Investigating Potential Dose–Response Relationships between Vitamin D Status and Cognitive Performance: A Cross-Sectional Analysis in Middle- to Older-Aged Adults in the Busselton Healthy Ageing Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. BHAS Study Cohort
2.2. Serum 25-Hydroxyvitamin D Measurement
2.3. Cognition
2.4. Covariates
2.5. Statistical Analysis
3. Results
3.1. Characteristics of the Study Cohort
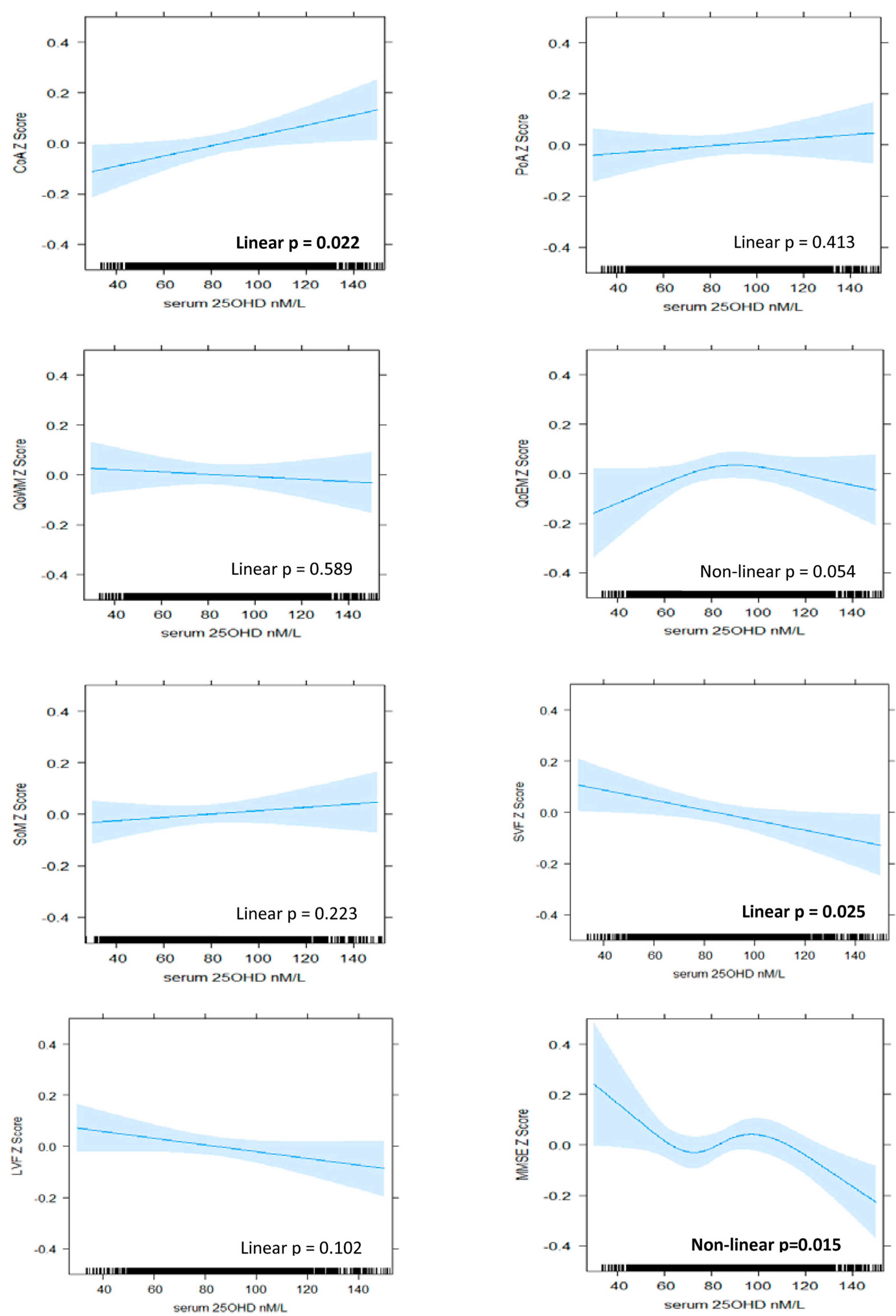
3.2. Patterns of Association between Serum 25OHD Level and Cognitive Performance
3.2.1. Attention
3.2.2. Memory
3.2.3. Verbal Fluency
3.2.4. Global Cognition
3.3. Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goodwill, A.M.; Szoeke, C. A Systematic Review and Meta-Analysis of The Effect of Low Vitamin D on Cognition. J. Am. Geriatr. Soc. 2017, 65, 2161–2168. [Google Scholar] [CrossRef] [PubMed]
- Bartali, B.; Devore, E.; Grodstein, F.; Kang, J.H. Plasma vitamin D levels and cognitive function in aging women: The nurses’ health study. J. Nutr. Health Aging. 2014, 18, 400–406. [Google Scholar] [CrossRef] [Green Version]
- Wilson, V.K.; Houston, D.K.; Kilpatrick, L.; Lovato, J.; Yaffe, K.; Cauley, J.A.; Harris, T.B.; Simonsick, E.M.; Ayonayon, H.N.; Kritchevsky, S.B.; et al. Relationship between 25-hydroxyvitamin D and cognitive function in older adults: The Health, Aging and Body Composition Study. J. Am. Geriatr. Soc. 2014, 62, 636–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Handing, E.P. Mediation and Moderation Analysis of Nutrition, Inflammatory Biomarkers, and Cognition in Older Adults. Ph.D. Thesis, University of South Florida, Tampa, FL, USA, January 2015. [Google Scholar]
- Annweiler, C.; Milea, D.; Whitson, H.E.; Cheng, C.Y.; Wong, T.Y.; Ikram, M.K.; Lamoureux, E.L.; Sabanayagam, C. Vitamin D insufficiency and cognitive impairment in Asians: A multi-ethnic population-based study and meta-analysis. J. Intern. Med. 2016, 280, 300–311. [Google Scholar] [CrossRef]
- Buell, J.S.; Scott, T.M.; Dawson-Hughes, B.; Dallal, G.E.; Rosenberg, I.H.; Folstein, M.F.; Tucker, K.L. Vitamin D is associated with cognitive function in elders receiving home health services. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2009, 64, 888–895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Annweiler, C.; Maby, E.; Meyerber, M.; Beauchet, O. Hypovitaminosis D and executive dysfunction in older adults with memory complaint: A memory clinic-based study. Dement. Geriatr. Cogn. Disord. 2014, 37, 286–293. [Google Scholar] [CrossRef]
- Pettersen, J.A. Vitamin D and executive functioning: Are higher levels better? J. Clin. Exp. Neuroipsychol. 2016, 38, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Karakis, I.; Pase, M.P.; Beiser, A.; Booth, S.L.; Jacques, P.F.; Rogers, G.; DeCarli, C.; Vasan, R.S.; Wang, T.J.; Himali, J.J.; et al. Association of serum Vitamin D with the risk of incident dementia and subclinical indices of brain aging: The Framingham Heart Study. J. Alzheimer’s Dis. 2016, 51, 451–461. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.M.; Tajar, A.; Ulubaev, A.; Pendleton, N.; O’Neill, T.W.; O’Connor, D.B.; Bartfai, G.; Boonen, S.; Bouillon, R.; Casanueva, F.F.; et al. Association between 25-hydroxyvitamin D levels and cognitive performance in middle-aged and older European men. J. Neurol. Neurosurg. 2009, 80, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Kueider, A.M.; Tanaka, T.; An, Y.; Kitner-Triolo, M.H.; Palchamy, E.; Ferrucci, L.; Thambisetti, M. State- and trait-dependent associations of vitamin-D with brain function during aging. Neurobiol. Aging. 2016, 39, 38–45. [Google Scholar] [CrossRef] [Green Version]
- Brouwer-Brolsma, E.M.; van de Rest, O.; Tieland, M.; van der Zwaluw, N.L.; Steegenga, W.T.; Adam, J.J.; van Loon, L.J.C.; Feskens, E.J.M.; de Groot, L.C.P.G.M. Serum 25-hydroxyvitamin D is associated with cognitive executive function in Dutch prefrail and frail elderly: A cross-sectional study exploring the associations of 25-hydroxyvitamin D with glucose metabolism, cognitive performance and depression. J. Am. Med. Dir. Assoc. 2013, 14, 852.e9–852.e17. [Google Scholar] [CrossRef] [PubMed]
- Granic, A.; Hill, T.; Kirkwood, T.; Davies, K.; Collerton, J.; Martin-Ruiz, C.; von Zglinicki, T.; Saxby, B.; Wesnes, K.; Collerton, D.; et al. Serum 25-hydroxyvitamin D and cognitive decline in the very old: The Newcastle 85+ Study. Eur. J. Neurol. 2015, 22, 106–115. [Google Scholar] [CrossRef] [Green Version]
- Breitling, L.P.; Perna, L.; Muller, H.; Raum, E.; Kliegel, M.; Brenner, H. Vitamin D and cognitive functioning in the elderly population in Germany. Exp. Gerontol. 2012, 47, 122–127. [Google Scholar] [CrossRef]
- Van Schoor, N.; Comijs, H.; Llewellyn, D.; Lips, P. Cross-sectional and longitudinal associations between serum 25-hydroxyvitamin D and cognitive functioning. Int. Psychogeriatr. 2016, 28, 759–768. [Google Scholar] [CrossRef]
- McGrath, J.; Scragg, R.; Chant, D.; Eyles, D.; Burne, T.; Obradovic, D. No association between serum 25-hydroxyvitamin D3 level and performance on psychometric tests in NHANES III. Neuroepidemiology 2007, 29, 49–54. [Google Scholar] [CrossRef]
- Maddock, J.; Geoffroy, M.C.; Power, C.; Hypponen, E. 25-Hydroxyvitamin D and cognitive performance in mid-life. Br. J. Nutr. 2014, 111, 904–914. [Google Scholar] [CrossRef] [Green Version]
- Lam, V.; Albrecht, M.; Takechi, R.; Prasopsang, P.; Lee, Y.; Foster, J.; Mamo, J. Serum 25-hydroxyvitamin D is associated with reduced verbal episodic memory in healthy, middle-aged and older adults. Eur. J. Nutr. 2016, 55, 1503–1513. [Google Scholar] [CrossRef]
- Garcion, E.; Wion-Barbot, N.; Montero-Menei, C.N.; Berger, F.; Wion, D. New clues about vitamin D functions in the nervous system. Trends Endocrinol. Metab. 2002, 13, 100–105. [Google Scholar] [CrossRef]
- Annweiler, C.; Schott, A.-M.; Berrut, G.; Chauviré, V.; Le Gall, D.; Inzitari, M.; Beauchet, O. Vitamin D and Ageing: Neurological Issues. Neuropsychobiology. 2010, 62, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Eyles, D.W.; Smith, S.; Kinobe, R.; Hewison, M.; McGrath, J.J. Distribution of the Vitamin D receptor and 1α-hydroxylase in human brain. J. Chem. Neuroanat. 2005, 29, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Landel, V.; Annweiler, C.; Millet, P.; Morello, M.; Feron, F. Vitamin D, Cognition and Alzheimer’s Disease: The Therapeutic Benefit is in the D-Tails. J. Alzheimer’s Dis. 2016, 53, 419–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masoumi, A.; Goldenson, B.; Ghirmai, S.; Avagyan, H.; Zaghi, J.; Abel, K.; Zheng, X.; Espinosa-Jeffrey, A.; Mahanian, M.; Liu, P.T.; et al. 1alpha,25-dihydroxyvitamin D3 interacts with curcuminoids to stimulate amyloid-beta clearance by macrophages of Alzheimer’s disease patients. J. Alzheimer’s Dis. 2009, 17, 703–717. [Google Scholar] [CrossRef] [PubMed]
- Ibi, M.; Sawada, H.; Nakanishi, M.; Kume, T.; Katsuki, H.; Kaneko, S.; Shimohama, S.; Akaike, A. Protective effects of 1α,25-(OH)2D3 against the neurotoxicity of glutamate and reactive oxygen species in mesencephalic culture. Neuropharmacology 2001, 40, 761–771. [Google Scholar] [CrossRef]
- Brewer, L.D.; Thibault, V.; Chen, K.-C.; Langub, M.C.; Landfield, P.W.; Porter, N.M. Vitamin D hormone confers neuroprotection in parallel with downregulation of L-type calcium channel expression in hippocampal neurons. J. Neurosci. 2001, 21, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, Treatment, and Prevention of Vitamin D Deficiency: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mozos, I.; Marginean, O. Links between Vitamin D deficiency and Cardiovascular Diseases. BioMed Res. Int. 2015, 2015, 109275–109312. [Google Scholar] [CrossRef]
- Caraba, A.; Crişan, V.; Romoşan, I.; Mozoş, I.; Murariu, M. Vitamin D status, disease activity, and endothelial dysfunction in early rheumatoid arthritis patients. Dis. Markers. 2017, 2017, 5241012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spina, C.S.; Tangpricha, V.; Uskokovic, M.; Adorinic, L.; Maehr, H.; Holick, M.F. Vitamin D and cancer. Anticancer Res. 2006, 26, 2515–2524. [Google Scholar] [PubMed]
- Sofianopoulou, E.; Kaptoge, S.K.; Afzal, S.; Jiang, T.; Gill, D.; Gundersen, T.E.; Bolton, T.R.; Allara, E.; Arnold, M.G.; Mason, A.M.; et al. Estimating dose-response relationships for vitamin D with coronary heart disease, stroke, and all-cause mortality: Observational and Mendelian randomisation analyses. Lancet Diabetes Endcrinol. 2021, 9, 837–846. [Google Scholar] [CrossRef]
- Zhu, K.; Knuiman, M.; Divitini, M.; Hung, J.; Lim, E.M.; Cooke, B.R.; Walsh, J.P. Serum 25-hydroxyvitamin D as a predictor of mortality and cardiovascular events: A 20-year study of a community-based cohort. Clin. Endocrinol. 2018, 88, 154–163. [Google Scholar] [CrossRef]
- Harrell, F.E. Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis; Springer: New York, NY, USA, 2001. [Google Scholar]
- James, A.; Hunter, M.; Straker, L.; Beilby, J.; Bucks, R.; Davis, T.; Eikelboom, R.; Hillman, D.; Hui, J.; Hung, J.; et al. Rationale, design and methods for a community-based study of clustering and cumulative effects of chronic disease processes and their effects on ageing: The Busselton healthy ageing study. BMC Public Health. 2013, 13, 936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooke, D.J.; Cooke, B.R.; Bell, D.A.; Vasikaran, S.D.; Glendenning, P. 25-Hydroxyvitamin D C3-epimer is universally present in neonatal Western Australian samples but is unlikely to contribute to diagnostic misclassification. Ann. Clin. Biochem. 2015, 53, 593–598. [Google Scholar] [CrossRef] [Green Version]
- Keith, M.S.; Stanislav, S.W.; Wesnes, K.A. Validity of a cognitive computerized assessment system in brain injured patients. Brain Inj. 1998, 12, 1037–1043. [Google Scholar] [CrossRef]
- Wesnes, K.A.; Ward, T.; McGinty, A.; Petrini, O. The memory enhancing effects of a Ginkgo biloba/Panax ginseng combination in healthy middle-aged volunteers. Psychopharmacology 2000, 152, 353–361. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Nelson, H.E.; Willison, J. National Adult Reading Test Manual, 2nd ed.; NFER-Nelson: Windsor, UK, 1991. [Google Scholar]
- Booth, M. Assessment of Physical Activity: An International Perspective. Res. Q. Exerc. Sport. 2015, 71, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B. The PHQ-9: Validity of a brief depression severity measure. J. Gen. Intern. Med. 2001, 16, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Spitzer, R.L.; Kroenke, K.; Williams, J.B.W.; Löwe, B. A Brief Measure for Assessing Generalized Anxiety Disorder: The GAD-7. Arch. Intern. Med. 2006, 166, 1092–1097. [Google Scholar] [CrossRef] [Green Version]
- Lovibond, S.H.; Lovibond, P.F. Manual for the Depression Anxiety Stress Scales, 2nd ed.; Psychology Foundation: Sydney, Australia, 1995. [Google Scholar]
- Shoben, A.B.; Kestenbaum, B.; Levin, G.; Hoofnagle, A.N.; Psaty, B.M.; Siscovick, D.S.; de Boer, I.H. Seasonal Variation in 25-Hydroxyvitamin D Concentrations in the Cardiovascular Health Study. Am. J. Epidemiol. 2011, 174, 1363–1372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sachs, M.C.; Shoben, A.; Levin, G.P.; Robinson-Cohen, C.; Hoofnagle, A.N.; Swords-Jenny, N.; Ix, J.H.; Budoff, M.; Lutsey, P.L.; Siscovick, D.S.; et al. Estimating mean annual 25-hydroxyvitamin D concentrations from single measurements: The Multi-Ethnic Study of Atherosclerosis. Am. J. Clin. Nutr. 2013, 97, 1243–1251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watt, S.; Ong, B.; Crowe, S.F. Developing a regression equation for predicting premorbid functioning in an Australian sample using the National Adult Reading Test: Predicting premorbid functioning. Aust. J. Psychol. 2018, 70, 186–195. [Google Scholar] [CrossRef]
- Lerchbaum, E. Vitamin D and menopause—A narrative review. Maturitas 2014, 79, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Peterson, A.; Mattek, N.; Clemons, A.; Bowman, G.L.; Buracchio, T.; Kaye, J.; Quinn, J. Serum vitamin D concentrations are associated with falling and cognitive function in older adults. J. Nutr. Health Aging 2012, 16, 898–901. [Google Scholar] [CrossRef] [Green Version]
- Feart, C.; Helmer, C.; Merle, B.; Herrmann, F.R.; Annweiler, C.; Dartigues, J.F.; Delcourt, C.; Samieri, C. Associations of lower vitamin D concentrations with cognitive decline and long-term risk of dementia and Alzheimer’s disease in older adults. Alzheimers. Dememt. 2017, 13, 1207–1216. [Google Scholar] [CrossRef]
- Llewellyn, D.J.; Langa, K.M.; Lang, I.A. Serum 25-hydroxyvitamin D concentration and cognitive impairment. J. Geriatr. Psychiatry Neurol. 2009, 22, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Jorde, R.; Mathiesen, E.B.; Rogne, S.; Wilsgaard, T.; Kjaergaard, M.; Grimnes, G.; Schirmer, H. Vitamin D and cognitive function: The Tromso Study. J. Neurol. Sci. 2015, 355, 155–161. [Google Scholar] [CrossRef]
- Miller, J.; Harvey, D.; Beckett, L.A.; Green, R.; Farias, S.T.; Reed, B.; Olichney, J.; Mungas, D.; DeCarli, C. Vitamin D status predicts rates of cognitive decline in a multi-ethnic cohort of older adults. JAMA Neurol. 2015, 72, 1295–1303. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Gooch, H.; Groves, N.J.; Sah, P.; Burne, T.H.; Eyles, D.W.; McGrath, J.J. Vitamin D and the brain: Key questions for future research. J. Steriod Biochem. Mol. 2015, 148, 305–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nabe-Nielsen, K.; Holtermann, A.; Gyntelberg, F.; Garde, A.H.; Islamoska, S.; Prescott, E.; Schnohr, P.; Hansen, Å.M. The effect of occupational physical activity on dementia: Results from the Copenhagen Male Study. Scand. J. Med. Sci. Sports 2021, 31, 446–455. [Google Scholar] [CrossRef] [PubMed]

| Women (N = 2678) | Men (N = 2194) | |
|---|---|---|
| Age (years) | 57.9 ± 5.7 | 58.1 ± 5.9 |
| De-seasonalised serum 25OHD (nM/L) | 78.3 ± 24.3 | 84.9 ± 24.6 |
| Vitamin D deficient (<50 nM/L), n (%) | 255 (9.5) | 83 (3.8) |
| Estimated IQ | 102.6 ± 9.6 | 102.1 ± 10.9 |
| Body mass index (kg/m2) | 27.9 ± 5.5 | 28.5 ± 4.1 |
| Smoking status, n (%) | ||
| Never | 1350 (50.4) | 931 (42.4) |
| Ex | 1094 (40.8) | 1014 (46.2) |
| Current <15 cigarettes per day | 121 (4.5) | 101 (4.6) |
| Current ≥15 cigarettes per day | 113 (4.2) | 148 (6.8) |
| Alcohol consumption (glasses per week), n (%) | ||
| Nil | 256 (9.6) | 133 (6.1) |
| 0 to 2.5 | 843 (31.5) | 286 (13.0) |
| 2.6 to 8.5 | 715 (26.7) | 386 (17.6) |
| 8.6 to 17.9 | 597 (22.3) | 509 (23.2) |
| 18+ | 267 (10.0) | 880 (40.1) |
| Physical activity category (MET minutes/week), n (%) | ||
| 0–599 | 633 (23.6) | 330 (15.0) |
| 600–2999 | 1084 (40.5) | 657 (30.0) |
| 3000+ | 960 (35.9) | 1207 (55.0) |
| Sitting hours per day | 4.3 ± 2.5 | 4.7 ± 2.7 |
| Employment status, n (%) | ||
| Employed | 1567 (58.5) | 1603 (73.1) |
| Retired | 680 (25.4) | 427 (19.5) |
| Other | 431 (16.1) | 164 (7.5) |
| Use of vitamin D supplements, n (%) | 435 (16.2) | 135 (6.2) |
| Self-reported health status, n (%) | ||
| Poor/fair | 218 (8.1) | 210 (9.6) |
| Good | 986 (36.8) | 907 (41.3) |
| Very good/excellent | 1474 (55.1) | 1077 (49.1) |
| Medical history, n (%) | ||
| Depression | 596 (22.3) | 335 (15.4) |
| Anxiety | 158 (5.9) | 72 (3.3) |
| CVD | 98 (3.7) | 178 (8.1) |
| Diabetes | 161 (6.0) | 155 (7.1) |
| Hypertension | 1023 (38.2) | 988 (45.0) |
| Education (level completed), n (%) | ||
| Primary or less | 30 (1.1) | 27 (1.2) |
| Secondary, including TAFE college | 2109 (78.8) | 1747 (79.6) |
| Tertiary | 539 (20.1) | 420 (19.1) |
| Raw cognitive scores | ||
| Continuity of attention factor | 90.6 ± 4.2 | 90.4 ± 3.8 |
| Power of attention factor | 1244.1 ± 138.4 | 1235.2 ± 139.4 |
| Quality of working memory factor | 187.7 ± 16.5 | 187.8 ± 15.2 |
| Quality of episodic memory factor | 190.1 ± 46.2 | 172.6 ± 44.7 |
| Speed of memory factor | 4319.6 ± 859.1 | 4359.9 ± 885.3 |
| Semantic verbal fluency | 19.0 ± 4.8 | 18.1 ± 4.6 |
| Letter verbal fluency | 40.1 ± 11.1 | 35.6 ± 10.8 |
| MMSE | 28.6 ± 1.6 | 28.4 ± 1.6 |
| Cognitive Scores * | Model ^ | Quartile 1 (53.0 nM/L) | Quartile 2 (68.6 nM/L) | Quartile 3 (82.9 nM/L) | Quartile 4 (104.2 nM/L) | p-Values # | Best Fit (Non Linear vs. Linear) | |
|---|---|---|---|---|---|---|---|---|
| Overall | Nonlinear | |||||||
| Continuity of attention factor | 1 | −0.08 (−0.15, −0.02) a | 0.00 (−0.04, 0.05) b | 0.05 (−0.01, 0.10) c | 0.05 (0.00, 0.11) b,c | 0.006 | 0.028 | Nonlinear 3 knots |
| 2 | −0.10 (−0.16, −0.04) a | 0.00 (−0.04, 0.04) b | 0.05 (0.00, 0.10) c | 0.06 (0.01, 0.12) c | <0.001 | 0.009 | ||
| 3 | −0.08 (−0.15, −0.02) a | 0.00 (−0.04, 0.04) b | 0.05 (0.00, 0.09) c | 0.06 (0.00, 0.11) b,c | 0.010 | 0.018 | ||
| 4 | −0.08 (−0.14, −0.01) a | 0.00 (−0.04, 0.04) b | 0.04 (−0.01, 0.09) c | 0.05 (0.00, 0.11) b,c | 0.021 | 0.035 | ||
| Power of attention factor | 1 | −0.01 (−0.07, 0.04) | −0.01 (−0.05, 0.04) | 0.00 (−0.04, 0.04) | 0.01 (−0.04, 0.07) | 0.497 | 0.366 | Linear |
| 2 | 0.00 (−0.05, 0.05) | 0.00 (−0.04, 0.04) | 0.00 (−0.04, 0.04) | 0.00 (−0.05, 0.05) | 0.970 | 0.182 | ||
| 3 | 0.00 (−0.05, 0.06) | 0.00 (−0.04, 0.04) | 0.00 (−0.08, 0.02) | 0.00 (−0.06, 0.05) | 0.842 | 0.101 | ||
| 4 | 0.00 (−0.05, 0.06) | 0.00 (−0.04, 0.04) | 0.00 (−0.04, 0.04) | 0.00 (−0.06, 0.05) | 0.912 | 0.128 | ||
| Quality of working memory factor | 1 | −0.03 (−0.08, 0.03) | −0.01 (−0.05, 0.03) | 0.00 (−0.03, 0.04) | 0.03 (−0.03, 0.08) | 0.176 | 0.131 | Linear |
| 2 | −0.03 (−0.09, 0.02) | −0.01 (−0.05, 0.03) | 0.01 (−0.03, 0.04) | 0.03 (−0.02, 0.09) | 0.085 | 0.078 | ||
| 3 | −0.02 (−0.08, 0.03) | −0.01 (−0.05, 0.03) | 0.00 (−0.03, 0.04) | 0.02 (−0.03, 0.08) | 0.262 | 0.096 | ||
| 4 | −0.02 (−0.08, 0.03) | −0.01 (−0.05, 0.03) | 0.00 (−0.03, 0.04) | 0.02 (−0.03, 0.08) | 0.280 | 0.121 | ||
| Quality of episodic memory factor | 1 | 0.02 (−0.03, 0.08) | 0.01 (−0.03, 0.05) | −0.01 (−0.04, 0.03) | −0.03 (−0.08, 0.03) | 0.214 | 0.995 | Linear |
| 2 | 0.01 (−0.04, 0.06) | 0.00 (−0.04, 0.04) | 0.00 (−0.04, 0.03) | −0.01 (−0.06, 0.04) | 0.085 | 0.642 | ||
| 3 | 0.00 (−0.05, 0.06) | 0.00 (−0.04, 0.04) | 0.00 (−0.04, 0.04 | 0.00 (−0.06, 0.05) | 0.883 | 0.447 | ||
| 4 | 0.00 (−0.05, 0.06) | 0.00 (−0.04, 0.04) | 0.00 (−0.04, 0.04) | −0.01(−0.06, 0.05) | 0.823 | 0.440 | ||
| Speed of memory factor | 1 | −0.04 (−0.10, 0.01) a | −0.02 (−0.06, 0.02) b | 0.01 (−0.03, 0.05) c | 0.04 (−0.01, 0.10) d | 0.032 | 0.736 | Linear |
| 2 | −0.03 (−0.08, 0.02) | −0.01 (−0.05, 0.03) | 0.01 (−0.03, 0.04) | 0.03 (−0.03, 0.08) | 0.150 | 0.894 | ||
| 3 | −0.01 (−0.07, 0.04) | −0.01 (−0.04, 0.03) | 0.00 (−0.03, 0.04) | 0.01 (−0.04, 0.07) | 0.469 | 0.510 | ||
| 4 | −0.02 (−0.07, 0.04) | −0.01 (−0.05, 0.03) | 0.00 (−0.03, 0.04) | 0.02 (−0.04, 0.07) | 0.416 | 0.540 | ||
| Semantic verbal fluency | 1 | 0.03 (−0.02, 0.09) | 0.01 (−0.03, 0.05) | −0.01 (−0.05, 0.03) | −0.04 (−0.09, 0.02) | 0.085 | 0.536 | Linear |
| 2 | 0.02 (−0.03, 0.07) | 0.01 (−0.03, 0.04) | 0.00 (−0.04, 0.03) | −0.02 (−0.07, 0.03) | 0.304 | 0.237 | ||
| 3 | 0.02 (−0.03, 0.07) | 0.01 (−0.03, 0.05) | 0.00 (−0.04, 0.03) | −0.02 (−0.08, 0.03) | 0.283 | 0.183 | ||
| 4 | 0.02 (−0.03, 0.07) | 0.01 (−0.03, 0.05) | 0.00 (−0.04, 0.03) | −0.02 (−0.08, 0.03) | 0.287 | 0.269 | ||
| Letter verbal fluency | 1 | −0.01 (−0.07, 0.04) | 0.00 (−0.05, 0.04) | 0.00 (−0.04, 0.04) | 0.01 (−0.04, 0.07) | 0.577 | 0.075 | Linear |
| 2 | −0.02 (−0.07, 0.03) | −0.01 (−0.05, 0.03) | 0.00 (−0.03, 0.04) | 0.02 (−0.03, 0.07) | 0.216 | 0.071 | ||
| 3 | −0.03 (−0.08, 0.03) | −0.01 (−0.05, 0.03) | 0.00 (−0.03, 0.04) | 0.02 (−0.03, 0.08) | 0.200 | 0.062 | ||
| 4 | −0.03 (−0.08, 0.02) | −0.01 (−0.05, 0.03) | 0.00 (−0.03, 0.04) | 0.03 (−0.03, 0.08) | 0.177 | 0.058 | ||
| Mini-Mental State Examination | 1 | −0.03 (−0.09, 0.02) | −0.01 (−0.05, 0.03) | 0.01 (−0.03, 0.04) | 0.03 (−0.02, 0.09) | 0.105 | 0.536 | Linear |
| 2 | −0.05 (−0.09, 0.00) a | −0.02 (−0.05, 0.02) a | 0.01 (−0.03, 0.04) a | 0.05 (−0.01, 0.10) b | 0.015 | 0.787 | ||
| 3 | −0.05 (−0.10. 0.00) a | −0.02 (−0.06, 0.02) a | 0.01 (−0.03, 0.04) a | 0.05 (−0.00, 0.10) b | 0.016 | 0.991 | ||
| 4 | −0.04 (−0.10, 0.01) a | −0.02 (−0.05, 0.02) a | 0.01 (−0.03, 0.04) a | 0.04 (−0.01, 0.10) b | 0.023 | 0.814 | ||
| Cognitive Scores * | Model ^ | Quartile 1 (59.8 nM/L) | Quartile 2 (75.4 nM/L) | Quartile 3 (89.2 nM/L) | Quartile 4 (110.9 nM/L) | p-Values # | Best Fit (Non Linear vs. Linear) | |
|---|---|---|---|---|---|---|---|---|
| Overall | Nonlinear | |||||||
| Continuity of attention factor | 1 | −0.04 (−0.10, 0.02) | −0.02 (−0.06, 0.03) | 0.01 (−0.04, 0.05) | 0.04 (−0.02, 0.10) | 0.067 | 0.811 | Linear |
| 2 | −0.06 (−0.12, 0.00) a | −0.02 (−0.07, 0.02) b | 0.01 (−0.03, 0.05) c | 0.06 (0.00, 0.12) d | 0.007 | 0.671 | ||
| 3 | −0.05 (−0.11, 0.01) a | −0.02 (−0.06, 0.02) b | 0.00 (−0.03, 0.05) c | 0.05 (−0.01, 0.11) d | 0.021 | 0.591 | ||
| 4 | −0.04 (−0.11, 0.03) a | −0.02 (−0.07, 0.02) b | 0.00 (−0.05, 0.05) c | 0.05 (−0.01, 0.11) d | 0.022 | 0.592 | ||
| Power of attention factor | 1 | −0.03 (−0.09, 0.03) | −0.01 (−0.06, 0.03) | 0.00 (−0.04, 0.05) | 0.03 (−0.03, 0.09) | 0.195 | 0.455 | Linear |
| 2 | −0.01 (−0.07, 0.05) | 0.00 (−0.05, 0.04) | 0.00 (−0.04, 0.04) | 0.01 (−0.05, 0.07) | 0.656 | 0.366 | ||
| 3 | −0.01 (−0.07, 0.04) | −0.01 (−0.05, 0.04) | 0.00 (−0.04, 0.04) | 0.02 (−0.05, 0.08) | 0.510 | 0.306 | ||
| 4 | −0.02 (−0.08, 0.04) | −0.01 (−0.05, 0.04) | 0.00 (−0.04, 0.04) | 0.02 (−0.04, 0.08) | 0.413 | 0.274 | ||
| Quality of working memory factor | 1 | 0.02 (−0.04, 0.08) | 0.01 (−0.04, 0.05) | 0.00 (−0.05, 0.04) | −0.02 (−0.08, 0.04) | 0.401 | 0.518 | Linear |
| 2 | 0.00 (−0.05, 0.06) | 0.00 (−0.04, 0.05) | 0.00 (−0.04, 0.04) | 0.00 (−0.06, 0.06) | 0.831 | 0.495 | ||
| 3 | 0.01 (−0.05, 0.07) | 0.01 (−0.04, 0.05) | 0.00 (−0.04, 0.04) | −0.01 (−0.07, 0.05) | 0.574 | 0.379 | ||
| 4 | 0.01 (−0.05, 0.07) | 0.00 (−0.04, 0.05) | 0.00 (−0.04, 0.04) | −0.01 (−0.07, 0.05) | 0.589 | 0.392 | ||
| Quality of episodic memory factor | 1 | −0.03 (−0.09, 0.04) | 0.03 (−0.02, 0.07) | 0.04 (−0.02, 0.09) | −0.01 (−0.07, 0.05) | 0.084 | 0.032 | Nonlinear 3 knots |
| 2 | −0.04 (−0.11, 0.02) a | 0.02 (−0.03, 0.06) b | 0.04 (−0.02, 0.09) a,b | 0.01 (−0.04, 0.07) a,b | 0.108 | 0.040 | ||
| 3 | −0.04 (−0.11, 0.02) | 0.01 (−0.03, 0.06) | 0.04 (−0.02, 0.09) | 0.01 (−0.05, 0.07) | 0.136 | 0.050 | ||
| 4 | −0.04 (−0.10, 0.03) | 0.02 (−0.03, 0.06) | 0.04 (−0.02, 0.09) | 0.01 (−0.05, 0.07) | 0.151 | 0.054 | ||
| Speed of memory factor | 1 | −0.06 (−0.11, 0.00) a | −0.02 (−0.07, 0.02) b | 0.01 (−0.03, 0.05) c | 0.06 (0.00, 0.12) d | 0.010 | 0.269 | Linear |
| 2 | −0.04 (−0.09, 0.02) | −0.01 (−0.06, 0.03) | 0.01 (−0.03, 0.05) | 0.04 (−0.02, 0.10) | 0.080 | 0.202 | ||
| 3 | −0.02 (−0.08, 0.03) | −0.01 (−0.05, 0.03) | 0.01 (−0.04, 0.05) | 0.03 (−0.03, 0.09) | 0.249 | 0.200 | ||
| 4 | −0.03 (−0.09, 0.03) | −0.01 (−0.05, 0.03) | 0.01 (−0.04, 0.05) | 0.03 (−0.03, 0.09) | 0.223 | 0.196 | ||
| Semantic verbal fluency | 1 | 0.06 (0.00, 0.12) a | 0.02 (−0.02, 0.07) b | −0.01 (−0.05, 0.03) c | −0.06 (−0.12, 0.00) d | 0.005 | 0.192 | Linear |
| 2 | 0.04 (−0.02, 0.10) | 0.01 (−0.03, 0.06) | −0.01 (−0.05, 0.03) | −0.04 (−0.10, 0.02) | 0.057 | 0.195 | ||
| 3 | 0.05 (−0.01, 0.11) a | 0.02 (−0.02, 0.06) b | −0.01 (−0.05, 0.03) c | −0.05 (−0.11, 0.01) d | 0.021 | 0.219 | ||
| 4 | 0.05 (−0.01, 0.11) a | 0.02 (−0.02, 0.06) b | −0.01 (−0.05, 0.03) c | −0.05 (−0.11, 0.01) d | 0.025 | 0.254 | ||
| Letter verbal fluency | 1 | 0.05 (−0.01, 0.11) a | 0.02 (−0.03, 0.06) b | −0.01 (−0.05, 0.03) c | −0.05 (−0.11, 0.01) d | 0.021 | 0.737 | Linear |
| 2 | 0.02 (−0.03, 0.07) | 0.01 (−0.03, 0.05) | 0.00 (−0.04, 0.03) | −0.02 (−0.08, 0.03) | 0.284 | 0.643 | ||
| 3 | 0.03 (−0.04, 0.09) | 0.02 (−0.03, 0.06) | 0.00 (−0.05, 0.05) | −0.04 (−0.09, 0.02) | 0.082 | 0.648 | ||
| 4 | 0.03 (−0.04, 0.09) | 0.01 (−0.03, 0.06) | 0.00 (−0.05, 0.05) | −0.03 (−0.09, 0.02) | 0.102 | 0.741 | ||
| Mini-Mental State Examination | 1 | 0.02 (−0.05, 0.09) | −0.03 (−0.09, 0.04) | 0.04 (−0.02, 0.09) | 0.00 (−0.07, 0.08) | <0.001 | 0.006 | Nonlinear 4 knots |
| 2 | 0.00 (−0.07, 0.06) a,b | −0.03 (−0.09, 0.03) a | 0.04 (−0.01, 0.09) b | 0.02 (−0.05, 0.09) a,b | 0.004 | 0.007 | ||
| 3 | 0.02 (−0.05, 0.08) | −0.03 (−0.09, 0.03) | 0.03 (−0.02, 0.08) | 0.01 (−0.06, 0.08) | 0.002 | 0.010 | ||
| 4 | 0.02 (−0.05, 0.08) | −0.03 (−0.09, 0.03) | 0.03 (−0.02, 0.08) | 0.01 (−0.06, 0.08) | 0.003 | 0.015 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harse, J.D.; Zhu, K.; Bucks, R.S.; Hunter, M.; Lim, E.M.; Cooke, B.R.; Walsh, J.P.; Murray, K. Investigating Potential Dose–Response Relationships between Vitamin D Status and Cognitive Performance: A Cross-Sectional Analysis in Middle- to Older-Aged Adults in the Busselton Healthy Ageing Study. Int. J. Environ. Res. Public Health 2022, 19, 450. https://doi.org/10.3390/ijerph19010450
Harse JD, Zhu K, Bucks RS, Hunter M, Lim EM, Cooke BR, Walsh JP, Murray K. Investigating Potential Dose–Response Relationships between Vitamin D Status and Cognitive Performance: A Cross-Sectional Analysis in Middle- to Older-Aged Adults in the Busselton Healthy Ageing Study. International Journal of Environmental Research and Public Health. 2022; 19(1):450. https://doi.org/10.3390/ijerph19010450
Chicago/Turabian StyleHarse, Janis D., Kun Zhu, Romola S. Bucks, Michael Hunter, Ee Mun Lim, Brian R. Cooke, John P. Walsh, and Kevin Murray. 2022. "Investigating Potential Dose–Response Relationships between Vitamin D Status and Cognitive Performance: A Cross-Sectional Analysis in Middle- to Older-Aged Adults in the Busselton Healthy Ageing Study" International Journal of Environmental Research and Public Health 19, no. 1: 450. https://doi.org/10.3390/ijerph19010450
APA StyleHarse, J. D., Zhu, K., Bucks, R. S., Hunter, M., Lim, E. M., Cooke, B. R., Walsh, J. P., & Murray, K. (2022). Investigating Potential Dose–Response Relationships between Vitamin D Status and Cognitive Performance: A Cross-Sectional Analysis in Middle- to Older-Aged Adults in the Busselton Healthy Ageing Study. International Journal of Environmental Research and Public Health, 19(1), 450. https://doi.org/10.3390/ijerph19010450






