Extremely Acidic Eukaryotic (Micro) Organisms: Life in Acid Mine Drainage Polluted Environments—Mini-Review
Abstract
:1. Introduction
2. Acid Mine Drainage
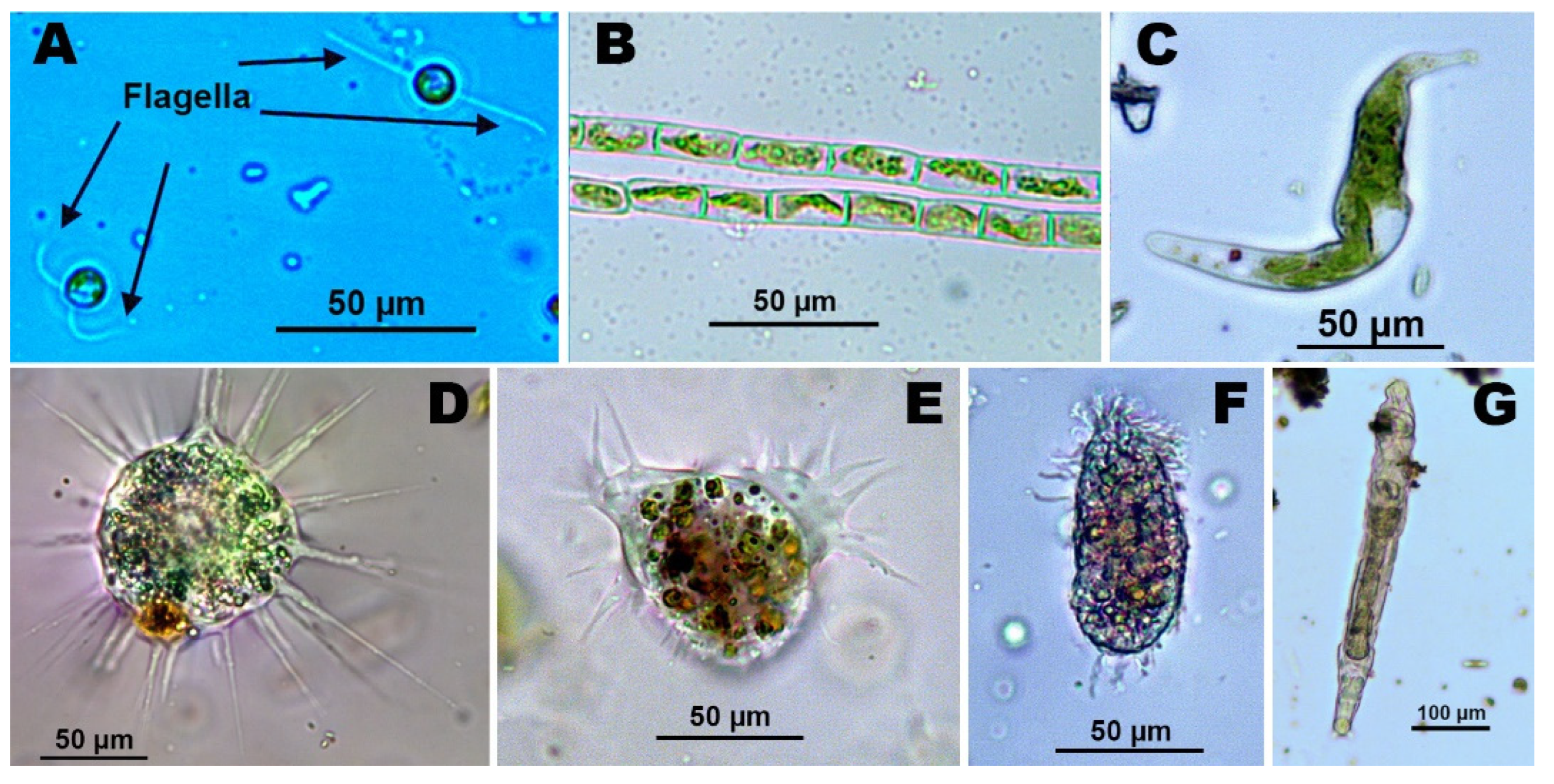
3. Eukaryotic Organisms in AMD-Polluted Extreme Environments
3.1. Diatoms
3.2. Unicellular and Filamentous Green Algae
3.3. Protozoa, Fungi and Yeasts in AMD-Polluted Waters
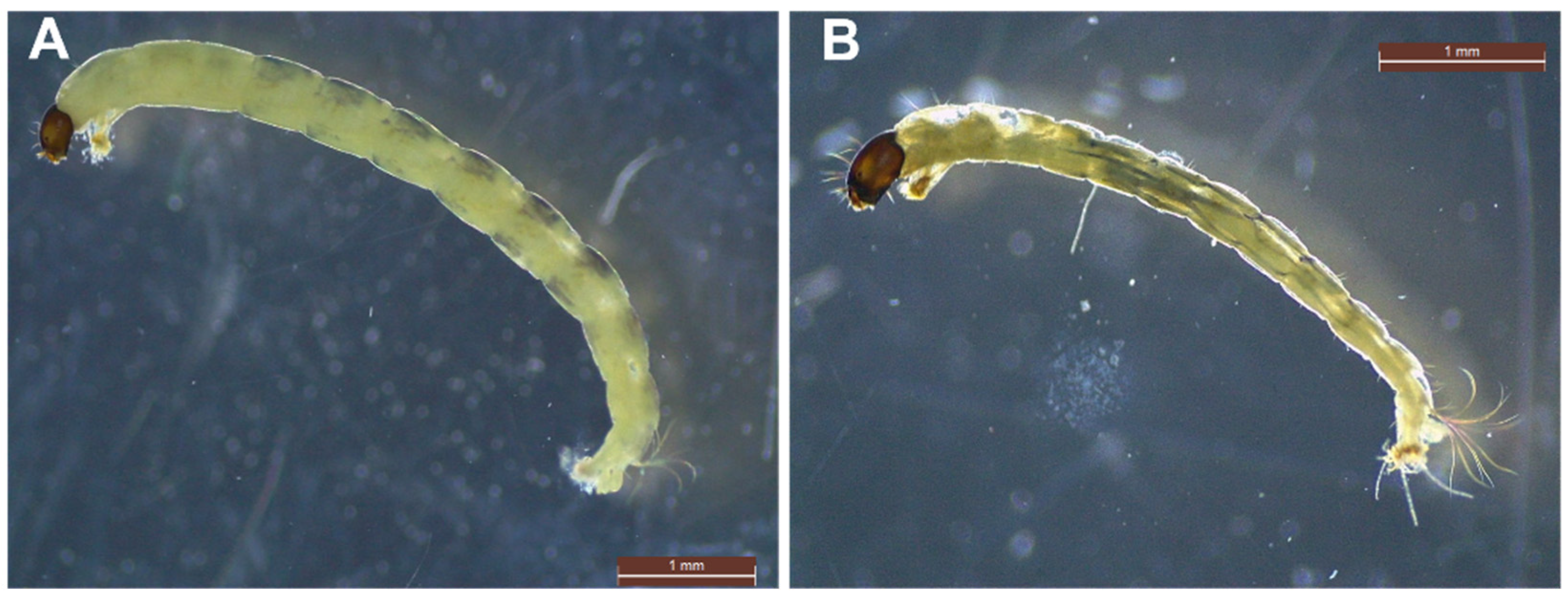
3.4. The Impact of AMD on Micro-Macroinvertebrates
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Kleinmann, R.L.P.; Crerar, D.A.; Pacelli, R.R. Biogeochemistry of acid mine drainage and a method to control acid formation. Miner. Eng. 1981, 33, 300–305. [Google Scholar]
- Gray, N.F. Environmental impact and remediation of acid-mine drainage: A management problem. Environ. Geol. 1997, 30, 62–71. [Google Scholar] [CrossRef]
- Nicholson, R.V. Iron-sulfide oxidation mechanism. In Chemical Weathering Rates of Silicate Minerals; White, A.F., Brantley, R.J., Eds.; Mineralogical Society of America: Chantilly, VA, USA, 1994; Volume 31, pp. 173–225. [Google Scholar]
- Grande, J.A. Drenaje Ácido de Mina en la Faja Pirítica Ibérica: Técnicas de estudio e inventario de explotaciones. Serv. Publ. 2016, 1, 345. [Google Scholar]
- Dogan, P.A. Characterization of mine waste for prediction of acid mine drainage. In Environmental Impacts of Mining Activities; Azcue, J.M., Ed.; Springer: Berlin, Germany, 1999; pp. 19–38. [Google Scholar]
- USEPA. Technical Document: Acid Mine Drainage Prediction; USEPA: Washington, DC, USA, 1994; p. 52. [Google Scholar]
- Grande, J.A.; Beltrán, R.; Santos, J.C.; de la Torre, M.L.; Borrego, J. Acid mine drainage and acid rock drainage in the environment of Herrerías Mine (Iberian Pyrite Belt, Huelva-Spain) and impact on the Andévalo dam. Environ. Geol. 2005, 47, 185–196. [Google Scholar] [CrossRef]
- Okabayashi, A.; Wakai, S.; Kanao, T.; Sugio, T.; Kamimura, K. Diversity of 16S ribosomal DNA-defined bacterial population in acid rock drainage from Japanese pyrite mine. J. Biosci. Bioeng. 2005, 100, 644–652. [Google Scholar] [CrossRef]
- DeNicola, D.M.; Stapleton, M.G. Benthic diatoms as indicators of long-term changes in a watershed receiving passive treatment for acid mine drainage. Hydrobiologia 2014, 732, 29–48. [Google Scholar] [CrossRef]
- Gray, N.F.; Delaney, E. Comparison of benthic macroinvertebrate indices for the assessment of the impact of acid mine drainage on an Irish river below an abandoned Cu-S mine. Environ. Pollut. 2007, 155, 31–40. [Google Scholar] [CrossRef]
- Clapcott, J.E.; Goodwin, E.O.; Harding, J.S. Identifying catchment-scale predictors of coal mining impacts on New Zealand stream communities. Environ. Manag. 2016, 57, 711–721. [Google Scholar] [CrossRef]
- Ruggiu, D.; Luglié, A.; Cattaneo, A.; Panzani, P. Paleoecological evidence for diatom response to metal pollution in Lake Orta (N. Italy). J. Paleolimnol. 1998, 20, 333–345. [Google Scholar] [CrossRef]
- Luís, A.T.; Coelho, H.; Almeida, S.F.P.; Ferreira da Silva, E.A.; Serôdio, J. Photosynthetic activity and ecology of benthic diatom communities from streams affected by Acid Mine Drainage (AMD) in pyritic mines. Fundam. Appl. Limnol. 2013, 182, 47–59. [Google Scholar] [CrossRef]
- Luís, A.T.; Teixeira, P.; Almeida, S.F.P.; Ector, L.; Matos, J.X.; Ferreira da Silva, E.A. Impact of acid mine drainage (AMD) on water quality, stream sediments and periphytic diatom communities in the surrounding streams of Aljustrel mining area (Portugal). Water Air Soil Pollut. 2009, 200, 147–167. [Google Scholar] [CrossRef]
- Luís, A.T.; Durães, N.; Almeida, S.F.P.; Ferreira da Silva, E.A. Integrating geochemical (surface waters, stream sediments) and biological (diatoms) approaches to assess AMD environmental impact in a pyritic mining area: Aljustrel (Alentejo, Portugal. J. Environ. Sci. 2016, 42, 215–226. [Google Scholar] [CrossRef]
- Valente, T.; Rivera, M.J.; Almeida, S.F.P.; Delgado, C.; Gomes, P.; Grande, J.A.; de la Torre, M.L. Characterization of water reservoirs affected by acid mine drainage: Geochemical, mineralogical and biological (diatoms) properties of the water. Environ. Sci. Pollut. Res. Int. 2016, 23, 6002–6011. [Google Scholar] [CrossRef]
- Luís, A.T.; Teixeira, P.; Almeida, S.F.P.; Matos, J.X.; Ferreira da Silva, E. Environmental impact of mining activities in the Lousal area (Portugal): Chemical and diatom characterization of metal-contaminated stream sediments and surface water of Corona stream. Sci. Total Environ. 2011, 409, 4312–4325. [Google Scholar] [CrossRef] [PubMed]
- Freitas, A.P.P.; Schneider, I.A.H.; Schwartzbold, A. Biosorption of heavy metals by algal communities in water streams affected by the acid mine drainage in the coal-mining region of Santa Catarina state. Brazil Miner. Eng. 2011, 24, 1215–1218. [Google Scholar] [CrossRef]
- Sabater, S.; Buchaca, T.; Cambra, J.; Catalan, J.; Guasch, H.; Ivorra, N.; Munoz, I.; Navarro, E.; Real, M.; Romaní, A. Structure and function of benthic algal communities in an extremely acid river. J. Phycol. 2003, 39, 481–489. [Google Scholar] [CrossRef] [Green Version]
- DeNicola, D.M. A review of diatoms found in highly acidic environments. Hydrobiologia 2000, 433, 111–122. [Google Scholar] [CrossRef]
- Bisthoven, L.J.; Nuyts, P.; Goddeeris, B.; Ollevier, F. Sublethal parameters in deformed Chironomus larvae: Clues to understanding their biomarker value. Freshw. Biol. 1998, 39, 179–191. [Google Scholar] [CrossRef]
- Hogsden, K.L.; Harding, J.S. Consequences of acid mine drainage for the structure and function of benthic stream communities: A, review. Freshw. Sci. 2012, 31, 108–120. [Google Scholar] [CrossRef]
- Bisthoven, J.L.; Gerhardt, A.; Soares, A.M.V.M. Chironomidae as bioindicators of an acid mine drainage in S. Portugal. Hydrobiologia 2005, 532, 181–191. [Google Scholar] [CrossRef]
- Montero, I.C.; Brimhall, G.H.; Alpers, C.N.; Swayze, G.A. Characterization of waste rock associated with acid drainage at the Penn Mine, California, by ground-based visible to short-wave infrared reflectance spectroscopy assisted by digital mapping. Chem. Geol. 2005, 215, 452–472. [Google Scholar] [CrossRef] [Green Version]
- Lottermoser, B. Mine Wastes Characterization, Treatment and Environmental Impacts, 2nd ed.; Springer Publisher: Heidelberg, Germany, 2007; p. 400. [Google Scholar]
- Pearce, J.; Weber, P.; Pearce, S.; Scott, P. Acid and metalliferous drainage contaminant load prediction for operational or legacy mines at closure. In Mine Closure; Fourie, A.B., Tibbett, M., Eds.; Australian Centre for Geomechanics: Perth, Australia, 2016; pp. 663–676. [Google Scholar]
- Quatrini, R.; Johnson, D.B. Microbiomes in extremely acidic environments: Functionalities and interactions that allow survival and growth of prokaryotes at low pH. Curr. Opin. Microbiol. 2018, 43, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Yaacob, W.Z.W.; Pauzi, N.S.M.; Mutalib, H.A. Acid mine drainage and heavy metals contamination at abandoned and active mine sites in Pahang. Bull. Geol. Soc. Malays. 2009, 55, 15–20. [Google Scholar] [CrossRef] [Green Version]
- Bodenan, F.; Baranger, P.; Piantone, P.; Lassin, A.; Azaroual, M. Arsenic behaviour in gold-ore mill tailings, Massif Central, France: Hydrogeochemical study and investigation of in situ redox signatures. Appl. Geochem. 2004, 19, 1785–1800. [Google Scholar] [CrossRef]
- Kang, J.-K.; Song, Y.; Moon, J.-W.; Moon, H.-S. Water quality impact of mining in the Wolmyoung area of Korea, and its short-term changes. Water Air Soil Pollut. 2001, 129, 349–367. [Google Scholar] [CrossRef]
- Rivera, M.J.; Luís, A.T.; Grande, J.A.; Sarmiento, A.M.; Dávila, J.M.; Fortes, J.C.; Córdoba, F.; Diaz-Curiel, J.; Santisteban, M. Physico-Chemical Influence of Surface Water Contaminated by Acid Mine Drainage on the Populations of Diatoms in Dams (Iberian Pyrite Belt, SW Spain). Int. J. Environ. Res. Public Health 2019, 16, 4516. [Google Scholar] [CrossRef] [Green Version]
- Nocete, F.; Sáez, R.; Nieto, J.M.; Cruz-Aunon, R.; Cabrero, R.; Alex, E.; Bayona, M.R. Circulation of silicified oolitic limestone blades in South-Iberia (Spain and Portugal) during the third millennium B.C.: An expression of a core/periphery framework. J. Anthropol. Archaeol. 2005, 24, 62–81. [Google Scholar] [CrossRef]
- Sarmiento, A.M.; Nieto, J.M.; Olías, M.; Cánovas, C.R. Hydrochemical characteristics and seasonal influence on the pollution by acid mine drainage in the Odiel river Basin (SW Spain). Appl. Geochem. 2009, 24, 697–714. [Google Scholar] [CrossRef]
- Sarmiento, A.M.; DelValls, A.; Nieto, J.M.; Salamanca, M.J.; Caraballo, M.A. Toxicity and potential risk assessment of a river polluted by acid mine drainage in the Iberian Pyrite Belt (SW Spain). Sci. Total Environ. 2011, 409, 4763–4771. [Google Scholar] [CrossRef] [PubMed]
- Grande, J.A.; Valente, T.; de la Torre, M.; Santisteban, M.; Cerón, J.C.; Pérez-Ostalé, E. Characterization of acid mine drainage sources in the Iberian Pyrite Belt: Base methodology for quantifying affected areas and for environmental management. Environ. Earth. Sci. 2014, 71, 2729–2738. [Google Scholar] [CrossRef]
- Sáinz, A.; Grande, J.A.; de la Torre, L. Characterization of heavy metal discharge into the Ria of Huelva. Environ. Int. 2004, 30, 557–566. [Google Scholar] [CrossRef]
- Younger, P.L. Hydrogeochemistry of minewaters flowing from abandoned coal workings in the Durham coalfield. Q. J. Eng. Geol. Hydrogeol. 1995, 28, 101–113. [Google Scholar] [CrossRef]
- Caruccio, F.T.; Ferm, J.C. Paleoenvironment—predictor of Acid Mine Drainage Problems. In Proceedings of the 5th Coal Mine Drainage Research Symposium, National Coal Association (USA), Louisville, KY, USA; 1974; pp. 5–9. [Google Scholar]
- Kim, J.-Y.; Chon, H.-T. Pollution of a water course impacted by acid mine drainage in the Imgok creek of the Gangreung coal field, Korea. Appl. Geochem. 2001, 16, 1387–1396. [Google Scholar] [CrossRef]
- Herlihy, A.T.; Kaufmann, P.R.; Mitch, M.E.; Brown, D.D. Regional estimates of acid mine drainage impact on streams in the mid-Atlantic and southeastern United States. Water Air Soil Pollut. 1990, 50, 91–107. [Google Scholar] [CrossRef]
- Pennsylvania Department of Environmental Protection. Pennsylvania Water Quality Assessment 305(b) Report: Harrisburg, P.A., Pennsylvania Department of Environmental Protection, 3800-BK-DEP2530 5/2/2002. Available online: www.dep.pa.gov (accessed on 7 November 2021).
- Bell, F.G.; Halbich, T.F.J.; Bullock, S.E.T. The effects of acid mine drainage from an old mine in the Witbank Coalfield, South Africa. Q. J. Eng. Geol. Hydrogeol. 2002, 35, 265–278. [Google Scholar] [CrossRef]
- Luís, A.T.; Teixeira, M.; Durães, N.; Pinto, R.; Almeida, S.F.P.; Ferreira da Silva, E.A.; Figueira, E. Extremely acidic environment: Biogeochemical effects on algal biofilms. Ecotoxicol. Environ. Saf. 2019, 177, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.B.; Vis, M.L. Reference diatom assemblage response to restoration of an acid mine drainage stream. Ecol. Indic. 2013, 29, 234–245. [Google Scholar] [CrossRef]
- Hill, B.H.; Herlihy, A.T.; Kaufmann, P.R.; Stevenson, R.J.; McCormick, F.H.; Johnson, C.B. Use of periphyton assemblage data as an index of biotic integrity. J. N. Am. Benthol. Soc. 2000, 19, 50–67. [Google Scholar] [CrossRef]
- Zalack, J.T.; Smucker, N.J.; Vis, M.L. Development of a Diatom Index of biotic integrity for acid mine drainage impacted streams. Ecol. Indic. 2010, 10, 287–295. [Google Scholar] [CrossRef]
- Smucker, N.J.; Vis, M.L. Use of diatoms to assess agricultural and coal mining impacts on streams and a multiassemblage case study. J. N. Am. Benthol. Soc. 2009, 28, 659–675. [Google Scholar] [CrossRef] [Green Version]
- Van Dam, H.; Merten, A.; Sinkeldam, J. A coded checklist and ecological indicator values of freshwater diatoms from The Netherlands. Netherland J. Aquat. Ecol. 1994, 28, 117–133. [Google Scholar]
- Luís, A.T.; Novais, M.H.; Van de Vijver, B.; Almeida, S.F.P.; Ferreira da Silva, E.A.; Hoffmann, L.; Ector, L. Pinnularia aljustrelica sp. nov. (Bacillariophyceae), a new diatom species found in acidic waters in the Aljustrel mining area (Portugal), and further observations on the taxonomy, morphology and ecology of P. acidophila HOFMANN et KRAMMER and P. acoricola HUSTEDT. Fottea 2012, 12, 27–40. [Google Scholar]
- Ponader, K.C.; Potapova, M.G. Diatoms from the genus Achnanthidium in flowing waters of the Appalachian Mountains (North America): Ecology, distribution and taxonomic notes. Limnologica 2007, 37, 227–241. [Google Scholar] [CrossRef] [Green Version]
- Verb, R.G.; Vis, M.L. Comparison of benthic diatom assemblages from streams draining abandoned and reclaimed coal mines and nonimpacted sites. J. N. Am. Benthol. Soc. 2000, 19, 274–288. [Google Scholar] [CrossRef]
- Sabater, S. Diatom communities as indicators of environmental stress in the Guadiamar River, S.-W. Spain, following a major mine tailings spill. J. Appl. Phycol. 2000, 12, 113–124. [Google Scholar] [CrossRef]
- Peterson, C.G.; Stevenson, R.J. Resistance and resilience of lotic algal communities: Importance of disturbance timing and current. Ecology 1992, 73, 1445–1461. [Google Scholar] [CrossRef]
- Gerhardt, A.; Bisthoven, L.J.; Guhr, K.; Soares, A.M.V.M.; Pereira, M.J. Phytoassessment of acid mine drainage: Lemna gibba bioassay and diatom community structure. Ecotoxicology 2008, 17, 47–58. [Google Scholar] [CrossRef]
- Luís, A.T.; Grande, J.A.; Dávila, J.M.; Aroba, J.; Durães, N.; Almeida, S.F.P.; de la Torre, M.L.; Sarmiento, A.M.; Fortes, J.C.; Ferreira da Silva, E.; et al. Application of fuzzy logic tools for the biogeochemical characterisation of (un)contaminated waters from Aljustrel mining area (South Portugal). Chemosphere 2018, 211, 736–744. [Google Scholar] [CrossRef]
- Dong, X.; Jian, X.; Jiang, W.; Wu, N.; Tang, T.; Cai, Q. Development and testing of a diatom-based index of biotic integrity for river ecosystems impacted by acid mine drainage in Gaolan river, China. Fresen. Environ. Bull. 2015, 24, 4114–4124. [Google Scholar]
- Bray, J.P.; Broady, P.A.; Niyogi, D.K.; Harding, J.S. Periphyton communities in New Zealand streams impacted by acid mine drainage. Mar. Freshw. Res. 2008, 59, 1084–1091. [Google Scholar] [CrossRef]
- Jia, X.; Jiang, W.; Li, F.; Tang, T.; Duan, S.; Cai, Q. The response of benthic algae to the impact of acid mine drainage. Acta Ecol. Sin. 2009, 29, 4620–4629. [Google Scholar]
- Urrea-Clos, G.; Sabater, S. Comparative study of algal communities in acid and alkaline waters from Tinto, Odiel and Piedras river basins (SW Spain). Limnetica 2009, 28, 261–272. [Google Scholar]
- Aguilera, A. Eukaryotic organisms in extreme acid environments. Life 2013, 3, 363–374. [Google Scholar] [CrossRef] [Green Version]
- Rivera, M.J.; Santisteban, M.; Aroba, J.; Grande, J.A.; Dávila, J.M.; Sarmiento, A.M.; Fortes, J.C.; Diaz-Curiel, J.; Luís, A.T. Application of Fuzzy Logic Techniques for Biogeochemical Characterization of Dams Affected by Acid Mine Drainage (AMD) Processes in the Iberian Pyrite Belt (IPB), Spain. Water Air Soil Pollut. 2020, 231, 142. [Google Scholar] [CrossRef]
- Tiwari, A.; Marella, T.K. Potential and application of diatoms for industry-specific wastewater treatment. In Application of Microalgae in Wastewater Treatment; Gupta, S., Bux, F., Eds.; Springer: Cham, Germany, 2019; pp. 321–339. [Google Scholar]
- Marella, T.K.; Saxena, A.; Tiwari, A. Diatom mediated heavy metal remediation: A review. Bioresour. Technol. 2020, 305, 123068. [Google Scholar] [CrossRef] [PubMed]
- Morin, S.; Vivas-Nogues, M.; Duong, T.T.; Boudou, A.; Coste, M.; Delmas, F. Dynamics of benthic diatom colonization in a cadmium/zinc-polluted river (Riou-Mort, France). Fundam. Appl. Limnol. 2007, 168, 179–187. [Google Scholar] [CrossRef]
- Morin, S.; Cordonier, A.; Lavoie, I.; Arini, A.; Blanco, S.; Duong, T.T.; Tornés, E.; Bonet, B.; Corcoll, N.; Faggiano, L.; et al. Consistency in diatom response to metal-contaminated environments. In Handbook of Environmental Chemistry, Emerging and Priority Pollutants in Rivers; Guasch, H., Ginebreda, A., Geiszinger, A., Eds.; Springer: Heidelberg, Germany, 2012; pp. 117–146. [Google Scholar]
- Falasco, E.; Bona, F.; Badino, G.; Hoffmann, L.; Ector, L. Diatom teratological forms and environmental alterations: A review. Hydrobiologia 2009, 623, 1–35. [Google Scholar] [CrossRef]
- Gold, C.; Feurtet-Mazel, A.; Coste, M.; Boudou, A. Effects of cadmium stress on periphytic diatom communities in indoor artificial streams. Freshw. Biol. 2003, 48, 316–328. [Google Scholar] [CrossRef]
- Hernández-Ávila, J.; Salinas-Rodríguez, E.; Cerecedo-Sáenz, E.; Reyes-Valderrama, I.; Arenas-Flores, A.; Román-Gutiérrez, A.D.; Rodríguez-Lugo, V. Diatoms and their capability for heavy metal removal by cationic exchange. Metals 2017, 7, 169. [Google Scholar] [CrossRef]
- Ferreira da Silva, E.F.; Almeida, S.F.P.; Nunes, M.L.; Luís, A.T.; Borg, F.; Hedlund, M.; Marques de Sá, C.; Patinha, C.; Teixeira, P. Heavy metal pollution downstream the abandoned Coval da Mó mine (Portugal) and associated effects on epilithic diatom communities. Sci. Total Environ. 2009, 407, 5620–5636. [Google Scholar] [CrossRef]
- Stewart, P.M.; Smith, E.P.; Cairns-Jr, J. Relationship of the physicochemical environment to diatom and protozoan communities: A multivariate approach. Arch. Für Protistenkd. 1987, 134, 331–341. [Google Scholar] [CrossRef]
- Cattaneo, A.; Couillard, Y.; Wunsam, S.; Courcelles, M. Diatom taxonomic and morphological changes as indicators of metal pollution and recovery in Lac Dufault (Québec, Canada). J. Paleolimnol. 2004, 32, 163–175. [Google Scholar] [CrossRef]
- Coste, M.; Boutry, S.; Tison-Rosebery, J.; Delmas, F. Improvements of the Biological Diatom Index (BDI): Description and efficiency of the new version (BDI-2006). Ecol. Indic. 2009, 9, 621–650. [Google Scholar] [CrossRef]
- Amaral-Zettler, L.A.; Gomez, F.; Zettler, E.; Keenan, B.G.; Amils, R.; Sogin, M.L. Eukaryotic diversity in Spain’s River of Fire. Nature 2002, 417, 137. [Google Scholar] [CrossRef]
- Aguilera, A.; Souza-Egipsy, V.; Gonzalez-Toril, E. La vida en Río Tinto; Centro de Astrobiología-CSIC-INTA, Ministerio de Defensa: Madrid, Spain, 2000; p. 100. [Google Scholar]
- Verb, R.G.; Vis, M.L. Macroalgal communities from an acid mine drainage impacted watershed. Aquat. Bot. 2001, 71, 93–107. [Google Scholar] [CrossRef]
- Niyogi, D.K.; Lewis, W.M., Jr.; McKnight, D.M. Effects of stress from mine drainage on diversity, biomass, and function of primary producers in mountain streams. Ecosystems 2002, 5, 554–567. [Google Scholar]
- Novis, P.M. Taxonomy of Klebsormidium (Klebsormidiales, Charophyceae) in New Zealand streams and the significance of low-pH habitats. Phycologia 2006, 45, 293–301. [Google Scholar] [CrossRef]
- Dean, A.P.; Hartley, A.; McIntosh, O.A.; Smith, A.; Feord, H.K.; Holmberg, N.H.; King, T.; Yardley, E.; White, K.N.; Pittman, J.K. Metabolic adaptation of a Chlamydomonas acidophila strain isolated from acid mine drainage ponds with low eukaryotic diversity. Sci. Total Environ. 2019, 647, 75–87. [Google Scholar] [CrossRef]
- Díaz, S.; de Francisco, P.; Olsson, S.; Aguilera, A.; González-Toril, E.; Martín, A.M. Toxicity, physiological, and ultrastructural effects of Arsenic and Cadmium on the extremophilic microalga Chlamydomonas acidophila. Int. J. Environ. Res. Public Health 2020, 17, 1650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aguilera, A.; Suominen, S.; Pétursdóttir, S.; Olgudóttir, E.; Guðmundsdóttir, E.E.; Altamirano, M.; González-Toril, E.; Hreggviðsson, G.O. Physiological plasticity of high-temperature intertidal cyanobacterial microbial mats to temperature and salinity: Daily and seasonal in situ photosynthetic performance. Eur. J. Phycol. 2020, 55, 223–233. [Google Scholar] [CrossRef]
- Stevens, A.E.; McCathy, B.C.; Vis, M.L. Metal content of Klebsormidium-dominated (Chlorophyta) algal mats from acid mine drainage waters in southeastern. J. Torrey Bot. Soc. 2001, 128, 226–233. [Google Scholar] [CrossRef]
- Vinebrooke, R.D. Abiotic and biotic regulation of periphyton in recovering acidified lakes. J. N. Am. Benthol. Soc. 1996, 15, 318–331. [Google Scholar] [CrossRef]
- Kalin, M.; Wheeler, W.N.; Olaveson, M.M. Response of phytoplankton to ecological engineering remediation of a Canadian Shield Lake affected by acid mine drainage. Ecol. Eng. 2006, 28, 296–310. [Google Scholar] [CrossRef]
- Novis, P.M. A taxonomic survey of microspora (Chlorophyceae, Chlorophyta) in New Zealand. N. Z. J. Bot. 2004, 42, 153–165. [Google Scholar] [CrossRef]
- Aguilera, A.; González-Toril, E. Eukaryotic life in extreme environments: Acidophilic fungi. In Fungi in Extreme Environments: Ecological Role and Biotechnological Significance; Tiquia-Arashiro, S.M., Grube, M., Eds.; Springer: NY city, NY, USA, 2019; pp. 21–38. [Google Scholar]
- López-Archilla, A.; González, A.E.; Terrón, M.C.; Amils, R. Ecological study of the fungal populations of the acidic Tinto River in Southwestern Spain. Can. J. Microbiol. 2004, 50, 923–934. [Google Scholar] [CrossRef] [PubMed]
- Duran, C.; Marin, I.; Amils, R. Specific metal sequestering acidophilic fungi. In Biohydrometallurgy and the Environment; Amils, R., Ballester, A., Eds.; Towards the Mining of the 21st Century, Proc. Int. Biohydrometal Symp; Elsevier: Amsterdam, The Netherlands, 1999; pp. 521–530. [Google Scholar]
- Oggerin, M.; Tornos, F.; Rodríguez, N.; del Moral, C.; Sánchez-Román, M.; Amils, R. Specific jarosite biomineralization by Purpureocillium lilacinum, an acidophilic fungus isolated from Río Tinto. Environ. Microbiol. 2013, 15, 2228–2237. [Google Scholar] [CrossRef] [PubMed]
- López-Archilla, A.I. Rio Tinto: Un universo de mundos microbianos. Ecosistemas 2005, 14, 52–65. [Google Scholar]
- Wright, I.A.; Paciuszkiewicz, K.; Belmer, N. Increased Water Pollution After Closure of Australia’s Longest Operating Underground Coal Mine: A 13-Month Study of Mine Drainage, Water Chemistry and River Ecology. Water Air Soil Pollut. 2018, 229, 55. [Google Scholar] [CrossRef]
- St. Louiss, V.L. Element concentrations in chironomids and their abundance in the littoral zone of acidified lakes in Northwestern Ontario. J. Fish. Aquat. Sci. 1993, 50, 953–963. [Google Scholar] [CrossRef]
- Deneke, R. Review of rotifers and crustaceans in highly acidic environments of pH values < 3. Hydrobiologia 2000, 433, 167–172. [Google Scholar]
- Amaral-Zettler, L.A. Eukaryotic diversity at pH extremes. Front. Microbiol. 2013, 3, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gower, A.M.; Myers, G.; Kent, M.; Foulkes, M.E. Relationships between macroinvertebrate communities and environmental variables in metal-contaminated streams in south-West England. Freshw. Biol 1994, 32, 199–221. [Google Scholar] [CrossRef]
- de Jonge, M.; de Vijuer, B.V.; Blust, R.; Bervoets, L. Responses of aquatic organisms to metal pollution in a lowland river in Flanders: A comparison of diatoms and macroinvertebrates. Sci. Total Environ. 2008, 407, 615–629. [Google Scholar] [CrossRef] [PubMed]
- Loayza-Muro, R.A.; Elias-Letts, R.; Marticorena-Ruiz, J.K.; Palomino, E.J.; Duivenvoorden, J.F.; Kraak, M.H.S.; Admiraal, W. Metal-induced shifts in benthic macroinvertebrate community composition in Andean high altitude streams. Environ. Toxicol. Chem. 2010, 29, 2761–2768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loayza-Muro, R.A.; de Baat, M.L.; Palomino, E.J.; Kuperus, P.; Kraak, M.H.S.; Admiraal, W.; Breeuwer, J.A.J. Metals and altitude drive genetic diversity of chironomids in Andean streams. Freshw. Biol. 2014, 59, 56–63. [Google Scholar] [CrossRef]
- Loayza-Muro, R.A.; Duivenvoorden, J.F.; Kraak, M.H.S.; Admiraal, W. 2014b Metal leaching, acidity, and altitude confine benthic macroinvertebrate community composition in Andean streams. Environ. Toxicol. Chem. 2014, 33, 404–411. [Google Scholar] [CrossRef]
- Beltman, D.J.; Clements, W.H.; Lipton, J.; Cacela, D. Benthic invertebrate metals exposure, accumulation and community-level effects downstream from a hard rock mine site. Environ. Toxicol. Chem. 1999, 18, 299–307. [Google Scholar] [CrossRef]
- Gerhardt, A.L.; Bisthoven, J.; Soares, A.M.V.M. Macroinvertebrate response to acid mine drainage: Community metrics and on-line behavioural toxicity bioassay. Environ. Pollut. 2004, 130, 263–274. [Google Scholar] [CrossRef]
- Canfield, T.J.; Kemble, N.E.; Brumbaugh, W.G.; Dwyer, F.J.; Ingersoll, C.G.; Fairchild, J.F. Use of benthic invertebrate community structure and the sediment quality triad to evaluate metal-contaminated sediment in the upper Clark Fork River, Montana. Environ. Toxicol. Chem. 1994, 13, 1999–2012. [Google Scholar] [CrossRef]
- Bisthoven, L.J.; Gerhardt, A. Chironomidae (Diptera, Nematocera) fauna in three small streams of Skania, Sweden. Environ. Monit. Assess. 2003, 83, 89–102. [Google Scholar] [CrossRef]
- Bisthoven, J.L.; Gerhardt, A.; Soares, A.M.V.M. Effects of Acid Mine Drainage on larval Chironomus (Diptera, Chironomidae) measured with the Multispecies Freshwater Biomonitor. Environ. Toxicol. Chem. 2004, 23, 1123–1128. [Google Scholar] [CrossRef]
- De Haas, E.M.; van Haaren, R.; Koelmans, A.A.; Kraak, M.H.S.; Admiraal, W. Analyzing the causes for the persistence of chironomids in floodplain lake sediments. Arch. Hydrobiol. 2005, 162, 211–228. [Google Scholar] [CrossRef]
- Löhr, A.J.; Sluik, R.; Olaveson, M.M.; Ivorra, N.; van Gestel, C.A.M.; van Straalen, N.M. Macroinvertebrate and algal communities in an extremely acidic river and the Kawah Ijen crater lake (pH < 0.3), Indonesia. Arch. Hydrobiol. 2006, 165, 1–21. [Google Scholar]
- Hamerlík, L.; Jacobsen, D. Chironomid (Diptera) distribution and diversity in Tibetan streams with different glacial influence. Insect Conserv. Divers. 2011, 5, 319–326. [Google Scholar] [CrossRef]
- Loayza-Muro, R.A.; Marticorena-Ruiz, J.K.; Palomino, E.J.; Merritt, C.; De Baat, M.L.; van Gemert, M.; Verweij, R.A.; Kraak, M.H.S.; Admiraal, W. Persistence of chironomids in metal polluted Andean high altitude streams: Does melanin play a role? Environ. Sci. Technol. 2013, 47, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Groenendijk, D.; Lücker, S.M.G.; Plans, M.; Kraak, M.H.S.; Admiraal, W. Dynamics of metal adaptation in riverine chironomids. Environ. Pollut. 2002, 117, 101–109. [Google Scholar] [CrossRef]
- Van Straalen, N.M.; Donker, M.H.; Vijver, M.G.; van Gestel, C.A.M. Bioavailability of contaminants estimated from uptake rates into soil invertebrates. Environ. Pollut. 2005, 136, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Buchwalter, D.B.; Cain, D.J.; Martin, C.A.; Xie, L.; Luoma, S.N.; Garland, T. Aquatic insect ecophysiological traits reveal phylogenetically based differences in dissolved cadmium susceptibility. Proc. Natl. Acad. Sci. USA 2008, 105, 8321–8326. [Google Scholar] [CrossRef] [Green Version]
- Sibly, R.M.; Calow, P. A life-cycle theory of responses to stress. Biol. J. Linn. Soc. 1989, 37, 101–116. [Google Scholar] [CrossRef]
- Michailova, P.; Ilkova, J.; Kerr, R.; White, K. Chromosome variability in Chironomus acidophilus Keyl, 1960 from the Afon Goch, UK—A river subject to long-term trace metal pollution. Aquat. Insects 2009, 31, 213–225. [Google Scholar] [CrossRef]
- Martinez, E.A.; Moore, B.C.; Schaumloffel, J.; Dasgupta, N. Morphological abnormalities in Chironomus tentans exposed to cadmium and copper-spiked sediments. Ecotoxicol. Environ. Saf. 2003, 55, 204–212. [Google Scholar] [CrossRef]
- di Veroli, A.; Goretti, E.; Paumen, M.L.; Kraak, M.H.S.; Admiraal, W. Induction of mouthpart deformities in chironomid larvae exposed to contaminated sediments. Environ. Pollut. 2012, 166, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Warwick, W.F. Morphological deformities in Chironomidae (Diptera) larvae as biological indicators of toxic stress. In Toxic Contaminants and Ecosystem Health: A Great Lakes Focus; Evans, M.S., Ed.; Wiley: New York, NY, USA, 1988; pp. 281–320. [Google Scholar]
- Warwick, W.F. Indexing deformities in ligulae and antennae of Procladius larvae (Diptera: Chironomidae): Application to contaminant-stressed environments. J. Fish. Aquat. Sci. 1991, 48, 1151–1166. [Google Scholar] [CrossRef]
- Beghelli, F.G.D.; Lopez-Doval, J.C.; Rosa, A.H.; Pompeo, M.; Carlos, V.M. Lethal and sublethal effects of metal-polluted sediments on Chironomus sancticaroli Strixino and Strixino, 1981. Ecotoxicology 2018, 27, 286–299. [Google Scholar] [CrossRef] [PubMed]
- di Veroli, A.; Santoro, F.; Pallottini, M.; Selvaggi, R.; Scardazza, F.; Cappelletti, D.; Goretti, E. Deformities of chironomid larvae and heavy metal pollution: From laboratory to field studies. Chemosphere 2014, 112, 9–17. [Google Scholar] [CrossRef]
- Martinez, E.A.; Moore, B.C.; Schaumloffel, J.; Dasgupta, N. Effects of exposure to a combination of zinc- and lead-spiked sediments on mouthpart development and growth in Chironomus tentans. Environ. Toxicol. Chem. 2004, 23, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Schlief, J.; Mutz, M. Palatability of leaves conditioned in streams affected by mine drainage: A feeding experiment with Gammarus pulex (L.). Hydrobiologia 2006, 563, 445–452. [Google Scholar] [CrossRef]
- Aluma, E.; Johnson, K.S.; Hassett, P. Mercury Bioaccumulation in Crayfish in Acid Mine-Impaired Appalachian Streams. Water Air Soil Pollut. 2017, 228, 200. [Google Scholar] [CrossRef]
- He, F.; Jiang, W.; Tang, T.; Cai, Q. Assessing impact of acid mine drainage on benthic macroinvertebrates: Can functional diversity metrics be used as indicators? J. Freshw. Ecol. 2015, 30, 513–524. [Google Scholar] [CrossRef] [Green Version]
- Luís, A.T.; Grande, J.A.; Durães, N.; Dávila, J.M.; Santisteban, M.; Almeida, S.F.P.; Sarmiento, A.M.; de la Torre, M.L.; Fortes, J.C.; Ferreira da Silva, E. Biogeochemical characterization of surface waters in the Aljustrel mining area (South Portugal). Environ. Geochem. Health 2019, 211, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Chacon-Baca, E.; Santos, A.; Sarmiento, A.M.; Luís, A.T.; Santisteban, M.; Fortes, J.C.; Dávila, J.M.; Curiel, J.; Grande, J.A. Acid Mine Drainage as energizing microbial niches for the formation of clastic iron stromatolites: The Tintillo river in SW Spain. Astrobiology 2021, 21, 443–463. [Google Scholar] [CrossRef] [PubMed]


| Species Name | pH Tolerance Range | Optimum pH | Metal Concentrations |
|---|---|---|---|
| Pinnularia aljustrelica | 2.0–5.0 | 2.0–3.0 | Fe 1300 to 6000 Cu 230–350 Zn 118–170 |
| Pinnularia acidophila | 2.0–4.5 | 2.0–2.2 | |
| Pinnularia acoricola | 2.0–6.0 | 2.0–3.0 | |
| Nitzschia thermalis | 2.0–7.0 | 3.0 | |
| Nitzschia hantzschiana | 2.0–6.8 | 2.0–2.2 | |
| Eunotia exigua | 3.0–5.0 | 3.0 | Similar metal concentrations as above, but species valves are morphologically affected by metals (teratologies) |
| Brachysira vitrea | 4.5–7.5 | 4.8 | Fe 1100 Zn 0.30 Cu 0.64 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luís, A.T.; Córdoba, F.; Antunes, C.; Loayza-Muro, R.; Grande, J.A.; Silva, B.; Diaz-Curiel, J.; Ferreira da Silva, E. Extremely Acidic Eukaryotic (Micro) Organisms: Life in Acid Mine Drainage Polluted Environments—Mini-Review. Int. J. Environ. Res. Public Health 2022, 19, 376. https://doi.org/10.3390/ijerph19010376
Luís AT, Córdoba F, Antunes C, Loayza-Muro R, Grande JA, Silva B, Diaz-Curiel J, Ferreira da Silva E. Extremely Acidic Eukaryotic (Micro) Organisms: Life in Acid Mine Drainage Polluted Environments—Mini-Review. International Journal of Environmental Research and Public Health. 2022; 19(1):376. https://doi.org/10.3390/ijerph19010376
Chicago/Turabian StyleLuís, Ana Teresa, Francisco Córdoba, Catarina Antunes, Raul Loayza-Muro, José Antonio Grande, Bruna Silva, Jesus Diaz-Curiel, and Eduardo Ferreira da Silva. 2022. "Extremely Acidic Eukaryotic (Micro) Organisms: Life in Acid Mine Drainage Polluted Environments—Mini-Review" International Journal of Environmental Research and Public Health 19, no. 1: 376. https://doi.org/10.3390/ijerph19010376





