Application of the ARIMA Model to Predict Under-Reporting of New Cases of Hansen’s Disease during the COVID-19 Pandemic in a Municipality of the Amazon Region
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Data Source
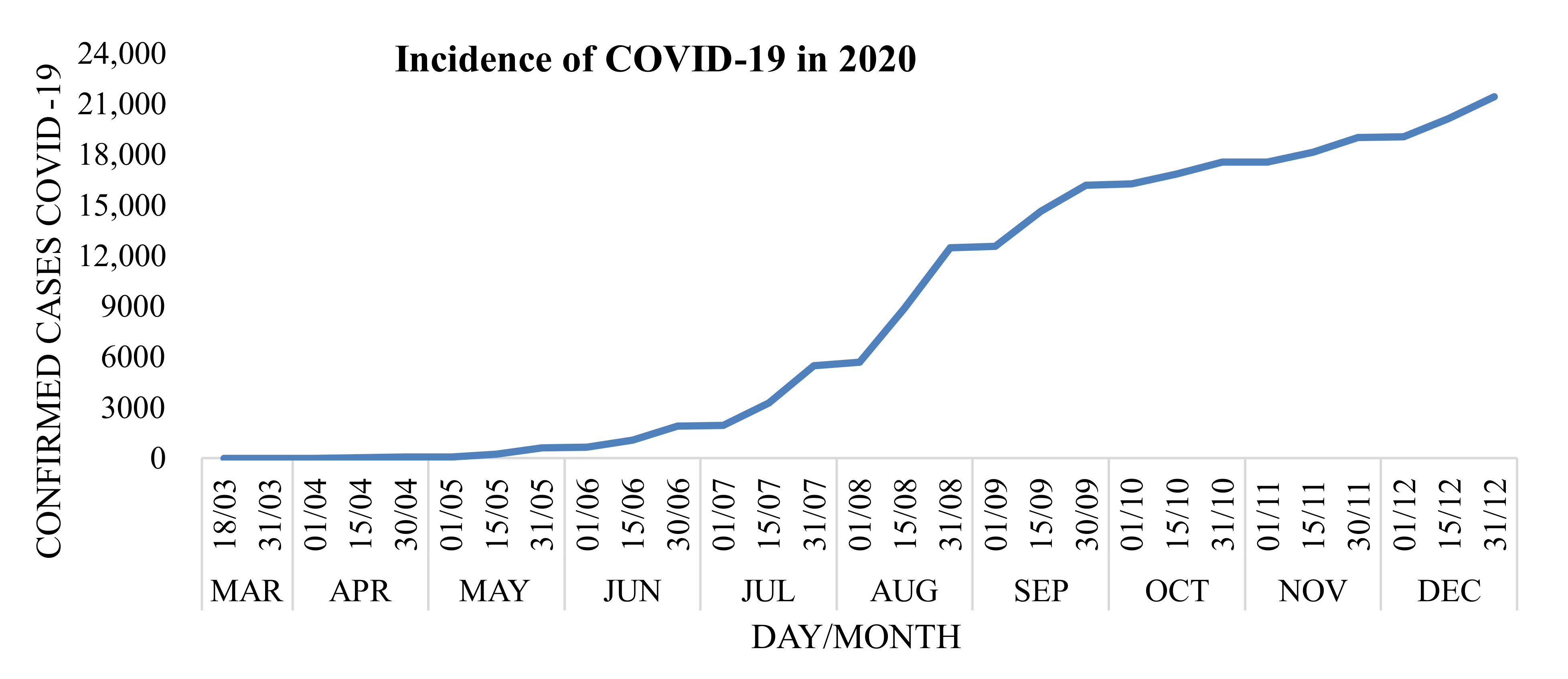
2.3. Exploratory Analysis
- Annual detection coefficient of new cases per 100,000 inhabitants, which assesses the magnitude of the disease and estimates the risk of the occurrence of new cases;
- Annual detection coefficient of new cases in people aged 0–14 years per 100,000 people, which measures the strength of recent transmission;
- Proportion of cases with grade II physical disability among the new cases detected in the year, which estimates the capacity for the early detection and the hidden endemic;
- Proportion of cured cases among new cases in the cohort years, which assesses the quality of attendance and treatment effectiveness.
2.4. Predictive Model
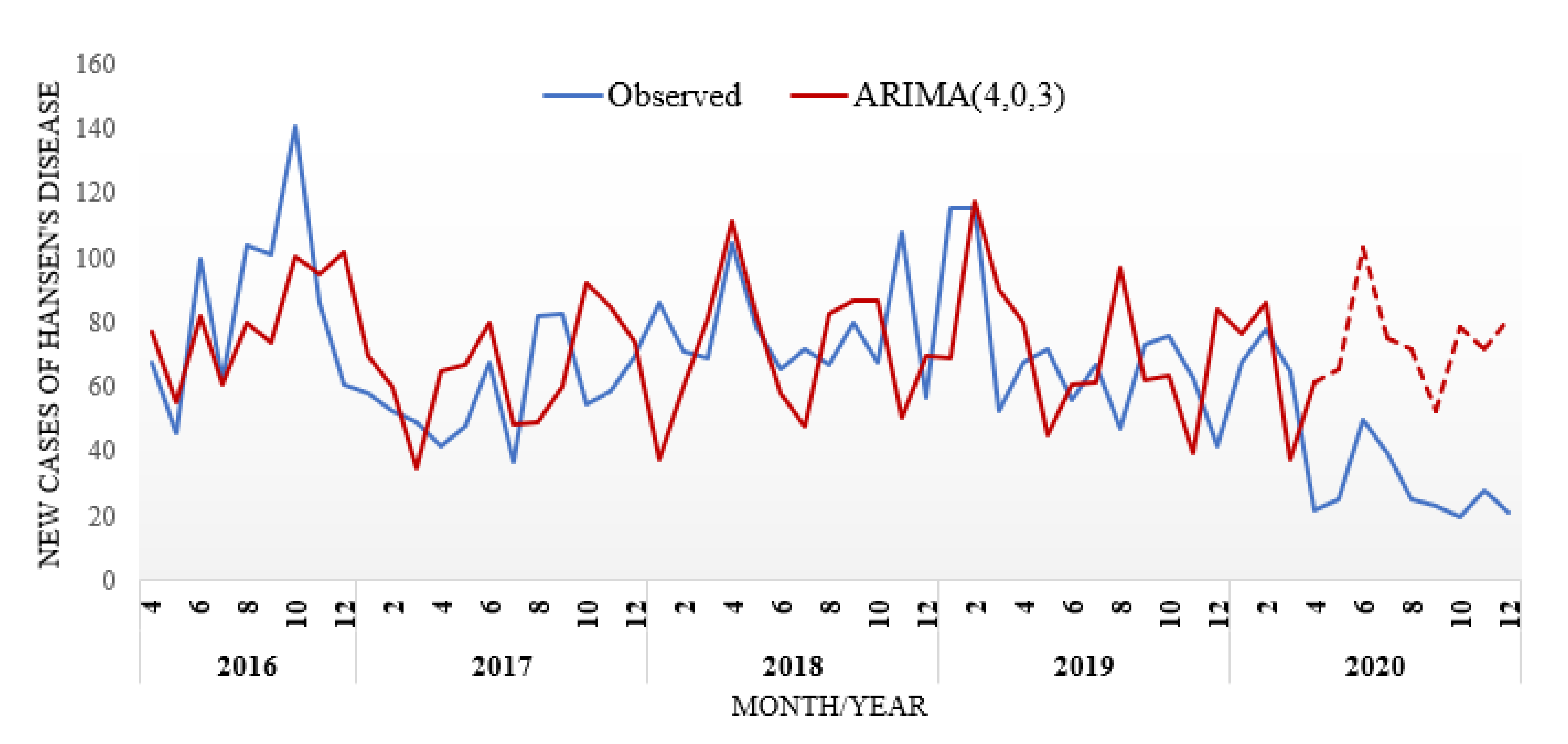
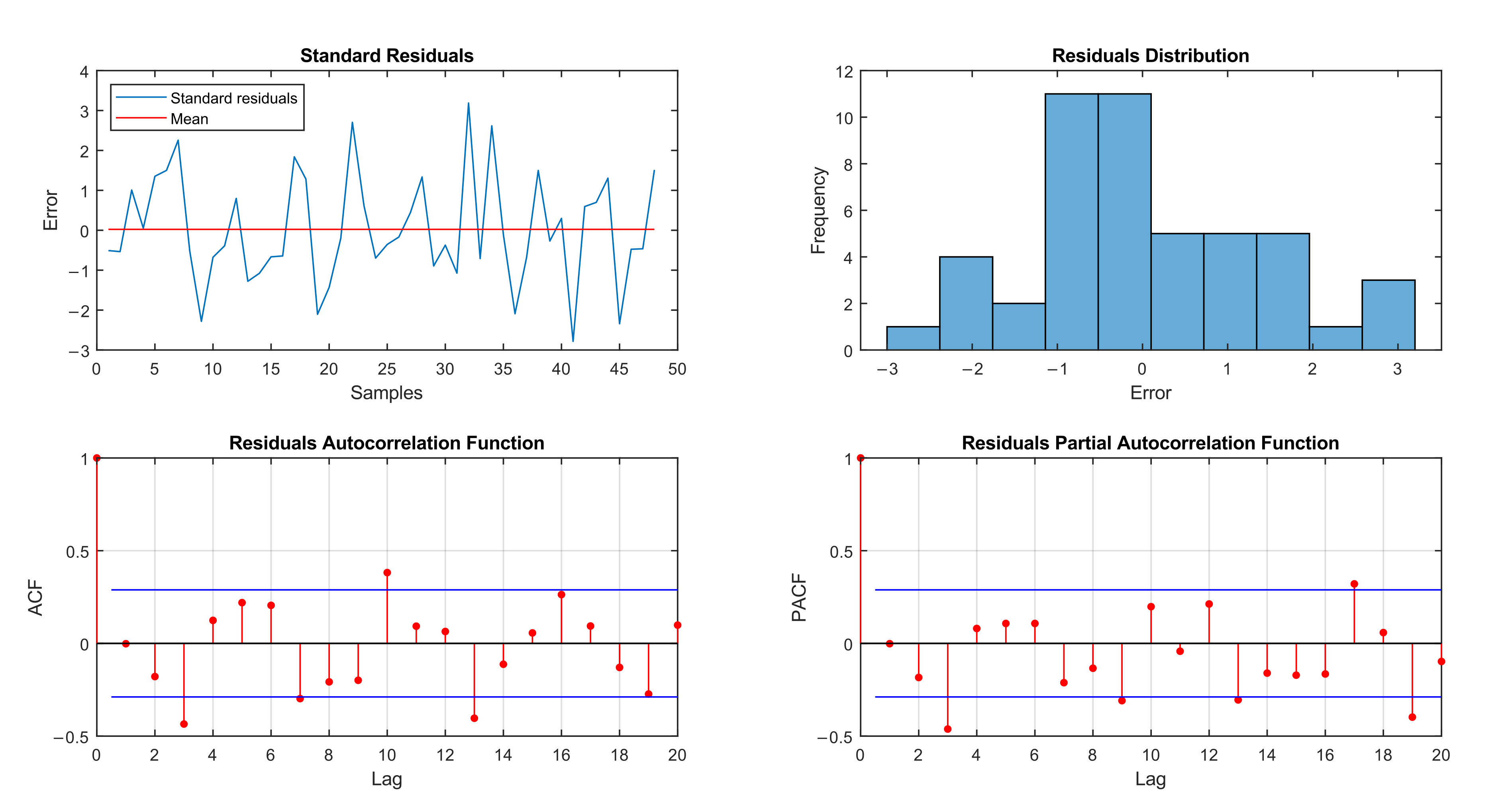
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- World Health Organization. Guidelines for the Diagnosis, Treatment and Prevention of Leprosy; WHO: New Delhi, India, 2019. [Google Scholar]
- World Health Organization. Leprosy (Hansen’s Disease). 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/leprosy (accessed on 15 September 2021).
- Centers for Disease Control and Prevention—CDC. Hansen’s Disease (Leprosy). 2017. Available online: https://www.cdc.gov/leprosy/index.html (accessed on 15 September 2021).
- Ministry of Health (Brazil). Epidemiological Bulletin—Hansen’s Disease 2021; Ministry of Health: Brasília, Brazil, 2021. Available online: https://www.gov.br/saude/pt-br/media/pdf/2021/fevereiro/12/boletim-hanseniase-_-25-01.pdf (accessed on 14 March 2021).
- Ministry of Health (Brazil). Epidemiological Situation and Hansen’s Disease. Anexo 3-Anexo 3—Epidemiological And Operational Indicators Of Hansen’s Disease, By States and Regions, Brazil, 2020; Ministry of Health: Brasília, Brazil, 2021. Available online: http://www.aids.gov.br/pt-br/hanseniase/situacao-epidemiologica (accessed on 15 July 2021).
- Monteiro, L.D.; Lopes, L.S.O.; Dos Santos, P.R.; Rodrigues, A.L.M.; Bastos, W.M.; Barreto, J.A. Tendências da hanseníase após implementação de um projeto de intervenção em uma capital da Região Norte do Brasil, 2002–2016. Cad. Saúde Pública 2018, 34, e00007818. [Google Scholar] [CrossRef] [PubMed]
- Aleves, L.; State of Tocantins. Tocantins Registers 1st Confirmed Case of COVID-19. Available online: https://www.to.gov.br/noticias/tocantins-registra-1o-caso-confirmado-do-covid-19/6edmfivjmco1 (accessed on 15 July 2021).
- City Hall of Palmas; Palmas Health Department. Official Information Page of COVID-19 Surveillance Actions in the City of Palmas. Available online: https://coronavirus.palmas.to.gov.br/ (accessed on 15 July 2021).
- Oliveira, W.K.; Duarte, E.; França, G.V.A.; Garcia, L.P. How Brazil can Contain COVID-19. Epidemiol. Health Serv. 2020, 28. [Google Scholar] [CrossRef]
- Cruz, A. For a Response to the Crisis That Guarantees the Right to Dignity, It Is Necessary to Raise Your Voices. The Teachings of the Fight against Hansen’s Disease to Face the COVID-19. 2020, pp. 6–12. Available online: http://www.morhan.org.br/views/upload/JPGS_Morhan/imagens_site/CadMorhanCOVIDportuguesFINAL.pdf (accessed on 10 April 2021).
- Brazilian Society of Hansenology. Ministry of Health Warns that There Will be A Lack of Medicines for Leprosy in Brazil. 2020. Available online: http://www.sbhansenologia.org.br/noticia/ministerio-da-saude-alerta-que-faltara-medicamentos-para-hanseniase-no-brasil (accessed on 10 April 2021).
- Brandão, P.S. People Affected by Leprosy and the COVID19 Pandemic: Reflections on the Context. 2020, pp. 25–30. Available online: http://www.morhan.org.br/views/upload/JPGS_Morhan/imagens_site/CadMorhanCOVIDportuguesFINAL.pdf (accessed on 10 July 2021).
- IBGE|Portal Do IBGE|IBGE Palmas (TO)|Cities and States|IBGE. Available online: https://www.ibge.gov.br/cidades-e-estados/to/palmas.html (accessed on 14 January 2021).
- Palmas Municipal Health Department (Tocantins/Brazil). Official Gazette of the Municipality of Palmas: Ordinance nº 518, de June 14, 2016. Palmas Municipal Health Department: Tocantins, Brazil. Available online: http://diariooficial.palmas.to.gov.br (accessed on 10 July 2021).
- Zheng, A.; Fang, Q.; Zhu, Y.; Jiang, C.; Jin, F.; Wang, X. An application of ARIMA model for predicting total health expenditure in China from 1978–2022. J. Glob. Health 2020, 10, 10803. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, S.I.; Aljamaan, I.A.; Al-Fakih, E.A. Forecasting the spread of the COVID-19 pandemic in Saudi Arabia using ARIMA prediction model under current public health interventions. J. Infect. Public Health 2020, 13, 914–919. [Google Scholar] [CrossRef] [PubMed]
- Sato, R.C. Gerenciamento de doenças utilizando séries temporais com o modelo ARIMA. Einstein 2013, 11, 128–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schoenberg, R. Constrained Maximum Likelihood. Comput. Econ. 1997, 10, 251–266. [Google Scholar] [CrossRef]
- Sumi, A.; Kamo, K.-I.; Ohtomo, N.; Mise, K.; Kobayashi, N. Time Series Analysis of Incidence Data of Influenza in Japan. J. Epidemiol. 2011, 21, 21–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheung, Y.-W.; Lai, K.S. Lag Order and Critical Values of the Augmented Dickey-Fuller Test. J. Bus. Econ. Stat. 1995, 13, 277–280. [Google Scholar] [CrossRef]
- Ismail, L.; Materwala, H.; Znati, T.; Turaev, S.; Khan, M.A. Tailoring time series models for forecasting coronavirus spread: Case studies of 187 countries. Comput. Struct. Biotechnol. J. 2020, 18, 2972–3206. [Google Scholar] [CrossRef] [PubMed]
- Akaike, H. A New Look at the Statistical Model Identification. IEEE Trans. Autom. Control 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Schwarz, G. Estimating the Dimension of a Model. Ann. Stat. 1978, 6, 461–464. [Google Scholar] [CrossRef]
- Box, G.E.P.; Jenkins, G.M.; Reinsel, G.C. Time Series Analysis: Forecasting and Control, 3rd ed.; Prentice Hall: Englewood Cliffs, NJ, USA, 1994. [Google Scholar]
- SBH–Brazilian Society of Hanseology. Guidelines for SBH Doctors on the Possibility of Coinfection Hansen’s Disease and COVID-19. Published 19-03-2020. March 2020. Available online: http://www.sbhansenologia.org.br/noticia/orientacoes-aos-medicos-da-sociedade-brasileira-de-hansenologia-sobre-a-possibilidade-de-coinfeccao-hanseniase-e-covid-19 (accessed on 10 March 2021).
- Ministry of Health (Brazil). Hansen’s Disease: What Is Hansen’s Disease? Ministry of Health: Brasília, Brazil, 2021. Available online: https://www.gov.br/saude/pt-br/assuntos/saude-de-a-a-z/h/hanseniase (accessed on 15 July 2021).
- Pires, C.A.A.; Malcher, C.M.S.R.; Júnior, J.M.C.A.; De Albuquerque, T.G.; Corrêa, I.R.S.; Daxbacher, E.L.R. Hanseníase em menores de 15 anos: A importância do exame de contato. Rev. Paul. Pediatr. 2012, 30, 292–295. [Google Scholar] [CrossRef] [Green Version]
- Monteiro, L.D.; Mello, F.R.M.; Miranda, T.P.; Heukelbach, J. Hanseníase em menores de 15 anos no estado do Tocantins, Brasil, 2001-2012: Padrão epidemiológico e tendência temporal. Rev. Bras. Epidemiol. 2019, 22, e190047. [Google Scholar] [CrossRef] [PubMed]
- Schneider, P.B.; Freitas, B. Tendência da hanseníase em menores de 15 anos no Brasil, 2001–2016. Cad. Saúde Pública 2018, 34, e00101817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujishima, M.A.; Lemos, L.X.D.O.; De Matos, H.J. Distribuição espacial da hanseníase em menores de 15 anos de idade, no município de Belém, estado do Pará, Brasil. Rev. Pan-Amaz. Saúde 2020, 11, 9. [Google Scholar] [CrossRef]
- Barreto, J.; Bisanzio, D.; Guimarães, L.D.S.; Spencer, J.; Vazquez-Prokopec, G.M.; Kitron, U.; Salgado, C.G. Spatial Analysis Spotlighting Early Childhood Leprosy Transmission in a Hyperendemic Municipality of the Brazilian Amazon Region. PLoS Negl. Trop. Dis. 2014, 8, e2665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Global Strategy of Hansen’s Disease 2021–2030— “Towards Zero Hansen’s Disease”. 2021. Available online: https://www.who.int/pt/publications/i/item/9789290228509 (accessed on 16 July 2021).
- Basso, M.E.M.; Andrade, R.F.; da Silva, R.L.F. Tendency of epidemiological indicators of leprosy in an endemic state of the Amazon region. Rev. Gaúcha Enferm. 2021, 42, e20190520. [Google Scholar] [CrossRef] [PubMed]
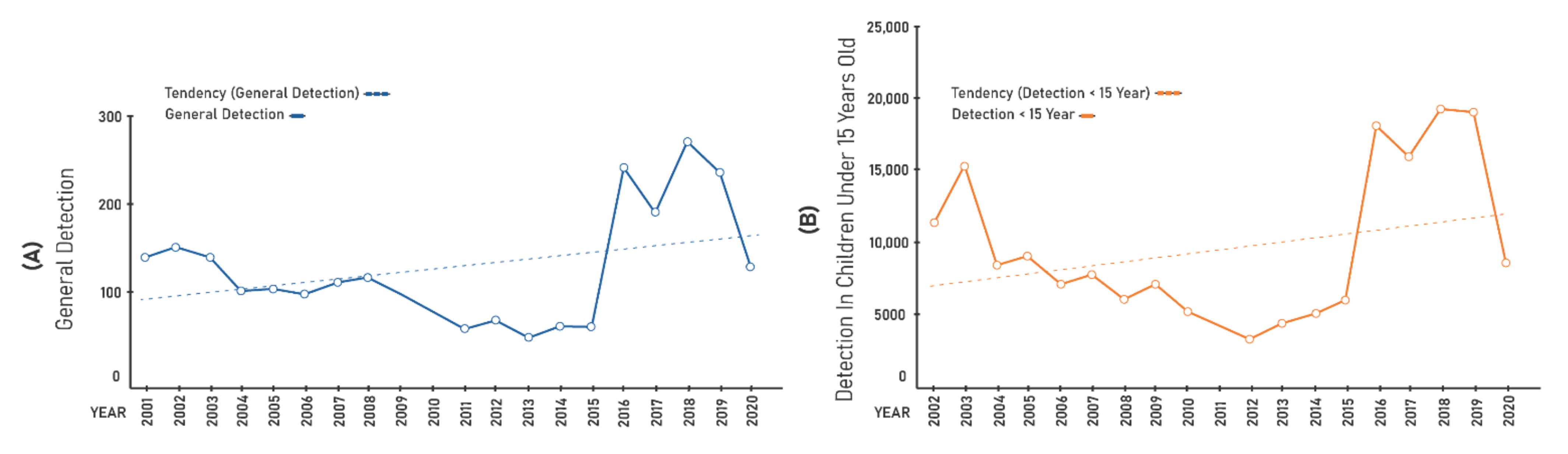
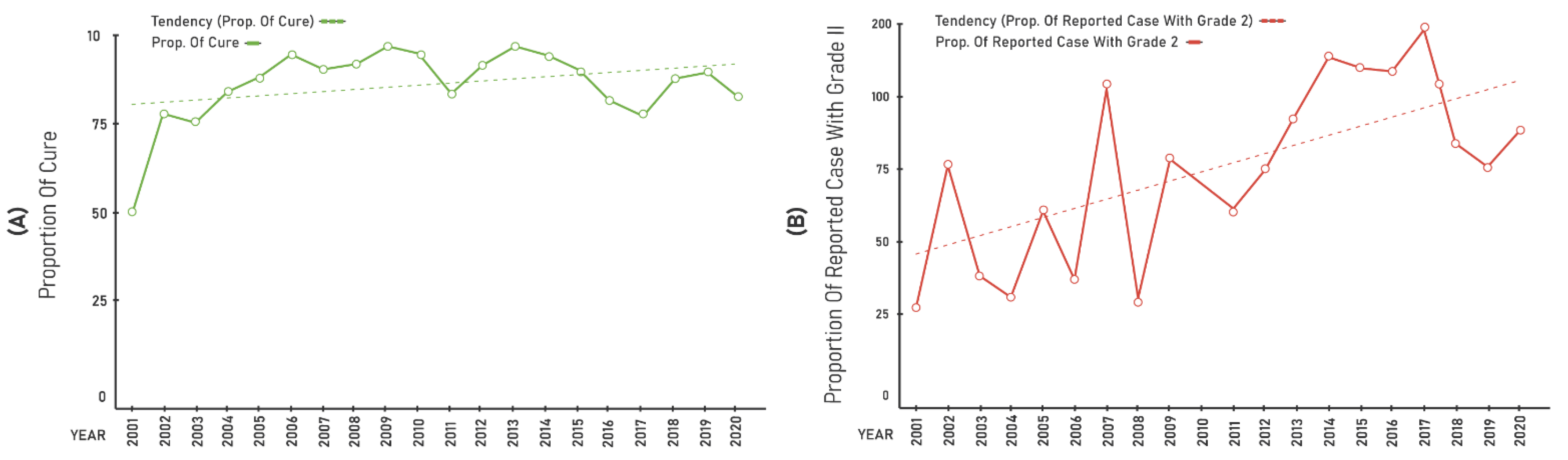
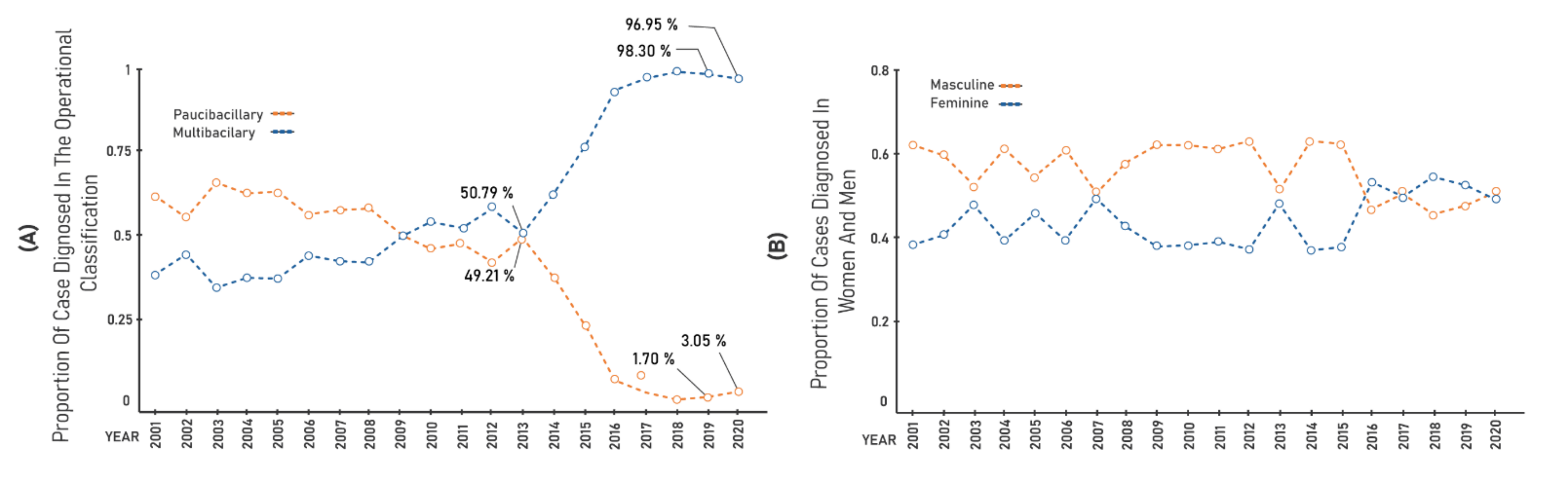

| Years | Gen.Det | <15 Years | Cure | Grade II | % PB | % MB | % F | % M |
|---|---|---|---|---|---|---|---|---|
| 2001 | 139.18 | 18.56 | 50.00 | 2.20 | 62% | 38% | 38% | 62% |
| 2002 | 150.80 | 11.23 | 77.68 | 6.13 | 56% | 44% | 40% | 60% |
| 2003 | 138.81 | 15.31 | 75.18 | 3.07 | 66% | 34% | 48% | 52% |
| 2004 | 101.26 | 8.37 | 83.87 | 2.46 | 63% | 37% | 39% | 61% |
| 2005 | 102.80 | 9.02 | 87.88 | 4.88 | 63% | 37% | 46% | 54% |
| 2006 | 97.33 | 7.07 | 94.29 | 2.95 | 56% | 44% | 39% | 61% |
| 2007 | 109.87 | 7.73 | 90.34 | 8.26 | 58% | 42% | 49% | 51% |
| 2008 | 115.75 | 6.02 | 91.59 | 2.48 | 58% | 42% | 42% | 58% |
| 2009 | 98.07 | 7.12 | 96.58 | 6.28 | 51% | 49% | 38% | 62% |
| 2010 | 77.08 | 5.15 | 94.69 | 5.58 | 46% | 54% | 38% | 62% |
| 2011 | 57.79 | 4.15 | 83.62 | 4.91 | 48% | 52% | 39% | 61% |
| 2012 | 66.92 | 3.22 | 91.21 | 5.98 | 42% | 58% | 37% | 63% |
| 2013 | 48.86 | 4.30 | 96.55 | 7.59 | 49% | 51% | 48% | 52% |
| 2014 | 59.15 | 4.94 | 94.05 | 9.09 | 38% | 62% | 37% | 63% |
| 2015 | 59.40 | 5.91 | 89.80 | 8.80 | 23% | 77% | 38% | 62% |
| 2016 | 241.20 | 18.02 | 81.11 | 8.70 | 7% | 93% | 53% | 47% |
| 2017 | 189.34 | 15.82 | 77.30 | 9.84 | 3% | 97% | 50% | 50% |
| 2018 | 270.68 | 19.19 | 87.45 | 6.70 | 1% | 99% | 55% | 45% |
| 2019 | 236.35 | 19.06 | 89.23 | 6.06 | 2% | 98% | 53% | 47% |
| 2020 | 128.31 | 8.49 | 82.40 | 7.06 | 3% | 97% | 49% | 51% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Cunha, V.P.; Botelho, G.M.; de Oliveira, A.H.M.; Monteiro, L.D.; de Barros Franco, D.G.; da Costa Silva, R. Application of the ARIMA Model to Predict Under-Reporting of New Cases of Hansen’s Disease during the COVID-19 Pandemic in a Municipality of the Amazon Region. Int. J. Environ. Res. Public Health 2022, 19, 415. https://doi.org/10.3390/ijerph19010415
da Cunha VP, Botelho GM, de Oliveira AHM, Monteiro LD, de Barros Franco DG, da Costa Silva R. Application of the ARIMA Model to Predict Under-Reporting of New Cases of Hansen’s Disease during the COVID-19 Pandemic in a Municipality of the Amazon Region. International Journal of Environmental Research and Public Health. 2022; 19(1):415. https://doi.org/10.3390/ijerph19010415
Chicago/Turabian Styleda Cunha, Valéria Perim, Glenda Michele Botelho, Ary Henrique Morais de Oliveira, Lorena Dias Monteiro, David Gabriel de Barros Franco, and Rafael da Costa Silva. 2022. "Application of the ARIMA Model to Predict Under-Reporting of New Cases of Hansen’s Disease during the COVID-19 Pandemic in a Municipality of the Amazon Region" International Journal of Environmental Research and Public Health 19, no. 1: 415. https://doi.org/10.3390/ijerph19010415






