Antimicrobial Resistance of Acetobacter and Komagataeibacter Species Originating from Vinegars
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains of Acetic Acid Bacteria
2.2. Analysis of Antimicrobial Resistance (AMR)
2.3. Bioinformatics
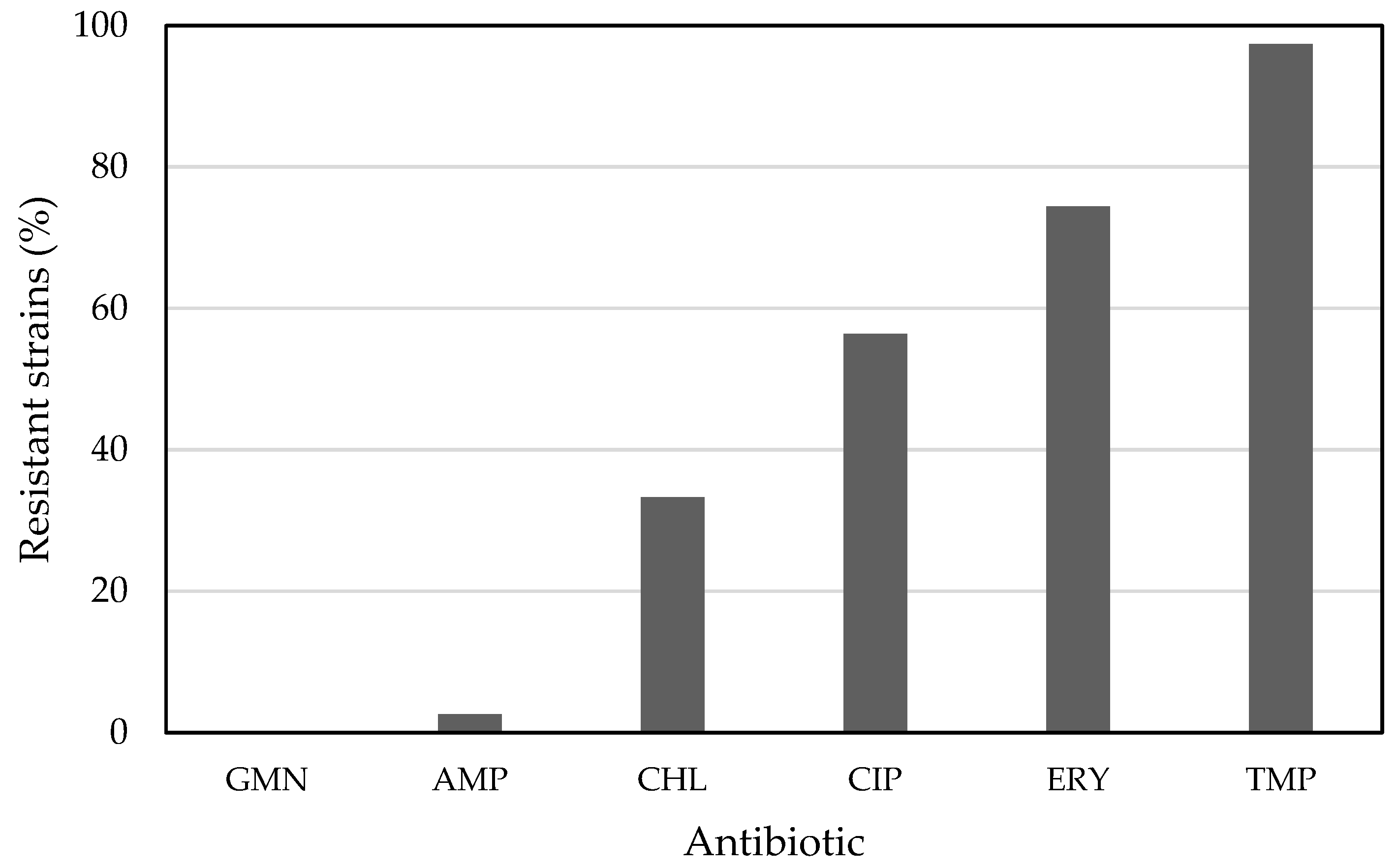
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davies, J.; Davies, D. Origins and Evolution of Antibiotic Resistance. Microbiol. Mol. Biol. Rev. 2010, 74, 417–433. [Google Scholar] [CrossRef] [PubMed]
- Aarts, H.; Margolles, A. Antibiotic resistance genes in food and gut (non pathogenic) bacteria. Bad genes in good bugs. Front. Microbiol. 2015, 5, 754. [Google Scholar] [CrossRef] [PubMed]
- Grevskott, D.H.; Ghavidel, F.Z.; Svanevik, C.S.; Marathe, N.P. Resistance profiles and diversity of β-lactamases in Escherichia coli strains isolated from city-scale sewage surveillance in Bergen, Norway mimic clinical prevalence. Ecotoxicol. Environ. Saf. 2021, 226, 112788. [Google Scholar] [CrossRef] [PubMed]
- Trček, J.; Mahnič, A.; Rupnik, M. Diversity of the microbiota involved in wine and organic apple cider submerged vinegar production as revealed by DHPLC analysis and next-generation sequencing. Int. J. Food Microbiol. 2016, 223, 57–62. [Google Scholar] [CrossRef]
- Škraban, J.; Cleenwerck, I.; Vandamme, P.; Fanedl, L.; Trček, J. Genome sequences and description of novel exopolysaccharides producing species Komagataeibacter pomaceti sp. nov. and reclassification of Komagataeibacter kombuchae (Dutta and Gachhui 2007) Yamada et al., 2013 as a later heterotypic synonym of Komagataeibacter hansenii (Gosselé et al. 1983) Yamada et al., 2013. Syst. Appl. Microbiol. 2018, 41, 581–592. [Google Scholar] [CrossRef]
- Štornik, A.; Skok, B.; Trček, J. Comparison of cultivable acetic acid bacterial microbiota in organic and conventional apple cider vinegar. Food Technol. Biotechnol. 2016, 54, 113–119. [Google Scholar] [CrossRef]
- Trcek, J.; Toyama, H.; Czuba, J.; Misiewicz, A.; Matsushita, K. Correlation between acetic acid resistance and characteristics of PQQ-dependent ADH in acetic acid bacteria. Appl. Microbiol. Biotechnol. 2006, 70, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Gouby, A.; Teyssier, C.; Vecina, F.; Marchandin, H.; Granolleras, C.; Zorgniotti, I.; Jumas-Bilak, E. Acetobacter cibinongensis bacteremia in human. Emerg. Infect. Dis. 2007, 13, 784–785. [Google Scholar] [CrossRef] [PubMed]
- Kohlmann, K.; Barenberg, K.; Anders, A.; Gatermann, S.G. Acetobacter indonesiensis Bacteremia in Child with Metachromatic Leukodystrophy. Emerg. Infect. Dis. 2016, 22, 1681–1683. [Google Scholar] [CrossRef]
- Basu, S.S.; Delaney, M.L.; Li, N.; Onderdonk, A.B.; Bry, L. Acetobacter indonesiensis Pneumonia after Lung Transplantation. Emerg. Infect. Dis. 2018, 24, 598–599. [Google Scholar] [CrossRef]
- Wu, L.; Xie, X.; Li, Y.; Liang, T.; Zhong, H.; Ma, J.; Yang, L.; Yang, J.; Li, L.; Xi, Y.; et al. Metagenomics-based analysis of the age-related cumulative effect of antibiotic resistance genes in gut microbiota. Antibiotics 2021, 10, 1006. [Google Scholar] [CrossRef] [PubMed]
- Trček, J.; Teuber, M. Genetic and restriction analysis of the 16S-23S rDNA internal transcribed spacer regions of the acetic acid bacteria. FEMS Microbiol. Lett. 2002, 208, 69–75. [Google Scholar] [CrossRef]
- Trček, J.; Barja, F. Updates on quick identification of acetic acid bacteria with a focus on the 16S-23S rRNA gene internal transcribed spacer and the analysis of cell proteins by MALDI-TOF mass spectrometry. Int. J. Food Microbiol. 2015, 196, 137–144. [Google Scholar] [CrossRef]
- Sokollek, S.J.; Hammes, W.P. Description of a Starter Culture Preparation for Vinegar Fermentation. Syst. Appl. Microbiol. 1997, 20, 481–491. [Google Scholar] [CrossRef]
- Antimicrobial Susceptibility Testing EUCAST Disk Diffusion Method Version 8.0. 2020, pp. 1–21. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Disk_test_documents/2020_manuals/Manual_v_8.0_EUCAST_Disk_Test_2020.pdf (accessed on 16 June 2020).
- Alcock, B.P.; Raphenya, A.R.; Lau, T.T.Y.; Tsang, K.K.; Bouchard, M.; Edalatmand, A.; Huynh, W.; Nguyen, A.L.V.; Cheng, A.A.; Liu, S.; et al. CARD 2020: Antibiotic resistome surveillance with the comprehensive antibiotic resistance database. Nucleic Acids Res. 2020, 48, D517–D525. [Google Scholar] [CrossRef] [PubMed]
- Gorgieva, S.; Trček, J. Bacterial cellulose: Production, modification and perspectives in biomedical applications. Nanomaterials 2019, 9, 1352. [Google Scholar] [CrossRef]
- AlRabiah, H.; Allwood, J.W.; Correa, E.; Xu, Y.; Goodacre, R. pH plays a role in the mode of action of trimethoprim on Escherichia coli. PLoS ONE 2018, 13, e0200272. [Google Scholar] [CrossRef]
- Jiang, M.; Kuang, S.; Lai, S.; Zhang, S.; Yang, J.; Peng, B.; Peng, X.; Chen, Z.G.; Li, H. Na+-NQR Confers Aminoglycoside Resistance via the Regulation of L-Alanine Metabolism. mBIO 2020, 11, e02086-20. [Google Scholar] [CrossRef]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Amyes, S.G.B.; Towner, K.J. Trimethoprim resistance; Epidemiology and molecular aspects. J. Med. Microbiol. 1990, 31, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Kahlmeter, G.; Menday, P. Cross-resistance and associated resistance in 2478 Escherichia coli isolates from the Pan-European ECO·SENS project surveying the antimicrobial susceptibility of pathogens from uncomplicated urinary tract infections. J. Antimicrob. Chemother. 2003, 52, 128–131. [Google Scholar] [CrossRef] [PubMed]
- Milanović, V.; Osimani, A.; Cardinali, F.; Litta-Mulondo, A.; Vignaroli, C.; Citterio, B.; Mangiaterra, G.; Aquilanti, L.; Garofalo, C.; Biavasco, F.; et al. Erythromycin-resistant lactic acid bacteria in the healthy gut of vegans, ovo-lacto vegetarians and omnivores. PLoS ONE 2019, 14, e0220549. [Google Scholar] [CrossRef] [PubMed]
- Brisaert, M.; Gabriëls, M.; Plaizier-Vercammen, J. Investigation of the chemical stability of an erythromycin-tretinoin lotion by the use of an optimization system. Int. J. Pharm. 2000, 197, 153–160. [Google Scholar] [CrossRef]
- Torniainen, K.; Tammilehto, S.; Ulvi, V. The effect of pH, buffer type and drug concentration on the photodegradation of ciprofloxacin. Int. J. Pharm. 1996, 131, 53–61. [Google Scholar] [CrossRef]
- Gavrilin, M.V.; Ushakova, L.S.; Gonyan, S.A. Stability of an infusion form of ciprofloxacin hydrochloride. Pharm. Chem. J. 2003, 37, 106–109. [Google Scholar] [CrossRef]
- D’Atanasio, N.; de Joannon, A.C.; Di Sante, L.; Mangano, G.; Ombrato, R.; Vitiello, M.; Bartella, C.; Magarò, G.; Prati, F.; Milanese, C.; et al. Antibacterial activity of novel dual bacterial DNA type II topoisomerase inhibitors. PLoS ONE 2020, 15, e0228509. [Google Scholar] [CrossRef]
- Papkou, A.; Hedge, J.; Kapel, N.; Young, B.; MacLean, R.C. Efflux pump activity potentiates the evolution of antibiotic resistance across S. aureus isolates. Nat. Commun. 2020, 11, 3970. [Google Scholar] [CrossRef]
- Trivedi, M.K.; Patil, S.; Shettigar, H.; Bairwa, K.; Jana, S. Spectroscopic Characterization of Chloramphenicol and Tetracycline: An Impact of Biofield Treatment. Pharm. Anal. Acta 2015, 6, 395. [Google Scholar] [CrossRef]
- Crofts, T.S.; Sontha, P.; King, A.O.; Wang, B.; Biddy, B.A.; Zanolli, N.; Gaumnitz, J.; Dantas, G. Discovery and Characterization of a Nitroreductase Capable of Conferring Bacterial Resistance to Chloramphenicol. Cell Chem. Biol. 2019, 26, 559–570.e6. [Google Scholar] [CrossRef]
- Gilbert, D.N.; Eubanks, N. Effect of pH and human serum on the susceptibility of group D streptococci (Enterococci) to ampicillin in vitro. Antimicrob. Agents Chemother. 1975, 7, 387–395. [Google Scholar] [CrossRef][Green Version]
- Garneau-Tsodikova, S.; Labby, K.J. Mechanisms of Resistance to Aminoglycoside Antibiotics: Overview and Perspectives. Med. Chem. Commun. 2016, 7, 11–27. [Google Scholar] [CrossRef]
- Munita, J.M.; Arias, C.A. Mechanisms of Antibiotic Resistance. Microbiol. Spectr. 2016, 4, 417–433. [Google Scholar] [CrossRef] [PubMed]
| Species and Strain Designation | Source of Isolation |
|---|---|
| Acetobacter aceti DSM 3508T | Beechwood shavings of vinegar plant |
| Acetobacter estunensis AV380 | Apple cider vinegar (company Apis Vita, Fram, Slovenia) |
| Acetobacter estunensis AV390 | Apple cider vinegar (company Apis Vita, Fram, Slovenia) |
| Acetobacter orleanensis IFO 13752T | Beer (Belgium) |
| Acetobacter pasteurianus DSM 3509T | Beer (The Netherlands) |
| Acetobacter pasteurianus AV366 | Apple cider vinegar (company Apis Vita, Fram, Slovenia) |
| Acetobacter pasteurianus JK_T6K1 | Apple cider vinegar (company Šampionka Renče, Volčja Draga, Slovenia) |
| Acetobacter pasteurianus JK_T1K1 | Apple cider vinegar (company Šampionka Renče, Volčja Draga, Slovenia) |
| Acetobacter pasteurianus BJK_1B | Apple cider vinegar (company Šampionka Renče, Volčja Draga, Slovenia) |
| Acetobacter pasteurianus SI3123 | Apple cider vinegar (company Kisarna Simonič, Zgornja Ščavnica, Slovenia) |
| Acetobacter pomorum LMG 18848T | Apple cider vinegar (Germany) |
| Acetobacter pomorum AV440 | Apple cider vinegar (company Apis Vita, Fram, Slovenia) |
| Acetobacter tropicalis IFO 16470T | Coconut (Indonesia) |
| Komagataeibacter europaeus LMG 18494 | Red wine vinegar produced in submerged bioreactor (company Kolinska, Ljubljana, Slovenia) |
| Komagataeibacter europaeus LMG 20956 | Apple cider vinegar (company Kolinska, Ljubljana, Slovenia) |
| Komagataeibacter hansenii DSM 5602T | Vinegar (Israel) |
| Komagataeibacter hansenii LMG 23726 | Kombucha (India) |
| Komagataeibacter kakiaceti LMG 26206T | Kaki vinegar (Japan) |
| Komagataeibacter maltaceti LMG 1529T | Malt vinegar brewery acetifiers |
| Komagataeibacter maltaceti SKU 1109 | Fruit (Thailand) |
| Komagataeibacter medellinensis LMG 1693T | Vinegar |
| Komagataeibacter melaceti AV382T | Apple cider vinegar (company Apis Vita, Fram, Slovenia) |
| Komagataeibacter melomenusus AV436T | Apple cider vinegar (company Apis Vita, Fram, Slovenia) |
| Komagataeibacter melomenusus SI3083 | Apple cider vinegar (company Kisarna Simonič, Zgornja Ščavnica, Slovenia) |
| Komagataeibacter nataicola LMG 1536T | Nata de coco (Philippines) |
| Komagateibacter oboediens AV371 | Apple cider vinegar (company Apis Vita, Fram, Slovenia) |
| Komagataeibacter oboediens BJK_8C | Apple cider vinegar (company Šampionka Renče, Volčja Draga, Slovenia) |
| Komagataeibacter oboediens SI3053 | Apple cider vinegar (company Kisarna Simonič, Zgornja Ščavnica, Slovenia) |
| Komagataeibacter pomaceti T5K1T | Apple cider vinegar (company Šampionka Renče, Volčja Draga, Slovenia) |
| Komagataeibacter pomaceti AV445 | Apple cider vinegar (company Apis Vita, Fram, Slovenia) |
| Komagataeibacter pomaceti AV446 | Apple cider vinegar (company Apis Vita, Fram, Slovenia) |
| Komagataeibacter pomaceti SI3133 | Apple cider vinegar (company Kisarna Simonič, Zgornja Ščavnica, Slovenia) |
| Komagataeibacter rhaeticus DSM 16663T | Organic apple juice (Italy) |
| Komagataeibacter saccharivorans LMG 1582T | Beet juice (Germany) |
| Komagataeibacter saccharivorans AV378 | Apple cider vinegar (company Apis Vita, Fram, Slovenia) |
| Komagataeibacter saccharivorans JK_3A | Apple cider vinegar (company Šampionka Renče, Volčja Draga, Slovenia) |
| Komagataeibacter swingsii LMG 22125T | Organic apple juice (Italy) |
| Gluconacetobacter entanii SI2035 | Apple cider vinegar (company Kisarna Simonič, Zgornja Ščavnica, Slovenia) |
| Gluconacetobacter entanii AV429 | Apple cider vinegar (company Apis Vita, Fram, Slovenia) |
| Species and Strain Designation | GMN | AMP | CHL | CIP | ERY | TMP |
|---|---|---|---|---|---|---|
| Acetobacter aceti DSM 3508T | X | X | X | |||
| Acetobacter estunensis AV380 | X | X | X | X | ||
| Acetobacter estunensis AV390 | X | X | X | X | ||
| Acetobacter orleanensis IFO 13752T | X | X | X | |||
| Acetobacter pasteurianus DSM 3509T | X | X | ||||
| Acetobacter pasteurianus AV366 | X | X | ||||
| Acetobacter pasteurianus JK_T6K1 | X | X | X | X | ||
| Acetobacter pasteurianus JK_T1K1 | X | X | X | X | ||
| Acetobacter pasteurianus BJK_1B | X | X | X | |||
| Acetobacter pasteurianus SI3123 | X | X | X | |||
| Acetobacter pomorum LMG 18848T | X | X | X | |||
| Acetobacter pomorum AV440 | X | X | X | |||
| Acetobacter tropicalis IFO 16470T | X | X | ||||
| Komagataeibacter europaeus LMG 18494 | X | X | ||||
| Komagataeibacter europaeus LMG 20956 | X | X | ||||
| Komagataeibacter hansenii DSM 5602T | X | X | ||||
| Komagataeibacter hansenii LMG 23726 | X | X | ||||
| Komagataeibacter kakiaceti LMG 26206T | X | X | X | X | ||
| Komagataeibacter maltaceti LMG 1529T | X | X | ||||
| Komagataeibacter maltaceti SKU 1109 | X | X | ||||
| Komagataeibacter medellinensis LMG 1693T | X | X | X | |||
| Komagataeibacter melaceti AV382T | X | |||||
| Komagataeibacter melomenusus AV436T | X | |||||
| Komagataeibacter melomenusus SI3083 | X | X | X | |||
| Komagataeibacter nataicola LMG 1536T | X | X | ||||
| Komagateibacter oboediens AV371 | X | X | X | X | ||
| Komagataeibacter oboediens BJK_8C | X | X | X | |||
| Komagataeibacter oboediens SI3053 | X | X | ||||
| Komagataeibacter pomaceti T5K1T | X | X | ||||
| Komagataeibacter pomaceti AV445 | X | X | ||||
| Komagataeibacter pomaceti AV446 | X | X | ||||
| Komagataeibacter pomaceti SI3133 | X | X | X | X | ||
| Komagataeibacter rhaeticus DSM 16663T | X | X | X | |||
| Komagataeibacter saccharivorans LMG 1582T | X | X | X | |||
| Komagataeibacter saccharivorans AV378 | X | X | X | |||
| Komagataeibacter saccharivorans JK_3A | X | X | X | |||
| Komagataeibacter swingsii LMG 22125T | X | X | ||||
| Gluconacetobacter entanii SI2035 | X | X | X | |||
| Gluconacetobacter entanii AV429 | X |
| Resistance Mechanism | AMR Gene Family | Number of Homologues |
|---|---|---|
| Antibiotic efflux | Major facilitator superfamily (MFS) antibiotic efflux pump, resistance-nodulation-cell division (RND) antibiotic efflux pump, multidrug and toxic compound extrusion (MATE) transporter, ATP-binding cassette (ABC) antibiotic efflux pump, small multidrug resistance (SMR) antibiotic efflux pump. | 648 |
| Antibiotic target alteration | Pmr phosphoethanolamine transferase, antibiotic-resistant murA transferase, rifampin glycosyltransferase, streptogramin vat acetyltransferase, MCR phosphoethanolamine transferase, fosfomycin thiol transferase, fluoroquinolone-resistant gyrB, daptomycin-resistant cls. | 259 |
| Antibiotic inactivation | LHK beta-lactamase, AmpC-type beta-lactamase, NmcA beta-lactamase, subclass B3 LRA beta-lactamase, AIM beta-lactamase, CRD3 beta-lactamase, DHT2 beta-lactamase, SRT beta-lactamase, CGA beta-lactamase, SST beta-lactamase, chloramphenicol phosphotransferase, chloramphenicol acetyltransferase, tetracycline inactivation enzyme, fosfomycin thiol transferase. | 62 |
| Reduced permeability to antibiotic | General bacterial porin with reduced permeability to beta-lactams, intrinsic peptide antibiotic resistant Lps, outer membrane porin (Opr). | 27 |
| Antibiotic target replacement | Methicillin-resistant PBP2. | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cepec, E.; Trček, J. Antimicrobial Resistance of Acetobacter and Komagataeibacter Species Originating from Vinegars. Int. J. Environ. Res. Public Health 2022, 19, 463. https://doi.org/10.3390/ijerph19010463
Cepec E, Trček J. Antimicrobial Resistance of Acetobacter and Komagataeibacter Species Originating from Vinegars. International Journal of Environmental Research and Public Health. 2022; 19(1):463. https://doi.org/10.3390/ijerph19010463
Chicago/Turabian StyleCepec, Eva, and Janja Trček. 2022. "Antimicrobial Resistance of Acetobacter and Komagataeibacter Species Originating from Vinegars" International Journal of Environmental Research and Public Health 19, no. 1: 463. https://doi.org/10.3390/ijerph19010463
APA StyleCepec, E., & Trček, J. (2022). Antimicrobial Resistance of Acetobacter and Komagataeibacter Species Originating from Vinegars. International Journal of Environmental Research and Public Health, 19(1), 463. https://doi.org/10.3390/ijerph19010463







