g rice powder × 14 days | Serial Sevens Test RT (visuomotor) Visual tracking (multiple object tracking) | Accuracy | 0.13 (−4.93; 4.67) | |
| Caffeine | Ali 2015 [54] | RCT | 10 | 6 mg·kg⸱BM−1 anhydrous caffeine (Fluka Sigma-Aldrich, St. Louis, MO, USA) | Artificial sweetener (Equal) | CRT Stroop task | Accuracy RT | 0.11 (−2.39; 2.61) 0.44 (−0.20; 1.08) |
| Baur et al. [55] | Parallel groups | PLA: 12 SUP: 14 | 400 and 200 g regular coffee (101 ± 0.6 mg caffeine·200 g) | Decaffeinated coffee (2.4 ± 0.05 mg caffeine·200 g) | PVT Visuospatial n-back task Letter n-back task Visual search task | Accuracy RT | −0.06 (−0.08; −0.04) −0.40 (−0.81; 0.01) | |
| Beaven 2013 [58] | RCT | 21 | 240 mg caffeine with/without blue light | Visually indistinguishable sugar PLA with/without blue light | PVT | Accuracy RT | 0.03 (−0.78; 0.84) 0.00 (−0.20; 0.20) | |
| dePauw 2017 [63] | RCT | 11 | Caffeine nasal spray (15 mg/mL) + HPMC (2 g) + mannitol (2.57 g) | Distilled water, benzalkonium chloride (40 mg), HPMC (2 g) and mannitol (2.57 g) | Stroop task | RT | 0.06 (−0.17; 0.29) | |
| Duncan 2018 [56] | RCT | 12 | 5 mg·kg⸱BM−1 caffeine capsules (Myprotein, Manchester, UK) | 5 mg·kg⸱BM−1 dextrose capsules (Myprotein, Manchester, UK) | Flanker task | Accuracy | −0.41 (−1.10; 0.28) | |
| Karayigit 2020 [57] | RCT | 17 | 0.16 g·kg⸱BM−1 caffeinated coffee (6 mg·kg−1 caffeine) 0.08 mg⸱kg−1⸱BM−1 caffeinated coffee (3 mg⸱kg−1⸱BM−1 caffeine) (Nescafé Gold, Nestlé, Istanbul, Turkey) | Equal volume decaffeinated coffee | Flanker task | Accuracy RT Accuracy RT | 3 mg⸱kg−1⸱BM−1: 0.23 (−0.86; 1.32) 6 mg⸱kg−1⸱BM−1: −0.39 (−0.59; −0.19) 3 mg⸱kg−1⸱BM−1: 0.41 (−3.56; 4.38) 6 mg⸱kg−1⸱BM−1: 0.4 (0.06; 0.84) | |
| Russell 2020 [44] | RCT | 14 | Caffeine gum (400 mg; 4.1 ± 0.5 mg⸱kg−1⸱BM−1) | Four pieces of PLA chewing gum | SRT Stroop task | Accuracy RT | 0.37 (−0.21; 0.95) 0.16 (−0.58; 0.90) | |
| Carbohydrate | de Pauw 2017 [63] | RCT | 11 | Glucose nasal spray (80 mg·mL−1) with HPMC (2 g) and mannitol (2.57 g) | Distilled water, benzalkonium chloride (40 mg), HPMC (2 g) and mannitol (2.57 g) | Stroop task | RT | 0.03 (−0.50; −0.56) |
| Harper 2017 [59] | RCT | 15 | 60 g CHO + 205 mg NA+ | PLA-electrolyte beverage or water (PLA beverage used as comparison in the meta-analysis) | COMPASS battery: Mean speed and accuracy scores (secondary and working memory, attention, and decision-making) Immediate and delayed word recall (# of correct responses) | Accuracy RT | −0.11 (−0.39; 0.17) 0.16 (−0.08; 0.40) | |
| Konishi 2017 [60] | RCT | 8 | 25 mL mouth-rinse × 5 s 6.4% maltodextrin (Maltodextrin; Body Plus International, Miyagi, Japan; 6% CHO solution | Water | Stroop task (incongruent) | Accuracy RT | −0.19 (−1.17; 0.79) 0.43 (−0.56; 1.42) | |
| Lee 2014 [64] | RCT | 12 | 68 g via a sports drink | 6 kcal, 0 g CHO PLA drink | CRT PVT Symbol digit matching Search and memory Digit span | RT | −0.01 (−0.12; 0.10) | |
| Energy drinks | Pomportes 2017 [42] | RCT | 10 | 6% CHO (fructose [89%] and maltodextrin [11%]; Isoxan Sport Pro, NHS, Rungis, France) 200 mg CAF (CAF: Prolab Nutrition, Chatsworth, CA) added with orange sugarless syrup, 3.4 g GUAc (300 mg guarana + 100 mg ginseng + 180 mg vitamins C; Isoxan Actiflash Booster, NHS, Rungis, France) | Tap water added with orange sugarless syrup | Simon task | RT CHO: 0.53 (−0.36; 1.42) Caffeine: 0.62 (−0.28; 1.52) Guarana: 0.89 (−0.04; 1.82) | |
| Lassiter et al. [65] | RCT | 15 | 54 g CHO, 160 mg caffeine, 2 g taurine, 400 mg Panax ginseng, and 5 g of other ingredients | 0 kcal beverage, trace ingredients (NR) | CRT Tapping task Go/No-Go (EF test) Stroop Test | RT −0.04 (−2.48; 2.40) |
4. Discussion
4.1. Beta-Alanine
4.2. Caffeine
4.3. Carbohydrate
4.4. Energy Drinks
5. Limitations and Future Directions
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Desmond, P.A.; Hancock, P.A. Active and passive fatigue states. Stress Workload Fatigue 2000, 1, 455–465. [Google Scholar]
- Russell, S.; Jenkins, D.; Smith, M.; Halson, S.; Kelly, V. The application of mental fatigue research to elite team sport performance: New perspectives. J. Sci. Med. Sport 2019, 22, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Piedra, C.; Rieiro, H.; Di Stasi, L.L. Monitoring army drivers’ workload during off-road missions: An experimental controlled field study. Saf. Sci. 2021, 134, 105092. [Google Scholar] [CrossRef]
- Smith, M.E.; Gevins, A. Neurophysiologic monitoring of mental workload and fatigue during operation of a flight simulator. In Proceedings of the Biomonitoring for Physiological and Cognitive Performance during Military Operations, Orlando, FL, USA, 23 May 2005; Volume 5797, pp. 116–126. [Google Scholar]
- Thompson, C.J.; Noon, M.; Towlson, C.; Perry, J.; Coutts, A.J.; Harper, L.D.; Skorski, S.; Smith, M.R.; Barrett, S.; Meyer, T. Understanding the presence of mental fatigue in English academy soccer players. J. Sports Sci. 2020, 38, 1524–1530. [Google Scholar] [CrossRef] [PubMed]
- Filipas, L.; Gallo, G.; Pollastri, L.; La Torre, A. Mental fatigue impairs time trial performance in sub-elite under 23 cyclists. PLoS ONE 2019, 14, e0218405. [Google Scholar] [CrossRef] [Green Version]
- Keramidas, M.E.; Siebenmann, C.; Norrbrand, L.; Gadefors, M.; Eiken, O. A brief pre-exercise nap may alleviate physical performance impairments induced by short-term sustained operations with partial sleep deprivation–A field-based study. Chronobiol. Int. 2018, 35, 1464–1470. [Google Scholar] [CrossRef] [Green Version]
- Reis, C.; Mestre, C.; Canhão, H. Prevalence of fatigue in a group of airline pilots. Aviat. Space Environ. Med. 2013, 84, 828–833. [Google Scholar] [CrossRef]
- Smith, M.R.; Chai, R.; Nguyen, H.T.; Marcora, S.M.; Coutts, A.J. Comparing the Effects of Three Cognitive Tasks on Indicators of Mental Fatigue. J. Psychol. 2019, 153, 759–783. [Google Scholar] [CrossRef]
- Forestier, C.; de Chanaleilles, M.; Boisgontier, M.P.; Chalabaev, A. Ego Depletion: A Review of Criticisms Along with New Perspectives for the Replicable Investigation of Self-Control Fatigue as a Multicomponent Phenomenon. PsyArXiv. 12 April 2021. Available online: https://psyarxiv.com/spm9a/ (accessed on 1 October 2021).
- Forestier, C.; Chalabaev, A. Ego-depletion or mental fatigue? Commentary: “Strong Effort Manipulations Reduce Response Caution: A Preregistered Reinvention of the Ego-Depletion Paradigm”. 20 May 2020, OSF Preprints. Available online: https://osf.io/ekzsv (accessed on 1 October 2021).
- Friese, M.; Loschelder, D.D.; Gieseler, K.; Frankenbach, J.; Inzlicht, M. Is ego depletion real? An analysis of arguments. Personal. Soc. Psychol. Rev. 2019, 23, 107–131. [Google Scholar] [CrossRef] [PubMed]
- Inzlicht, M.; Friese, M. The past, present, and future of ego depletion. Soc. Psychol. 2019, 50, 370–378. [Google Scholar] [CrossRef]
- Lurquin, J.H.; Miyake, A. Challenges to ego-depletion research go beyond the replication crisis: A need for tackling the conceptual crisis. Front. Psychol. 2017, 8, 568. [Google Scholar] [CrossRef] [Green Version]
- Inzlicht, M.; Marcora, S.M. The central governor model of exercise regulation teaches us precious little about the nature of mental fatigue and self-control failure. Front. Psychol. 2016, 7, 656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pageaux, B. The psychobiological model of endurance performance: An effort-based decision-making theory to explain self-paced endurance performance. Sports Med. 2014, 44, 1319. Available online: https://link.springer.com/article/10.1007%2Fs40279-014-0198-2 (accessed on 15 October 2021). [CrossRef] [PubMed]
- Lambert, E.V.; Gibson, A.S.C.; Noakes, T.D. Complex systems model of fatigue: Integrative homoeostatic control of peripheral physiological systems during exercise in humans. Br. J. Sports Med. 2005, 39, 52–62. [Google Scholar] [CrossRef] [Green Version]
- Read, G.J.M.; Shorrock, S.; Walker, G.H.; Salmon, P.M. State of science: Evolving perspectives on ‘human error’. Ergonomics 2021, 64, 1091–1114. [Google Scholar] [CrossRef] [PubMed]
- Russell, S.; Jenkins, D.; Rynne, S.; Halson, S.L.; Kelly, V. What is mental fatigue in elite sport? Perceptions from athletes and staff. Eur. J. Sport Sci. 2019, 19, 1367–1376. [Google Scholar] [CrossRef] [PubMed]
- Lopes, T.R.; Oliveira, D.M.; Simurro, P.B.; Akiba, H.T.; Nakamura, F.Y.; Okano, A.H.; Dias, Á.M.; Silva, B.M. No Sex Difference in Mental Fatigue Effect on High-Level Runners’ Aerobic Performance. Med. Sci. Sports Exerc. 2020, 52, 2207–2216. [Google Scholar] [CrossRef] [PubMed]
- Filipas, L.; Borghi, S.; La Torre, A.; Smith, M.R. Effects of mental fatigue on soccer-specific performance in young players. Sci. Med. Footb. 2021, 5, 150–157. [Google Scholar] [CrossRef]
- Van Cutsem, J.; De Pauw, K.; Marcora, S.; Meeusen, R.; Roelands, B. A caffeine-maltodextrin mouth rinse counters mental fatigue. Psychopharmacology 2018, 235, 947–958. Available online: https://link.springer.com/article/10.1007%2Fs00213-017-4809-0 (accessed on 15 October 2021). [CrossRef] [Green Version]
- Smith, M.R.; Zeuwts, L.; Lenoir, M.; Hens, N.; De Jong, L.M.S.; Coutts, A.J. Mental fatigue impairs soccer-specific decision-making skill. J. Sports Sci. 2016, 34, 1297–1304. [Google Scholar] [CrossRef]
- Van Cutsem, J.; De Pauw, K.; Vandervaeren, C.; Marcora, S.; Meeusen, R.; Roelands, B. Mental fatigue impairs visuomotor response time in badminton players and controls. Psychol. Sport Exerc. 2019, 45, 101579. [Google Scholar] [CrossRef]
- Head, J.; Tenan, M.S.; Tweedell, A.J.; LaFiandra, M.E.; Morelli, F.; Wilson, K.M.; Ortega, S.V.; Helton, W.S. Prior Mental Fatigue Impairs Marksmanship Decision Performance. Front. Physiol. 2017, 8, 680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, X.; Lodewijks, G. Detecting fatigue in car drivers and aircraft pilots by using non-invasive measures: The value of differentiation of sleepiness and mental fatigue. J. Saf. Res. 2020, 72, 173–187. [Google Scholar] [CrossRef]
- Tran, Y.; Craig, A.; Craig, R.; Chai, R.; Nguyen, H. The influence of mental fatigue on brain activity: Evidence from a systematic review with meta-analyses. Psychophysiology 2020, 57, e13554. [Google Scholar] [CrossRef] [PubMed]
- Petrides, M. The role of the mid-dorsolateral prefrontal cortex in working memory. Exp. Brain Res. 2000, 133, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Azab, H.; Hayden, B.Y. Correlates of decisional dynamics in the dorsal anterior cingulate cortex. PLoS Biol. 2017, 15, e2003091. [Google Scholar] [CrossRef] [Green Version]
- Rolls, E.T. The cingulate cortex and limbic systems for emotion, action, and memory. Brain Struct. Funct. 2019, 224, 3001–3018. [Google Scholar] [CrossRef] [Green Version]
- Sachdev, P.S.; Blacker, D.; Blazer, D.G.; Ganguli, M.; Jeste, D.V.; Paulsen, J.S.; Petersen, R.C. Classifying neurocognitive disorders: The DSM-5 approach. Nat. Rev. Neurol. 2014, 10, 634–642. [Google Scholar] [CrossRef]
- Schneider, W.J.; McGrew, K.S. The Cattell–Horn–Carroll theory of cognitive abilities. In Contemporary Intellectual Assessment: Theories, Tests, and Issues; Flanagan, D.P., McDonough, E.M., Eds.; The Guilford Press: New York, NY, USA, 2018; pp. 73–163. [Google Scholar]
- Harvey, P.D. Domains of cognition and their assessment. Dialogues Clin. Neurosci. 2019, 21, 227. [Google Scholar]
- Grillon, C.; Quispe-Escudero, D.; Mathur, A.; Ernst, M. Mental fatigue impairs emotion regulation. Emotion 2015, 15, 383. [Google Scholar] [CrossRef]
- Meeusen, R. Exercise, nutrition and the brain. Sports Med. 2014, 44, 47–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, K.; Périard, J.; Rattray, B.; Pyne, D.B. Physiological factors which influence cognitive performance in military personnel. Hum. Factors 2020, 62, 93–123. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, J.A.; Mallis, M.M.; Caldwell, J.L.; Paul, M.A.; Miller, J.C.; Neri, D.F. Fatigue countermeasures in aviation. Aviat. Space Environ. Med. 2009, 80, 29–59. [Google Scholar] [CrossRef]
- Azevedo, R.; Silva-Cavalcante, M.; Gualano, B.; Lima-Silva, A.; Bertuzzi, R. Effects of caffeine ingestion on endurance performance in mentally fatigued individuals. Eur. J. Appl. Physiol. 2016, 116, 2293–2303. [Google Scholar] [CrossRef] [PubMed]
- Furst, T.; Massaro, A.; Miller, C.; Williams, B.T.; LaMacchia, Z.M.; Horvath, P.J. β-Alanine supplementation increased physical performance and improved executive function following endurance exercise in middle aged individuals. J. Int. Soc. Sports Nutr. 2018, 15, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franco-Alvarenga, P.E.; Brietzke, C.; Canestri, R.; Goethel, M.F.; Hettinga, F.; Santos, T.M.; Pires, F.O. Caffeine improved cycling trial performance in mentally fatigued cyclists, regardless of alterations in prefrontal cortex activation. Physiol. Behav. 2019, 204, 41–48. [Google Scholar] [CrossRef]
- Newman, R.A.; Kamimori, G.H.; Wesensten, N.J.; Picchioni, D.; Balkin, T.J. Caffeine gum minimizes sleep inertia. Percept. Mot. Ski. 2013, 116, 280–293. [Google Scholar] [CrossRef]
- Pomportes, L.; Brisswalter, J.; Hays, A.; Davranche, K. Effects of carbohydrate, caffeine, and guarana on cognitive performance, perceived exertion, and shooting performance in high-level athletes. Int. J. Sports Physiol. Perform. 2019, 14, 576–582. [Google Scholar] [CrossRef]
- Tartar, J.L.; Kalman, D.; Hewlings, S. A prospective study evaluating the effects of a nutritional supplement intervention on cognition, mood states, and mental performance in video gamers. Nutrients 2019, 11, 2326. [Google Scholar] [CrossRef] [Green Version]
- Russell, M.; Reynolds, N.A.; Crewther, B.T.; Cook, C.J.; Kilduff, L.P. Physiological and Performance Effects of Caffeine Gum Consumed During a Simulated Half-Time by Professional Academy Rugby Union Players. J. Strength Cond. Res. 2020, 34, 145–151. [Google Scholar] [CrossRef] [Green Version]
- Trexler, E.T.; Smith-Ryan, A.E.; Stout, J.R.; Hoffman, J.R.; Wilborn, C.D.; Sale, C.; Kreider, R.B.; Jäger, R.; Earnest, C.P.; Bannock, L. International society of sports nutrition position stand: Beta-Alanine. J. Int. Soc. Sports Nutr. 2015, 12, 30. [Google Scholar] [CrossRef] [Green Version]
- Marcora, S.M.; Staiano, W.; Manning, V. Mental fatigue impairs physical performance in humans. J. Appl. Physiol. 2009, 106, 857–864. Available online: https://gateway.library.qut.edu.au/login?url=https://search.ebscohost.com/login.aspx?direct=true&db=sph&AN=37006772&site=ehost-live&scope=site (accessed on 15 October 2021). [CrossRef]
- Wallace, B.C.; Small, K.; Brodley, C.E.; Lau, J.; Trikalinos, T.A. Deploying an interactive machine learning system in an evidence-based practice center: Abstrackr. In Proceedings of the 2nd ACM SIGHIT International Health Informatics Symposium, Miami, FL, USA, 28–30 January 2012. [Google Scholar]
- Polanin, J.R.; Pigott, T.D.; Espelage, D.L.; Grotpeter, J.K. Best practice guidelines for Abstract screening large-evidence systematic reviews and meta-analyses. Res. Synth. Methods 2019, 10, 330–342. [Google Scholar] [CrossRef] [Green Version]
- Downs, S.H.; Black, N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J. Epidemiol. Community Health 1998, 52, 377–384. [Google Scholar] [CrossRef] [Green Version]
- Harrer, M.; Cuijpers, P.; Furukawa, T.; Ebert, D.D. Dmetar: Companion R package for the Guide ’Doing Meta-Analysis in R’. R package version 0.0 2019, 9000. Available online: https://bookdown.org/MathiasHarrer/Doing_Meta_Analysis_in_R/ (accessed on 24 September 2021).
- Balduzzi, S.; Rücker, G.; Schwarzer, G. How to perform a meta-analysis with R: A practical tutorial. Evid.-Based Ment. Health 2019, 22, 153–160. [Google Scholar] [CrossRef] [Green Version]
- Viechtbauer, W. Conducting meta-analyses in R with the metafor package. J. Stat. Softw. 2010, 36, 1–48. [Google Scholar] [CrossRef] [Green Version]
- Doma, K.; Gahreman, D.; Connor, J. Fruit supplementation reduces indices of exercise-induced muscle damage: A systematic review and meta-analysis. Eur. J. Sport Sci. 2021, 21, 562–579. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; O’Donnell, J.; Von Hurst, P.; Foskett, A.; Holland, S.; Starck, C.; Rutherfurd-Markwick, K. Caffeine ingestion enhances perceptual responses during intermittent exercise in female team-game players. J. Sports Sci. 2016, 34, 330–341. [Google Scholar] [CrossRef] [PubMed]
- Baur, D.M.; Lange, D.; Elmenhorst, E.-M.; Elmenhorst, D.; Bauer, A.; Aeschbach, D.; Landolt, H.-P. Coffee effectively attenuates impaired attention in ADORA2A C/C-allele carriers during chronic sleep restriction. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 109, 110232. [Google Scholar] [CrossRef] [PubMed]
- Duncan, M.J.; Dobell, A.P.; Caygill, C.L.; Eyre, E.; Tallis, J. The effect of acute caffeine ingestion on upper body anaerobic exercise and cognitive performance. Eur. J. Sport Sci. 2019, 19, 103–111. [Google Scholar] [CrossRef]
- Karayigit, R.; Naderi, A.; Akca, F.; Cruz CJG d Sarshin, A.; Yasli, B.C.; Ersoz, G.; Kaviani, M. Effects of Different Doses of Caffeinated Coffee on Muscular Endurance, Cognitive Performance, and Cardiac Autonomic Modulation in Caffeine Naive Female Athletes. Nutrients 2021, 13, 2. [Google Scholar] [CrossRef]
- Beaven, C.M.; Ekström, J. A comparison of blue light and caffeine effects on cognitive function and alertness in humans. PLoS ONE 2013, 8, e76707. [Google Scholar] [CrossRef] [Green Version]
- Harper, L.D.; Stevenson, E.J.; Rollo, I.; Russell, M. The influence of a 12% carbohydrate-electrolyte beverage on self-paced soccer-specific exercise performance. J. Sci. Med. Sport 2017, 20, 1123–1129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konishi, K.; Kimura, T.; Yuhaku, A.; Kurihara, T.; Fujimoto, M.; Hamaoka, T.; Sanada, K. Mouth rinsing with a carbohydrate solution attenuates exercise-induced decline in executive function. J. Int. Soc. Sports Nutr. 2017, 14, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wells, A.J.; Varanoske, A.N.; Coker, N.A.; Kozlowski, G.J.; Frosti, C.L.; Boffey, D.; Harat, I.; Jahani, S.; Gepner, Y.; Hoffman, J.R. Effect of β-Alanine Supplementation on Monocyte Recruitment and Cognition During a 24-Hour Simulated Military Operation. J. Strength Cond. Res. 2020, 34, 3042–3054. [Google Scholar] [CrossRef] [PubMed]
- Varanoske, A.N.; Wells, A.J.; Kozlowski, G.J.; Gepner, Y.; Frosti, C.L.; Boffey, D.; Coker, N.A.; Harat, I.; Hoffman, J.R. Effects of β-alanine supplementation on physical performance, cognition, endocrine function, and inflammation during a 24 h simulated military operation. Physiol. Rep. 2018, 6, e13938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Pauw, K.; Roelands, B.; Van Cutsem, J.; Decroix, L.; Valente, A.; Taehee, K.; Lettan, R.B.; Carrillo, A.E.; Meeusen, R. Do glucose and caffeine nasal sprays influence exercise or cognitive performance? Int. J. Sports Physiol. Perform. 2017, 12, 1186–1191. [Google Scholar] [CrossRef]
- Lee, J.K.W.; Ang, W.H.; Ng, J.W.X.; Fan, P.W.P.; Teo, Y.S.; Nolte, H.W.; Yeo, Y.Y.W. Effects of a carbohydrate-electrolyte solution on cognitive performance following exercise-induced hyperthermia in humans. J. Int. Soc. Sports Nutr. 2014, 11, 51. [Google Scholar] [CrossRef] [Green Version]
- Lassiter, D.G.; Kammer, L.; Burns, J.; Ding, Z.; Kim, H.; Lee, J.; Ivy, J.L. Effect of an energy drink on physical and cognitive performance in trained cyclists. J. Caffeine Res. 2012, 2, 167–175. [Google Scholar] [CrossRef]
- Kane, R.L.; Roebuck-Spencer, T.; Short, P.; Kabat, M.; Wilken, J. Identifying and monitoring cognitive deficits in clinical populations using Automated Neuropsychological Assessment Metrics (ANAM) tests. Arch. Clin. Neuropsychol. 2007, 22 (Suppl. 1), S115–S126. [Google Scholar] [CrossRef] [Green Version]
- Pickering, C.; Kiely, J. Are the current guidelines on caffeine use in sport optimal for everyone? Inter-individual variation in caffeine ergogenicity, and a move towards personalised sports nutrition. Sports Med. 2018, 48, 7–16. [Google Scholar] [CrossRef] [Green Version]
- Grgic, J.; Pickering, C.; Bishop, D.J.; Del Coso, J.; Schoenfeld, B.J.; Tinsley, G.M.; Pedisic, Z. ADORA2A C allele carriers exhibit ergogenic responses to caffeine supplementation. Nutrients 2020, 12, 741. [Google Scholar] [CrossRef] [Green Version]
- Queiros, V.S.D.; Dantas, M.; Fortes, L.D.S.; Silva, L.F.D.; Silva, G.M.D.; Dantas, P.M.S.; Cabral, B.G.D.A.T. Mental Fatigue Reduces Training Volume in Resistance Exercise: A Cross-Over and Randomized Study. Percept. Mot. Ski. 2021, 128, 409–423. [Google Scholar] [CrossRef] [PubMed]
- Morris, T.P.; Fried, P.J.; Macone, J.; Stillman, A.; Gomes-Osman, J.; Costa-Miserachs, D.; Tormos Munoz, J.M.; Santarnecchi, E.; Pascual-Leone, A. Light aerobic exercise modulates executive function and cortical excitability. Eur. J. Neurosci. 2020, 51, 1723–1734. [Google Scholar] [CrossRef] [PubMed]
- Kamimori, G.H.; Karyekar, C.S.; Otterstetter, R.; Cox, D.S.; Balkin, T.J.; Belenky, G.L.; Eddington, N.D. The rate of absorption and relative bioavailability of caffeine administered in chewing gum versus capsules to normal healthy volunteers. Int. J. Pharm. 2002, 234, 159–167. [Google Scholar] [CrossRef]
- Russell, S.; Jenkins, D.G.; Halson, S.L.; Juliff, L.E.; Kelly, V.G. How do elite female team sport athletes experience mental fatigue? Comparison between international competition, training and preparation camps. Eur. J. Sport Sci. 2021, Ahead of print. 1–11. [Google Scholar] [CrossRef]
- Rodriguez-Giustiniani, P.; Rollo, I.; Witard, O.C.; Galloway, S.D. Ingesting a 12% carbohydrate-electrolyte beverage before each half of a soccer match simulation facilitates retention of passing performance and improves high-intensity running capacity in academy players. Int. J. Sport Nutr. Exerc. Metab. 2019, 29, 397–405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sterne, J.A.C.; Jüni, P.; Schulz, K.F.; Altman, D.G.; Bartlett, C.; Egger, M. Statistical methods for assessing the influence of study characteristics on treatment effects in ‘meta-epidemiological’research. Stat. Med. 2002, 21, 1513–1524. [Google Scholar] [CrossRef] [PubMed]
- Balagué, N.; Hristovski, R.; Almarcha, M.D.C.; Garcia-Retortillo, S.; Ivanov, P.C. Network physiology of exercise: Vision and perspectives. Front. Physiol. 2020, 11, 1607. [Google Scholar] [CrossRef] [PubMed]
- Adikari, A.; Appukutty, M.; Kuan, G. Effects of Daily Probiotics Supplementation on Anxiety Induced Physiological Parameters among Competitive Football Players. Nutrients 2020, 12, 1920. [Google Scholar] [CrossRef]
- Sashihara, T.; Nagata, M.; Mori, T.; Ikegami, S.; Gotoh, M.; Okubo, K.; Uchida, M.; Itoh, H. Effects of Lactobacillus gasseri OLL2809 and α-lactalbumin on university-student athletes: A randomized, double-blind, placebo-controlled clinical trial. Appl. Physiol. Nutr. Metab. 2013, 38, 1228–1235. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.; Wylie, L.J.; Fulford, J.; Kelly, J.; Black, M.I.; McDonagh, S.T.J.; Jeukendrup, A.E.; Vanhatalo, A.; Jones, A.M. Dietary nitrate improves sprint performance and cognitive function during prolonged intermittent exercise. Eur. J. Appl. Physiol. 2015, 115, 1825–1834. [Google Scholar] [CrossRef]
- Thompson, K.G.; Turner, L.; Prichard, J.; Dodd, F.; Kennedy, D.O.; Haskell, C.; Blackwell, J.R.; Jones, A.M. Influence of dietary nitrate supplementation on physiological and cognitive responses to incremental cycle exercise. Respir. Physiol. Neurobiol. 2014, 193, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Fontani, G.; Lodi, L.; Migliorini, S.; Corradeschi, F. Effect of omega-3 and policosanol supplementation on attention and reactivity in athletes. J. Am. Coll. Nutr. 2009, 28 (Suppl. 4), 473S–481S. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, J.; Roelands, B.; Pluym, B.; Tassignon, B.; Verschueren, J.O.; De Pauw, K.; Meeusen, R. Can creatine combat the mental fatigue-associated decrease in visuomotor skills? Med. Sci. Sports Exerc. 2020, 52, 120–130. [Google Scholar] [CrossRef]
- Waldman, H.S.; Basham, S.A.; Price, F.G.; Smith, J.W.; Chander, H.; Knight, A.C.; Krings, B.M.; McAllister, M.J. Exogenous ketone salts do not improve cognitive responses after a high-intensity exercise protocol in healthy college-aged males. Appl. Physiol. Nutr. Metab. 2018, 43, 711–717. [Google Scholar] [CrossRef]
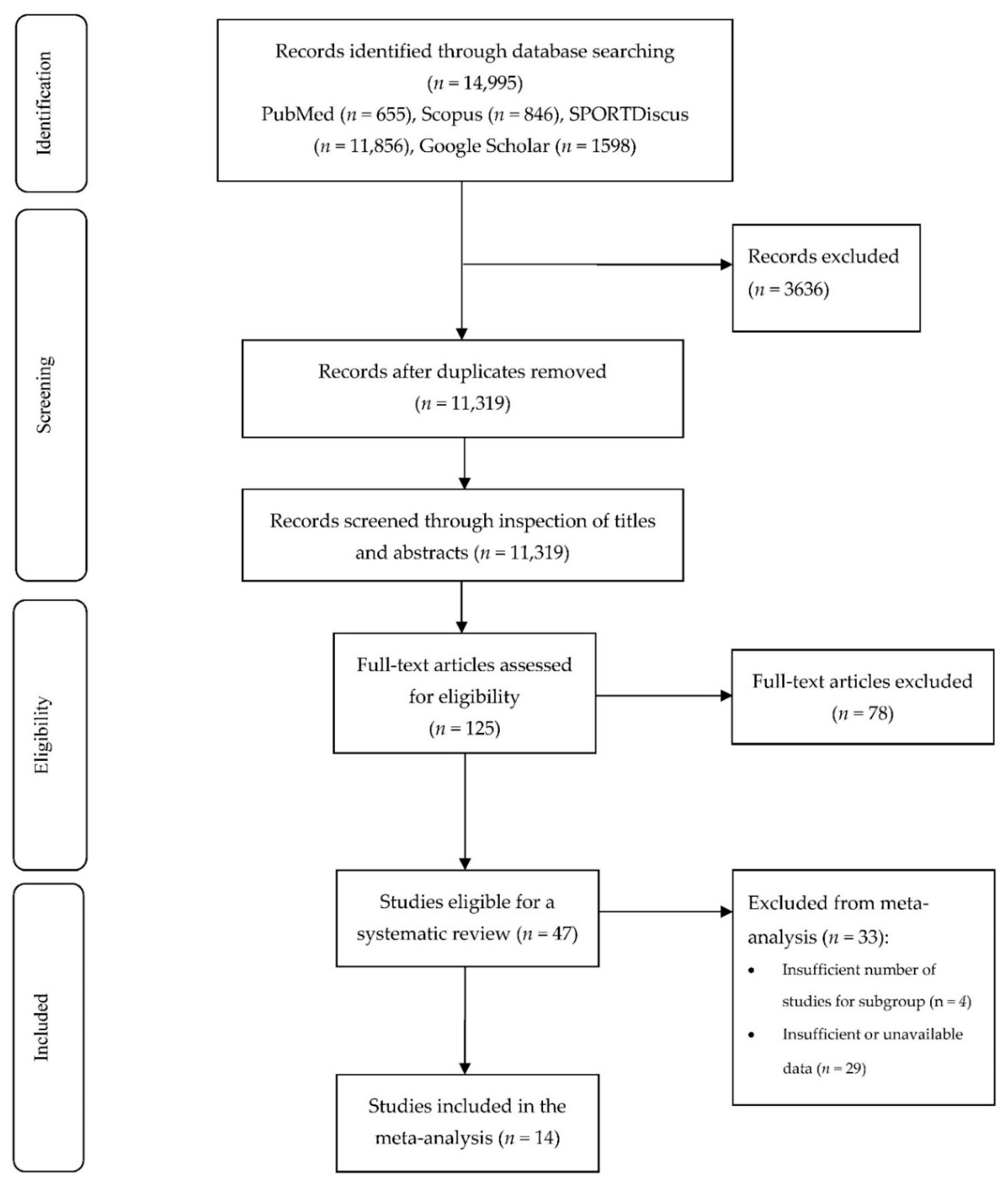
| Search Terms | PubMed | Scopus | SPORTDiscus | Google Scholar |
|---|---|---|---|---|
| Results | Results | Results | Results | |
| 23 | 731 | 614 | 980 |
| 3 | 29 | 1500 | 294 |
| 151 | 58 | 4386 | 37 |
| 378 | 6 | 2958 | 7 |
| 100 | 22 | 2398 | 80 |
| Total 14,955 | 655 | 846 | 11,856 | 1598 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliver, L.S.; Sullivan, J.P.; Russell, S.; Peake, J.M.; Nicholson, M.; McNulty, C.; Kelly, V.G. Effects of Nutritional Interventions on Accuracy and Reaction Time with Relevance to Mental Fatigue in Sporting, Military, and Aerospace Populations: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 307. https://doi.org/10.3390/ijerph19010307
Oliver LS, Sullivan JP, Russell S, Peake JM, Nicholson M, McNulty C, Kelly VG. Effects of Nutritional Interventions on Accuracy and Reaction Time with Relevance to Mental Fatigue in Sporting, Military, and Aerospace Populations: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health. 2022; 19(1):307. https://doi.org/10.3390/ijerph19010307
Chicago/Turabian StyleOliver, Liam S., John P. Sullivan, Suzanna Russell, Jonathan M. Peake, Mitchell Nicholson, Craig McNulty, and Vincent G. Kelly. 2022. "Effects of Nutritional Interventions on Accuracy and Reaction Time with Relevance to Mental Fatigue in Sporting, Military, and Aerospace Populations: A Systematic Review and Meta-Analysis" International Journal of Environmental Research and Public Health 19, no. 1: 307. https://doi.org/10.3390/ijerph19010307
APA StyleOliver, L. S., Sullivan, J. P., Russell, S., Peake, J. M., Nicholson, M., McNulty, C., & Kelly, V. G. (2022). Effects of Nutritional Interventions on Accuracy and Reaction Time with Relevance to Mental Fatigue in Sporting, Military, and Aerospace Populations: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health, 19(1), 307. https://doi.org/10.3390/ijerph19010307







